Investigation of Various Active Layers for Their Performance on Organic Solar Cells
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic applianceson plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Outlook brightens for plastic solar cells. Science 2011, 332, 293. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.W. Two-layer organic photovoltaic cell. Appl. Phys. Lett. 1986, 48, 183–185. [Google Scholar] [CrossRef]
- Cheyns, D.; Rand, B.P.; Heremans, P. Organic tandem solar cells with complementary absorbing layers and a high open-circuit voltage. Appl. Phys. Lett. 2010, 97. [Google Scholar] [CrossRef]
- You, J.; Dou, L.D.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Xu, Z.; Hong, Z.; Yang, Y. Interface investigation and engineering—Achieving high performance polymer photovoltaicdevices. J. Mater. Chem. 2010, 20, 2575–2598. [Google Scholar] [CrossRef]
- Zhang, M.; Irfan, I.M.; Ding, H.; Gao, Y.; Tang, C.W. Organic Schottky barrier photovoltaic cells based on MoOx/C60. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Li, C.Z.; Yip, H.L.; Jen, A.K.Y. Functional fullerenes for organic photovoltaics. J. Mater. Chem. 2012, 22, 4161–4177. [Google Scholar] [CrossRef]
- He, Y.; Li, Y. Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 2011, 13, 1970–1983. [Google Scholar] [CrossRef] [PubMed]
