Carbon Nanomaterials as Antibacterial Colloids
Abstract
:1. Introduction
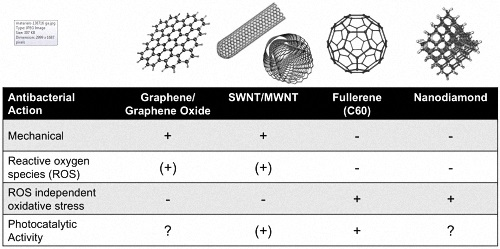
2. Inherent Antibacterial Properties of Carbon Nanomaterials
2.1. Graphene
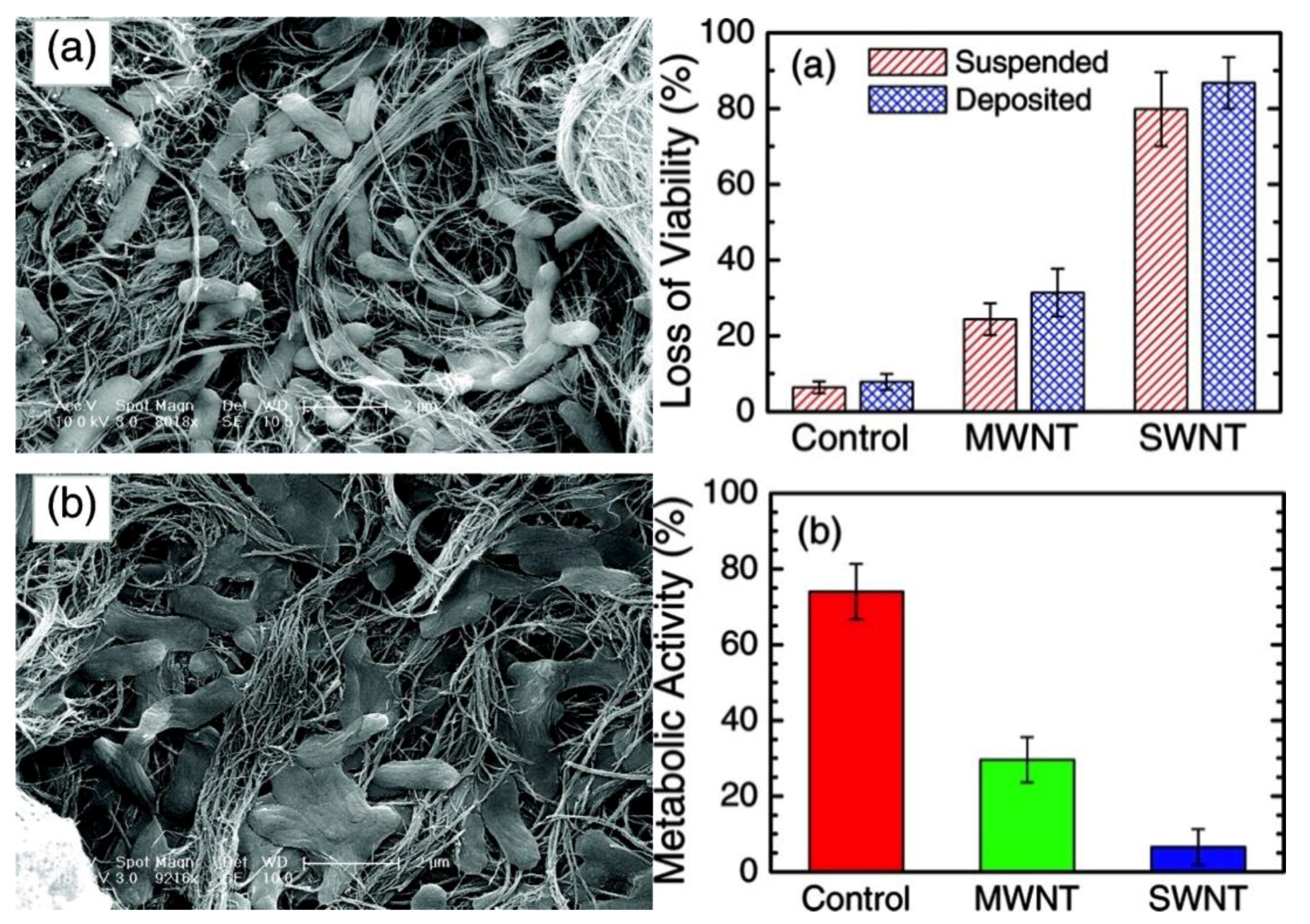
2.2. Carbon Nanotubes
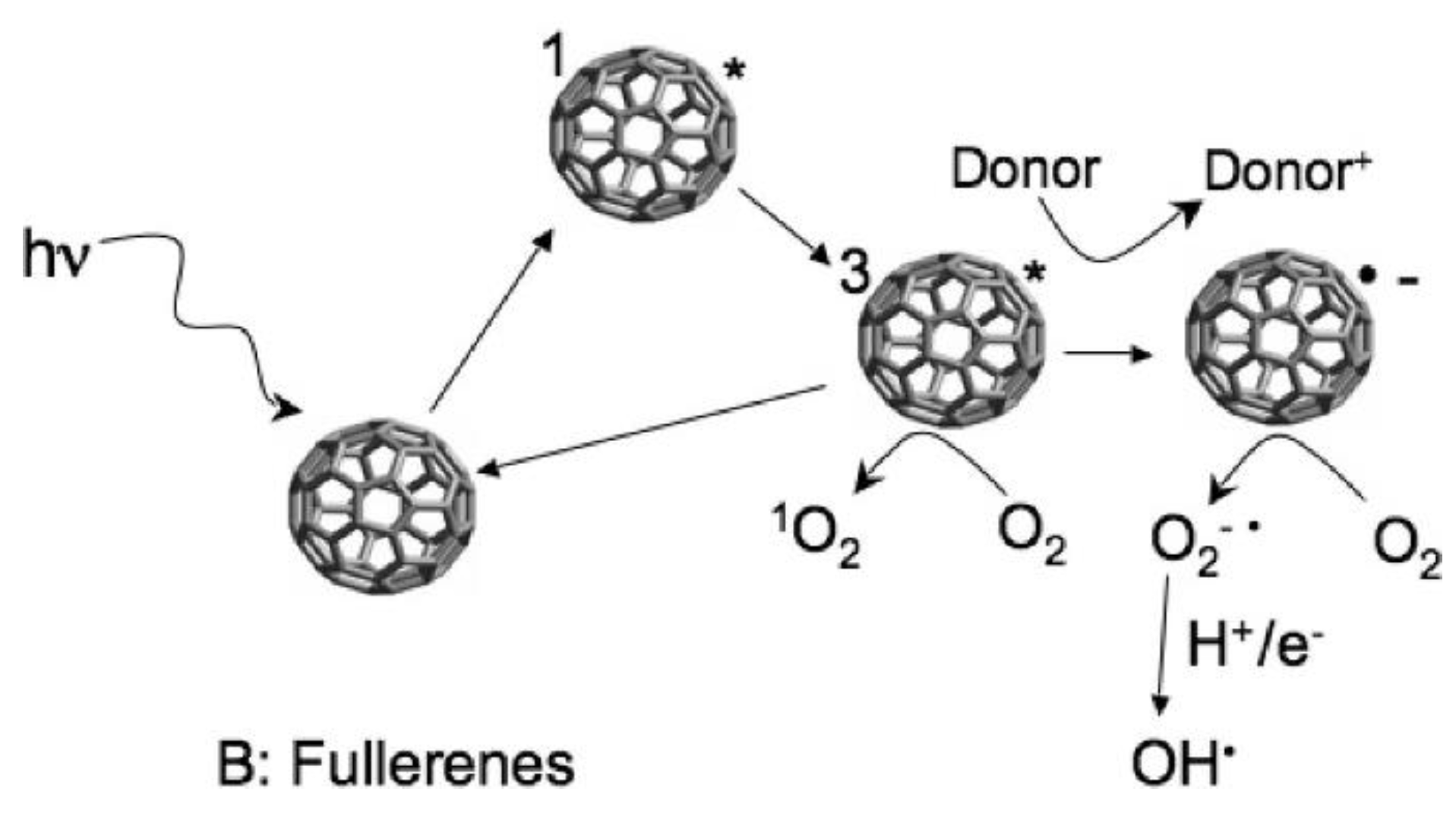
2.3. Fullerenes
2.4. Nanodiamond
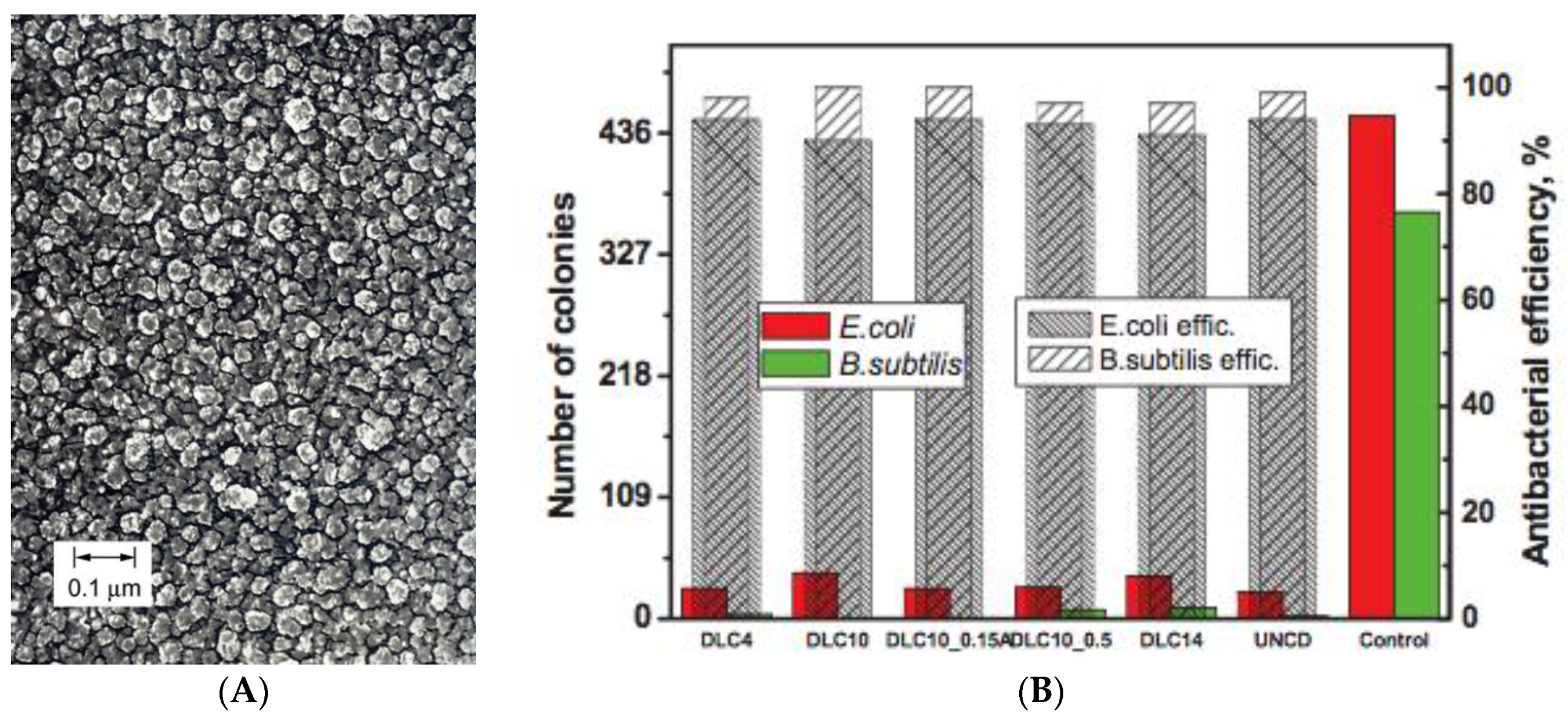
2.5. Diamond-Like Carbon, Diamond Thin Films
3. Functionalization of Carbon Nanomaterials for Tailoring Antibacterial Properties
3.1. Graphene
3.2. Carbon Nanotubes
3.3. Fullerenes
3.4. Nanodiamond
3.5. Diamond-Like Carbon, Diamond Thin Films
4. Summary and Outlook
Conflicts of Interest
References
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and Toxicity of Graphene and Its Functionalized Derivatives in Biological Systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Riviere, N.A.; Nemanich, R.J.; Inman, A.O.; Wang, Y.Y.; Riviere, J.E. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol. Lett. 2005, 155, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Pulskamp, K.; Diabaté, S.; Krug, H.F. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, N.R.; Pojana, G.; White, P.; Møller, P.; Cohn, C.A.; Korsholm, K.S.; Vogel, U.; Marcomini, A.; Loft, S.; Wallin, H. Genotoxicity, cytotoxicity, and reactive oxygen species induced by single-walled carbon nanotubes and C60 fullerenes in the FE1-MutaTMMouse lung epithelial cells. Environ. Mol. Mutagen. 2008, 49, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon nanotubes introduced into the abdominal cavity of mice show asbestos-like pathogenicity in a pilot study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Gass, M.; Muller, K.; Skepper, J.N.; Midgley, P.A.; Welland, M. Direct imaging of single-walled carbon nanotubes in cells. Nat. Nanotechnol. 2007, 2, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Krug, H.F. Nanosafety Research—Are We on the Right Track? 2014. Available online: http://onlinelibrary.wiley.com/doi/10.1002/anie.201403367/full (accessed on 17 October 2014).