- Hoke, E.T.; Vandewal, K.; Bartelt, J.A.; Mateker, W.R.; Douglas, J.D.; Noriega, R.; Graham, K.R.; Fréchet, J.M.J.; Salleo, A.; McGehee, M.D. Recombination in polymer:fullerene solar cells with open-circuit voltages approaching and exceeding 1.0 v. Adv. Energy Mater. 2012, 3, 220–230. [Google Scholar] [CrossRef]
- Gommans, H.; Aernouts, T.; Verreet, B.; Heremans, P.; Medina, A.; Claessens, C.G.; Torres, T. Perfluorinated subphthalocyanine as a new acceptor material in a small-molecule bilayer organic solar cell. Adv. Funct. Mater. 2009, 19, 3435–3439. [Google Scholar] [CrossRef]
- Verreet, B.; Rand, B.P.; Cheyns, D.; Hadipour, A.; Aernouts, T.; Heremans, P.; Medina, A.; Claessens, C.G.; Torres, T. A 4% efficient organic solar cell using a fluorinated fused subphthalocyanine dimer as an electron acceptor. Adv. Energy Mater. 2011, 1, 565–568. [Google Scholar] [CrossRef]
- Sullivan, P.; Durand, A.; Hancox, I.; Beaumot, N.; Mirri, G.; Tucker, J.H.R.; Hatton, R.A.; Shipman, M.; Jones, T.S. Halogenated boron subphthalocyanines as light harvesting electron acceptors in organic photovoltaics. Adv. Energy Mater. 2011, 1, 352–355. [Google Scholar] [CrossRef]
- Huang, C.J.; Ke, J.C.; Chen, W.R.; Meen, T.H.; Yang, C.F. Improved the efficiency of small molecule organic solar cell by double anode buffer layers. Sol. Energy Mater. Sol. 2011, 95, 3460–3464. [Google Scholar] [CrossRef]
- Ke, J.C.; Wang, Y.H.; Chen, K.L.; Huang, C.J. Effect of organic solar cells using various sheet resistances of indium tin oxide and different cathodes: Aluminum, silver. Synth. Met. 2015, 201, 25–29. [Google Scholar] [CrossRef]