- Wörle-Knirsch, J.M.; Pulskamp, K.; Krug, H.F. Oops They Did It Again! Carbon Nanotubes Hoax Scientists in Viability Assays. Nano Lett. 2006, 6, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Microbial Cytotoxicity of Carbon-Based Nanomaterials: Implications for River Water and Wastewater Effluent. Environ. Sci. Technol. 2009, 43, 2648–2653. [Google Scholar] [CrossRef] [PubMed]
- Oyelami, A.O.; Semple, K.T. Impact of carbon nanomaterials on microbial activity in soil. Soil Biol. Biochem. 2015, 86, 172–180. [Google Scholar] [CrossRef]
- Mauter, M.S.; Elimelech, M. Environmental Applications of Carbon-Based Nanomaterials. Environ. Sci. Technol. 2008, 42, 5843–5859. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Mahendra, S.; Lyon, D.Y.; Brunet, L.; Liga, M.V.; Li, D.; Alvarez, P.J.J. Antimicrobial nanomaterials for water disinfection and microbial control: Potential applications and implications. Water Res. 2008, 42, 4591–4602. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Deng, B.; Lv, M.; Li, J.; Zhang, Y.; Jiang, H.; Peng, C.; Li, J.; Shi, J.; Huang, Q.; et al. Graphene Oxide-Based Antibacterial Cotton Fabrics. Adv. Healthc. Mater. 2013, 2, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Kitahara, N.; Sato, T.; Isogawa, H.; Ohgoe, Y.; Masuko, S.; Shizuku, F.; Hirakuri, K.K. Antibacterial property of DLC film coated on textile material. Diam. Relat. Mater. 2010, 19, 690–694. [Google Scholar] [CrossRef]
- Lu, B.; Li, T.; Zhao, H.; Li, X.; Gao, C.; Zhang, S.; Xie, E. Graphene-based composite materials beneficial to wound healing. Nanoscale 2012, 4, 2978. [Google Scholar] [CrossRef] [PubMed]
- Sreeprasad, T.S.; Maliyekkal, M.S.; Deepti, K.; Chaudhari, K.; Xavier, P.L.; Pradeep, T. Transparent, Luminescent, Antibacterial and Patternable Film Forming Composites of Graphene Oxide/Reduced Graphene Oxide. ACS Appl. Mater. Interfaces 2011, 3, 2643–2654. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions: Effects of Preparation Method and Particle Size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Castañón, G.A.; Niño-Martínez, N.; Martínez-Gutierrez, F.; Martínez-Mendoza, J.R.; Ruiz, F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J. Nanopart. Res. 2008, 10, 1343–1348. [Google Scholar] [CrossRef]
- Xiu, Z.; Zhang, Q.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.P.; Walker, K.A.; Obare, S.O.; Docherty, K.M. Size-Dependent Antimicrobial Effects of Novel Palladium Nanoparticles. PLoS ONE 2014, 9, e85981. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Chng, E.L.K.; Pumera, M. The Toxicity of Graphene Oxides: Dependence on the Oxidative Methods Used. Chem. Eur. J. 2013, 19, 8227–8235. [Google Scholar] [CrossRef] [PubMed]
- Salas, E.C.; Sun, Z.; Lüttge, A.; Tour, J.M. Reduction of Graphene Oxide via Bacterial Respiration. ACS Nano 2010, 4, 4852–4856. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Tria, M.C.R.; Vergara, R.A.M.V.; Ahmed, F.; Advincula, R.C.; Rodrigues, D.F. Antimicrobial graphene polymer (PVK-GO) nanocomposite films. Chem. Commun. 2011, 47, 8892. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Mangadlao, J.; Ahmed, F.; Leon, A.; Advincula, R.C.; Rodrigues, D.F. Graphene nanocomposite for biomedical applications: Fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 2012, 23, 395101. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Ji, H.; Sun, H.; Qu, X. Antibacterial applications of graphene-based nanomaterials: Recent achievements and challenges. Adv. Drug Deliv. Rev. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping Bacteria by Graphene Nanosheets for Isolation from Environment, Reactivation by Sonication, and Inactivation by Near-Infrared Irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral Dimension-Dependent Antibacterial Activity of Graphene Oxide Sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Dallavalle, M.; Calvaresi, M.; Bottoni, A.; Melle-Franco, M.; Zerbetto, F. Graphene Can Wreak Havoc with Cell Membranes. ACS Appl. Mater. Interfaces 2015, 7, 4406–4414. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Zhang, L.H.; Yun, K.; Kim, S.J. Antibacterial Efficiency of Graphene Nanosheets against Pathogenic Bacteria via Lipid Peroxidation. J. Phys. Chem. C 2012, 116, 17280–17287. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Umasuthan, N.; Mohan, R.; Lee, J.; Kim, S.J. Antibacterial Activity of Graphene Oxide Nanosheets. Sci. Adv. Mater. 2012, 4, 1111–1117. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomedicine 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Pham, V.T.H.; Truong, V.K.; Quinn, M.D.J.; Notley, S.M.; Guo, Y.; Baulin, V.A.; al Kobaisi, M.; Crawford, R.J.; Ivanova, E.P. Graphene Induces Formation of Pores That Kill Spherical and Rod-Shaped Bacteria. ACS Nano 2015, 9, 8458–8467. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Escherichia coli bacteria reduce graphene oxide to bactericidal graphene in a self-limiting manner. Carbon 2012, 50, 1853–1860. [Google Scholar] [CrossRef]
- Dellieu, L.; Lawarée, E.; Reckinger, N.; Didembourg, C.; Letesson, J.J.; Sarrazin, M.; Deparis, O.; Matroule, J.Y.; Colomer, J.F. Do CVD grown graphene films have antibacterial activity on metallic substrates? Carbon 2015, 84, 310–316. [Google Scholar] [CrossRef]
- Ruiz, O.N.; Fernando, K.A.S.; Wang, B.; Brown, N.A.; Luo, P.G.; McNamara, N.D.; Vangsness, M.; Sun, Y.P.; Bunker, C.E. Graphene Oxide: A Nonspecific Enhancer of Cellular Growth. ACS Nano 2011, 5, 8100–8107. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Piao, J.G.; Auletta, J.; Hu, K.; Zhu, Y.; Meyer, T.; Liu, H.; Yang, L. Availability of the Basal Planes of Graphene Oxide Determines Whether It Is Antibacterial. ACS Appl. Mater. Interfaces 2014, 6, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, G.; Zhang, G.; Xu, X.; Yang, F. Using graphene oxide to enhance the activity of anammox bacteria for nitrogen removal. Bioresour. Technol. 2013, 131, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Monopoli, M.P.; Åberg, C.; Salvati, A.; Dawson, K.A. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 2012, 7, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Hegab, H.M.; ElMekawy, A.; Zou, L.; Mulcahy, D.; Saint, C.P.; Ginic-Markovic, M. The controversial antibacterial activity of graphene-based materials. Carbon 2016, 105, 362–376. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Terms of Endearment: Bacteria Meet Graphene Nanosurfaces, 2016. Available online: http://www.sciencedirect.com/science/article/pii/S0142961216001447 (accessed on 12 July 2016).