- Ke, J.C.; Wang, Y.H.; Chen, K.L.; Huang, C.J. Effect of open-circuit voltage in organic solar cells based on various electron donor materials by inserting molybdenum trioxide anode buffer layer. Sol. Energy Mater. Sol. 2015, 133, 248–254. [Google Scholar] [CrossRef]
- Brabec, C.J.; Cravino, A.; Meissner, D.; Sariciftci, N.S.; Fromherz, T.; Rispens, M.T.; Sanchez, L.; Hummelen, J.C. Origin of the open circuit voltage of plastic solar cells. Adv. Funct. Mater. 2001, 11, 374–380. [Google Scholar] [CrossRef]
- Spano, F.C. Modeling disorder in polymer aggregates: The optical spectroscopy of regioregular poly(3-hexylthiophene) thin films. J. Chem. Phys. 2005, 122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Belmonte, G.; Bisquert, J. Open-circuit voltage limit caused by recombination through tail states in bulk heterojunction polymer-fullerene solar cells. Appl. Phys. Lett. 2010, 96. [Google Scholar] [CrossRef]
- Förster, T. 10th Spiers Memorial Lecture. Transfer mechanisms of electronic excitation. Discuss. Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Scully, S.R.; Armstrong, P.B.; Edder, C.; Fre’chet, J.M.J.; McGehee, M.D. Long-range resonant energy transfer for enhanced exciton harvesting for organic solar cells. Adv. Mater. 2007, 19, 2961–2966. [Google Scholar] [CrossRef]