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef] [PubMed]
- Haung, C.F.; Chan, Y.H.; Chen, L.K.; Liu, C.M.; Huang, W.C.; Ou, S.F.; Ou, K.L.; Wang, D.J. Preparation, Characterization, and Properties of Anticoagulation and Antibacterial Films of Carbon-Based Nanowires Fabricated on Surfaces of Ti Implants. J. Electrochem. Soc. 2013, 160, H392–H397. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Chiaretti, M.; Carru, G.A.; Bellucci, S.; Mazzanti, G. Multi-walled carbon nanotubes: Lack of mutagenic activity in the bacterial reverse mutation assay. Toxicol. Lett. 2009, 184, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.R.; Watanabe, Y.; Wiesner, M.R. Comparative photochemical reactivity of spherical and tubular fullerene nanoparticles in water under ultraviolet (UV) irradiation. Water Res. 2011, 45, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurina, Y.Y.; Tyurin, V.A.; Konduru, N.V.; Potapovich, A.I.; Osipov, A.N.; Kisin, E.R.; Schwegler-Berry, D.; Mercer, R.; Castranova, V.; et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: Role of iron. Toxicol. Lett. 2006, 165, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Fortner, J.D.; Sayes, C.M.; Colvin, V.L.; Hughes, J.B. Bacterial cell association and antimicrobial activity of a C60 water suspension. Environ. Toxicol. Chem. 2005, 24, 2757–2762. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Brunet, L.; Hinkal, G.W.; Wiesner, M.R.; Alvarez, P.J.J. Antibacterial Activity of Fullerene Water Suspensions (nC60) Is Not Due to ROS-Mediated Damage. Nano Lett. 2008, 8, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Lyon, D.Y.; Alvarez, P.J.J. Fullerene Water Suspension (nC60) Exerts Antibacterial Effects via ROS-Independent Protein Oxidation. Environ. Sci. Technol. 2008, 42, 8127–8132. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lyon, D.Y.; Wiesner, M.R.; Dong, J.; Alvarez, P.J.J. Effect of a Fullerene Water Suspension on Bacterial Phospholipids and Membrane Phase Behavior. Environ. Sci. Technol. 2007, 41, 2636–2642. [Google Scholar] [CrossRef] [PubMed]
- Fortner, J.D.; Lyon, D.Y.; Sayes, C.M.; Boyd, A.M.; Falkner, J.C.; Hotze, E.M.; Alemany, L.B.; Tao, Y.J.; Guo, W.; Ausman, K.D.; et al. C60 in Water: Nanocrystal Formation and Microbial Response. Environ. Sci. Technol. 2005, 39, 4307–4316. [Google Scholar] [CrossRef] [PubMed]
- Badireddy, A.R.; Hotze, E.M.; Chellam, S.; Alvarez, P.; Wiesner, M.R. Inactivation of Bacteriophages via Photosensitization of Fullerol Nanoparticles. Environ. Sci. Technol. 2007, 41, 6627–6632. [Google Scholar] [CrossRef] [PubMed]
- Hotze, E.M.; Badireddy, A.R.; Chellam, S.; Wiesner, M.R. Mechanisms of Bacteriophage Inactivation via Singlet Oxygen Generation in UV Illuminated Fullerol Suspensions. Environ. Sci. Technol. 2009, 43, 6639–6645. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor Photocatalysis—Mechanistic and Synthetic Aspects. Angew. Chem. Int. Ed. 2013, 52, 812–847. [Google Scholar] [CrossRef] [PubMed]
- Brunet, L.; Lyon, D.Y.; Hotze, E.M.; Alvarez, P.J.J.; Wiesner, M.R. Comparative Photoactivity and Antibacterial Properties of C60 Fullerenes and Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009, 43, 4355–4360. [Google Scholar] [CrossRef] [PubMed]
- Schrand, A.M.; Huang, H.; Carlson, C.; Schlager, J.J.; Osawa, E.; Hussain, S.M.; Dai, L. Are diamond nanoparticles cytotoxic? J. Phys. Chem. B 2007, 111, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Dai, L. Nanodiamonds for nanomedicine. Nanomedicine 2009, 4, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Chow, E.K.; Zhang, X.Q.; Chen, M.; Lam, R.; Robinson, E.; Huang, H.; Schaffer, D.; Osawa, E.; Goga, A.; Ho, D. Nanodiamond Therapeutic Delivery Agents Mediate Enhanced Chemoresistant Tumor Treatment. Sci. Transl. Med. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Paget, V.; Sergent, J.A.; Grall, R.; Altmeyer-Morel, S.; Girard, H.A.; Petit, T.; Gesset, C.; Mermoux, M.; Bergonzo, P.; Arnault, J.C.; et al. Carboxylated nanodiamonds are neither cytotoxic nor genotoxic on liver, kidney, intestine and lung human cell lines. Nanotoxicology 2013, 8, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Vaijayanthimala, V.; Chang, H.C. Functionalized fluorescent nanodiamonds for biomedical applications. Nanomedicine 2008, 4, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Mohan, N.; Chen, C.S.; Hsieh, H.H.; Wu, Y.C.; Chang, H.C. In Vivo Imaging and Toxicity Assessments of Fluorescent Nanodiamonds in Caenorhabditis elegans. Nano Lett. 2010, 10, 3692–3699. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Xiong, W.; Zhu, L.; O̅sawa, E.; Hussin, S.; Dai, L. DNA Damage in Embryonic Stem Cells Caused by Nanodiamonds. ACS Nano 2011, 5, 2376–2384. [Google Scholar] [CrossRef] [PubMed]
- Chwalibog, A.; Sawosz, E.; Hotowy, A.; Szeliga, J.; Mitura, S.; Mitura, K.; Grodzik, M.; Orlowski, P.; Sokolowska, A. Visualization of interaction between inorganic nanoparticles and bacteria or fungi. Int. J. Nanomedicine 2010, 5, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Sawosz, E.; Chwalibog, A.; Mitura, K.; Mitura, S.; Szeliga, J.; Niemiec, T.; Rupiewicz, M.; Grodzik, M.; Sokołowska, A. Visualisation of Morphological Interaction of Diamond and Silver Nanoparticles with Salmonella Enteritidis and Listeria Monocytogenes. J. Nanosci. Nanotechnol. 2011, 11, 7635–7641. [Google Scholar] [CrossRef] [PubMed]
- Beranová, J.; Seydlová, G.; Kozak, H.; Potocký, Š.; Konopásek, I.; Kromka, A. Antibacterial behavior of diamond nanoparticles against Escherichia coli. Phys. Status Solidi B 2012, 249, 2581–2584. [Google Scholar] [CrossRef]
- Beranová, J.; Seydlová, G.; Kozak, H.; Benada, O.; Fišer, R.; Artemenko, A.; Konopásek, I.; Kromka, A. Sensitivity of bacteria to diamond nanoparticles of various size differs in gram-positive and gram-negative cells. FEMS Microbiol. Lett. 2014, 351, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wehling, J.; Dringen, R.; Zare, R.N.; Maas, M.; Rezwan, K. Bactericidal Activity of Partially Oxidized Nanodiamonds. ACS Nano 2014, 8, 6475–6483. [Google Scholar] [CrossRef] [PubMed]
- Voznyakovskii, A.P.; Dolmatov, V.Y.; Shumilov, F.A. The influence of detonation synthesis conditions on surface properties of detonation nanodiamonds. J. Superhard Mater. 2014, 36, 165–170. [Google Scholar] [CrossRef]
- Kromka, A.; Jira, J.; Stenclova, P.; Kriha, V.; Kozak, H.; Beranova, J.; Vretenar, V.; Skakalova, V.; Rezek, B. Bacterial response to nanodiamonds and graphene oxide sheets. Phys. Status Solidi B 2016. [Google Scholar] [CrossRef]
- Jang, D.M.; Myung, Y.; Im, H.S.; Seo, Y.S.; Cho, Y.J.; Lee, C.W.; Park, J.; Jee, A.Y.; Lee, M. Nanodiamonds as photocatalysts for reduction of water and graphene oxide. Chem. Commun. 2012, 48, 696. [Google Scholar] [CrossRef] [PubMed]
- Marciano, F.R.; Bonetti, L.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Diamond-like carbon films produced from high deposition rates exhibit antibacterial activity. Synth. Met. 2009, 159, 2167–2169. [Google Scholar] [CrossRef]
- Narayan, R.J.; Wei, W.; Jin, C.; Andara, M.; Agarwal, A.; Gerhardt, R.A.; Shih, C.C.; Shih, C.M.; Lin, S.J.; Su, Y.Y.; et al. Microstructural and biological properties of nanocrystalline diamond coatings. Diam. Relat. Mater. 2006, 15, 1935–1940. [Google Scholar] [CrossRef]