- Gommans, H.; Schols, S.; Kadashchuk, A.; Heremans, P. Exciton diffusion length and lifetime in subphthalocyanine films. J. Phys. Chem. C 2009, 113, 2974–2979. [Google Scholar] [CrossRef]
- Lunt, R.R.; Giebink, N.C.; Belak, A.A.; Benziger, J.B.; Forrest, S.R. Exciton diffusion lengths of organic semiconductor thin films measured by spectrally resolved photoluminescence quenching. J. Appl. Phys. 2009, 105. [Google Scholar] [CrossRef]
- Luhman, W.A.; Holmes, R.J. Investigation of energy transfer in organic photovoltaic cells and impact on exciton diffusion length measurements. Adv. Funct. Mater. 2011, 21, 764–771. [Google Scholar] [CrossRef]
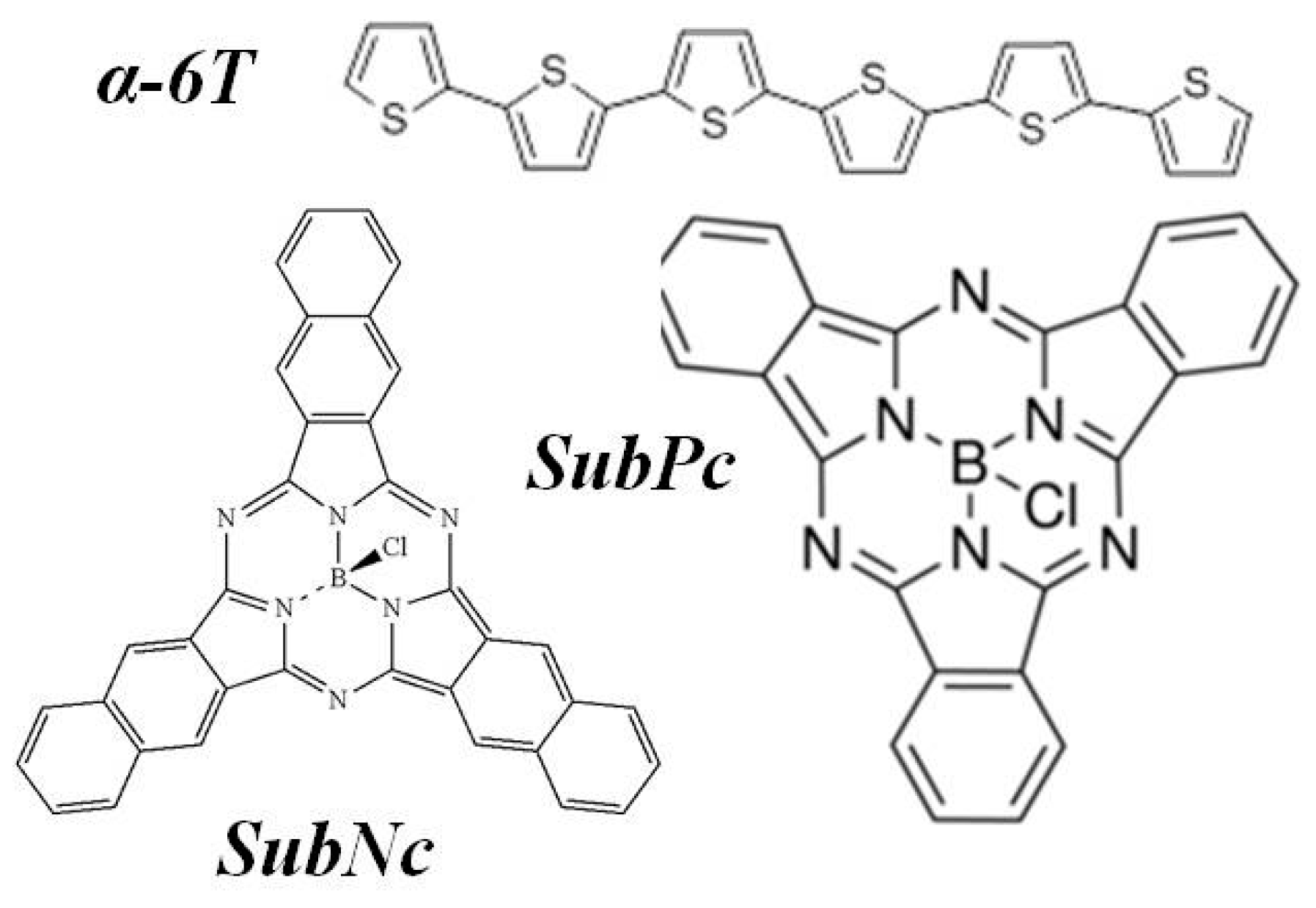


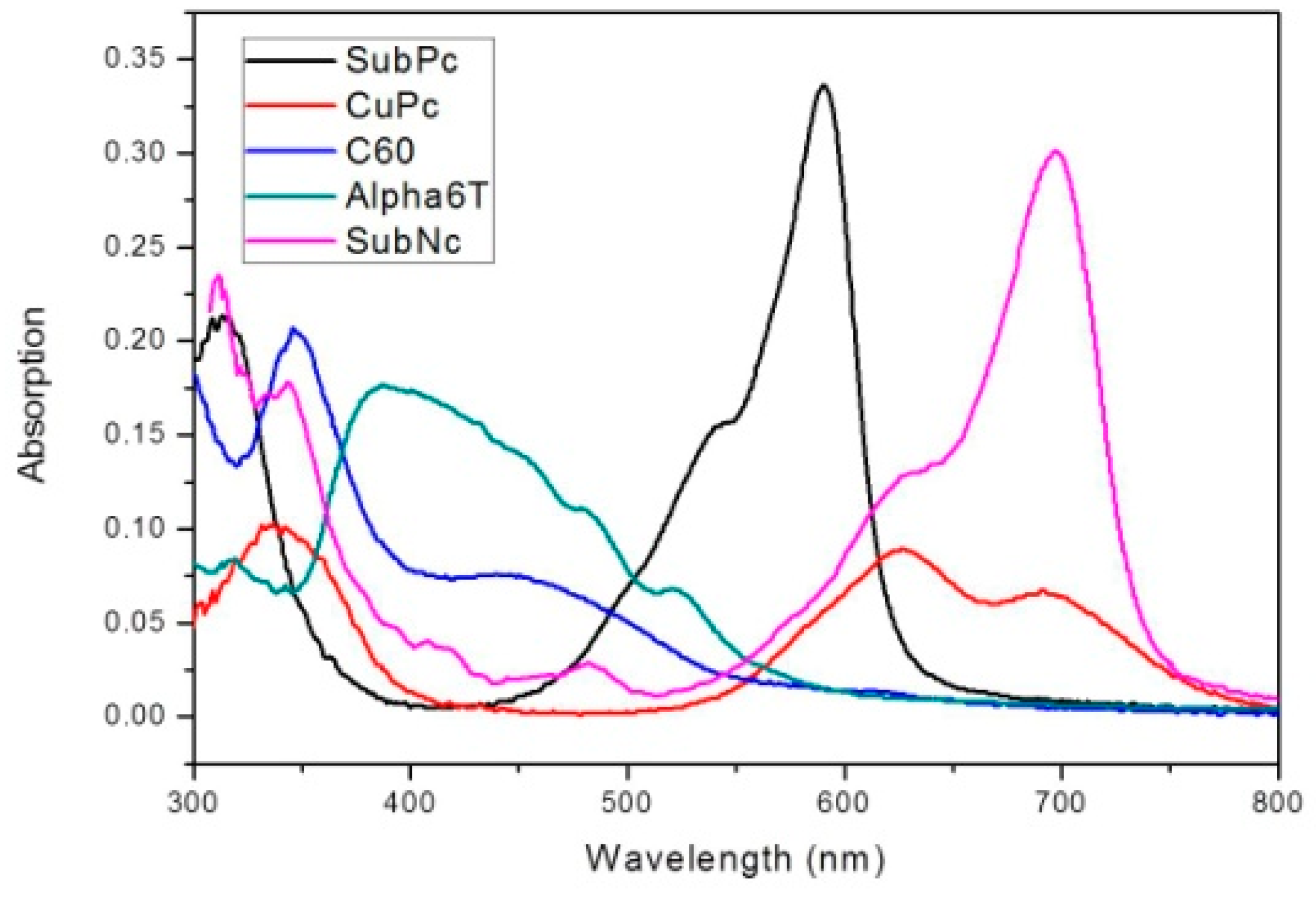
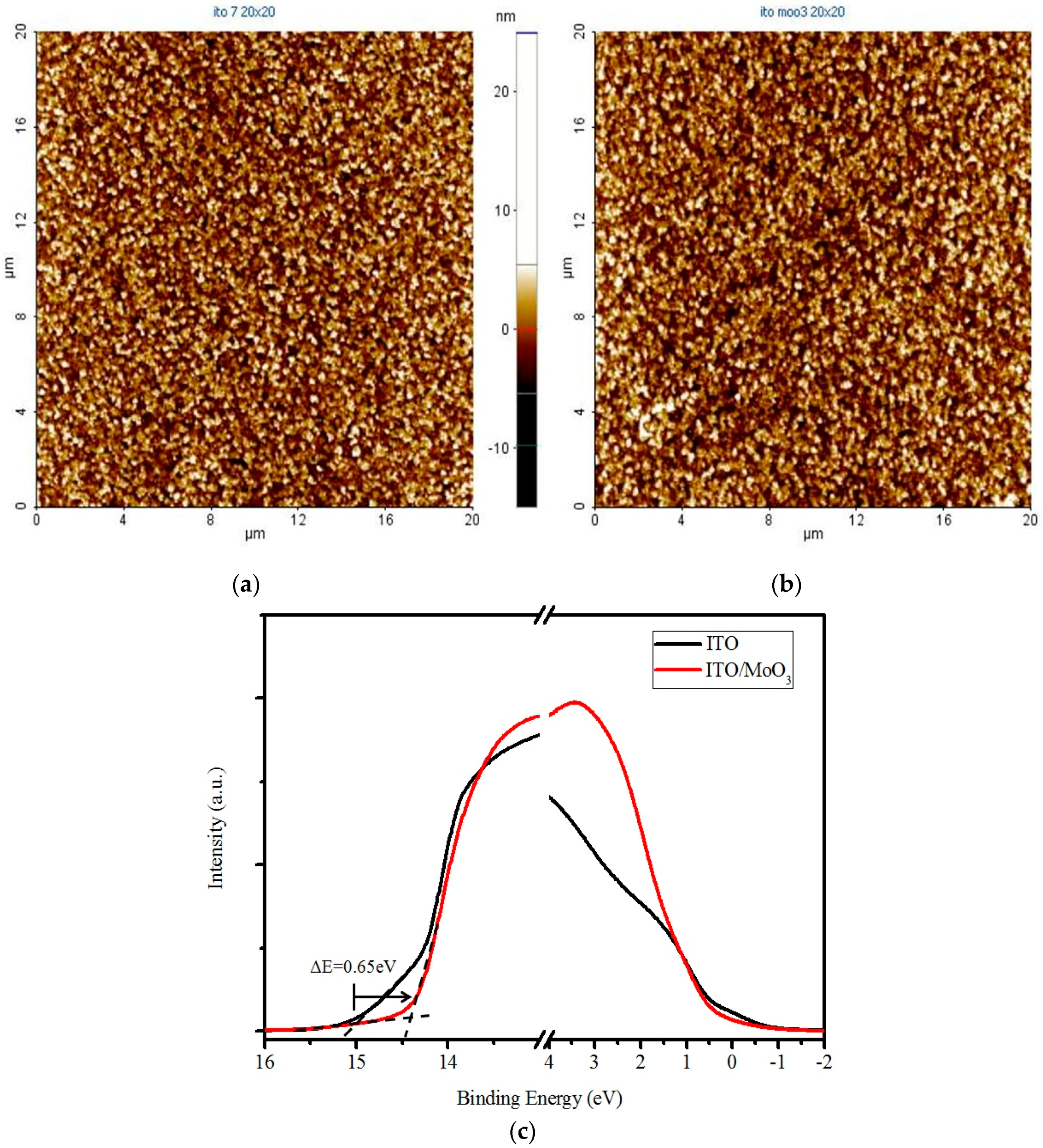
| Material Thickness (nm) | JSC (mA/cm2) | VOC (V) | FF (%) | η (%) | RS (Ω) | RSh (kΩ) |
|---|---|---|---|---|---|---|
| SubPc | 4.57 ± 0.02 | 1.00 ± 0.01 | 55.27 ± 1.22 | 2.52 ± 0.10 | 31.71 | 1.125 |
| 9 | ||||||
| CuPc | 3.41 ± 0.05 | 0.39 ± 0.02 | 54.00 ± 1.31 | 0.70 ± 0.08 | 23.63 | 0.96 |
| 20 | ||||||
| α-6T | 3.67 ± 0.08 | 0.35 ± 0.02 | 55.30 ± 1.17 | 0.71 ± 0.05 | 23.53 | 0.96 |
| 40 |
| Material Thickness (nm) | JSC (mA/cm2) | VOC (V) | FF (%) | η (%) | RS (Ω) | RSh (kΩ) |
|---|---|---|---|---|---|---|
| α-6T | ITO/MoO3/α-6T (X nm)/C70 (30 nm)/BCP/Ag | |||||
| 40 | 6.01 ± 0.07 | 0.36 ± 0.02 | 56.56 ± 1.02 | 1.23 ± 0.10 | 12.55 | 0.386 |
| 50 | 5.98 ± 0.02 | 0.38 ± 0.02 | 56.37 ± 1.04 | 1.28 ± 0.09 | 12.79 | 0.49 |
| 60 | 5.92 ± 0.05 | 0.39 ± 0.01 | 53.57 ± 1.42 | 1.24 ± 0.07 | 15.46 | 0.501 |
| C70 | ITO/MoO3/α-6T (40 nm)/C70 (X nm)/BCP/Ag | |||||
| 20 | 6.35 ± 0.05 | 0.34 ± 0.01 | 58.30 ± 0.02 | 1.26 ± 0.04 | 9.77 | 0.563 |
| 25 | 7.05 ± 0.05 | 0.35 ± 0.02 | 58.28 ± 0.03 | 1.44 ± 0.09 | 9.47 | 0.454 |
| 30 | 6.26 ± 0.02 | 0.35 ± 0.02 | 57.66 ± 0.07 | 1.26 ± 0.08 | 11.23 | 0.517 |
| 32 | 6.03 ± 0.07 | 0.33 ± 0.01 | 57.01 ± 0.11 | 1.14 ± 0.04 | 10.31 | 0.416 |
| SubNc | ITO/MoO3/SubNc (X nm)/C70 (30 nm)/BCP/Ag | |||||
| 10 | 7.15 ± 0.13 | 0.68 ± 0.02 | 44.12 ± 1.85 | 2.15 ± 0.19 | 24.95 | 0.311 |
| 40 | 6.73 ± 0.11 | 0.72 ± 0.01 | 29.71 ± 1.77 | 1.44 ± 0.13 | 82.81 | 0.178 |
| 50 | 4.58 ± 0.18 | 0.70 ± 0.02 | 25.11 ± 1.82 | 0.81 ± 0.11 | 123.32 | 0.184 |
| Material Thickness (nm) | JSC (mA/cm2) | VOC (V) | FF (%) | η (%) | RS (Ω) | RSh (kΩ) |
|---|---|---|---|---|---|---|
| SubPc | ITO/MoO3/α-6T (50 nm)/SubPc (X nm)/BCP/Ag | |||||
| 9 | 6.01 ± 0.03 | 0.36 ± 0.03 | 56.56 ± 0.04 | 1.23 ± 0.10 | 12.55 | 0.386 |
| 5 | 5.98 ± 0.02 | 0.38 ± 0.01 | 56.37 ± 0.07 | 1.28 ± 0.04 | 12.79 | 0.49 |
| 18 | 5.92 ± 0.06 | 0.39 ± 0.02 | 53.57 ± 1.01 | 1.24 ± 0.10 | 15.46 | 0.501 |
| α-6T | ITO/MoO3/α-6T (X nm)/SubPc (18 nm)/BCP/Ag | |||||
| 50 | 4.14 ± 0.07 | 0.87 ± 0.03 | 50.70 ± 1.72 | 1.83 ± 0.13 | 53.64 | 1.45 |
| 60 | 3.83 ± 0.07 | 1.04 ± 0.03 | 64.14 ± 1.98 | 2.56 ± 0.21 | 17.24 | 2.689 |
| SubNc | ITO/MoO3/α-6T (60 nm)/SubNc (X nm)/BCP/Ag | |||||
| 12 | 5.75 ± 0.34 | 0.73 ± 0.04 | 51.66 ± 1.42 | 2.17 ± 0.32 | 64.86 | 0.608 |
| 16 | 5.68 ± 0.26 | 0.61 ± 0.05 | 51.79 ± 1.58 | 1.80 ± 0.29 | 12.46 | 0.481 |
| SubNc Thickness (nm) | JSC (mA/cm2) | VOC (V) | FF (%) | η (%) | RS (Ω) | RSh (kΩ) |
|---|---|---|---|---|---|---|
| 0 | 3.83 ± 0.05 | 1.04 ± 0.02 | 64.14 ± 1.55 | 2.56 ± 0.14 | 17.24 | 2.689 |
| 3 | 7.19 ± 0.02 | 0.96 ± 0.02 | 43.61 ± 1.24 | 3.01 ± 0.16 | 25.94 | 0.878 |
| 6 | 8.28 ± 0.01 | 0.93 ± 0.03 | 45.20 ± 1.31 | 3.48 ± 0.12 | 19.29 | 0.576 |
| 12 | 10.12 ± 0.06 | 0.94 ± 0.01 | 44.12 ± 1.48 | 4.20 ± 0.19 | 17.41 | 0.449 |
| 15 | 9.88 ± 0.01 | 0.94 ± 0.02 | 46.81 ± 1.17 | 4.35 ± 0.20 | 12.95 | 0.443 |
| 18 | 6.14 ± 0.03 | 0.93 ± 0.02 | 48.65 ± 1.06 | 2.78 ± 0.13 | 20.48 | 0.615 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-H.; Wang, Y.-H.; Ke, J.-C.; Huang, C.-J. Investigation of Various Active Layers for Their Performance on Organic Solar Cells. Materials 2016, 9, 667. https://doi.org/10.3390/ma9080667
Huang P-H, Wang Y-H, Ke J-C, Huang C-J. Investigation of Various Active Layers for Their Performance on Organic Solar Cells. Materials. 2016; 9(8):667. https://doi.org/10.3390/ma9080667
Chicago/Turabian StyleHuang, Pao-Hsun, Yeong-Her Wang, Jhong-Ciao Ke, and Chien-Jung Huang. 2016. "Investigation of Various Active Layers for Their Performance on Organic Solar Cells" Materials 9, no. 8: 667. https://doi.org/10.3390/ma9080667