- Jelinek, M.; Voss, A.; Kocourek, T.; Mozafari, M.; Vymetalova, V.; Zezulova, M.; Pisarik, P.; Kotzianova, A.; Popov, C.; Miksovsky, J. Comparison of the surface properties of DLC and ultrananocrystalline diamond films with respect to their bio-applications. Phys. Status Solidi Appl. Mater. Sci. 2013, 210, 2106–2110. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Mangolin, J.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Investigation into the antibacterial property and bacterial adhesion of diamond-like carbon films. Vacuum 2011, 85, 662–666. [Google Scholar] [CrossRef]
- Marciano, F.R.; Bonetti, L.F.; Da-Silva, N.S.; Corat, E.J.; Trava-Airoldi, V.J. Wettability and antibacterial activity of modified diamond-like carbon films. Appl. Surf. Sci. 2009, 255, 8377–8382. [Google Scholar] [CrossRef]
- Jakubowski, W.; Bartosz, G.; Niedzielski, P.; Szymanski, W.; Walkowiak, B. Nanocrystalline diamond surface is resistant to bacterial colonization. Diam. Relat. Mater. 2004, 13, 1761–1763. [Google Scholar] [CrossRef]
- Mitura, K.; Niedzielski, P.; Bartosz, G.; Moll, J.; Walkowiak, B.; Pawłowska, Z.; Louda, P.; Kieć-Świerczyńska, M.; Mitura, S. Interactions between carbon coatings and tissue. Surf. Coat. Technol. 2006, 201, 2117–2123. [Google Scholar] [CrossRef]
- Medina, O.; Nocua, J.; Mendoza, F.; Gómez-Moreno, R.; Ávalos, J.; Rodríguez, C.; Morell, G. Bactericide and bacterial anti-adhesive properties of the nanocrystalline diamond surface. Diam. Relat. Mater. 2012, 22, 77–81. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, L.; Ogino, A.; Nagatsu, M. Investigation into the antibacterial property of carbon films. Diam. Relat. Mater. 2008, 17, 1416–1419. [Google Scholar] [CrossRef]
- Amaral, M.; Gomes, P.S.; Lopes, M.A.; Santos, J.; Silva, D.R.F.; Fernandes, M.H. Nanocrystalline Diamond as a Coating for Joint Implants: Cytotoxicity and Biocompatibility Assessment. J. Nanomater. 2008, 2008, e894352. [Google Scholar] [CrossRef]
- Kalbacova, M.; Michalikova, L.; Baresova, V.; Kromka, A.; Rezek, B.; Kmoch, S. Adhesion of osteoblasts on chemically patterned nanocrystalline diamonds. Phys. Status Solidi B 2008, 245, 2124–2127. [Google Scholar] [CrossRef]
- Gupta, S.; Weiner, B.R.; Morell, G. Room-temperature electrical conductivity studies of sulfur-modified microcrystalline diamond thin films. Appl. Phys. Lett. 2003, 83, 491–493. [Google Scholar] [CrossRef]
- Apátiga, L.M.; Castaño, V.M. Ultraviolet stimulated photocatalytic activity on polycrystalline diamond film surfaces. Appl. Phys. Lett. 2003, 83, 4542–4543. [Google Scholar] [CrossRef]
- Das, M.R.; Sarma, R.K.; Saikia, R.; Kale, V.S.; Shelke, M.V.; Sengupta, P. Synthesis of silver nanoparticles in an aqueous suspension of graphene oxide sheets and its antimicrobial activity. Colloids Surf. B BioInterfaces 2011, 83, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, J.; Wang, Y.; Yan, X.; Sun, D.D. Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity. New J. Chem. 2011, 35, 1418. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, J.; Xiong, Z.; Yong, Y.; Zhao, X.S. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J. Mater. Chem. 2011, 21, 3350. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, C.; Song, G.; Chang, W.; Wang, G.; Wu, Q.; Fu, H. A novel Ag/graphene composite: Facile fabrication and enhanced antibacterial properties. J. Mater. Sci. 2013, 48, 1980–1985. [Google Scholar] [CrossRef]
- Tang, J.; Chen, Q.; Xu, L.; Zhang, S.; Feng, L.; Cheng, L.; Xu, H.; Liu, Z.; Peng, R. Graphene Oxide-Silver Nanocomposite As a Highly Effective Antibacterial Agent with Species-Specific Mechanisms. ACS Appl. Mater. Interfaces 2013, 5, 3867–3874. [Google Scholar] [CrossRef] [PubMed]
- De Faria, A.F.; de Moraes, A.C.M.; Marcato, P.D.; Martinez, D.S.T.; Durán, N.; Filho, A.G.S.; Brandelli, A.; Alves, O.L. Eco-friendly decoration of graphene oxide with biogenic silver nanoparticles: Antibacterial and antibiofilm activity. J. Nanopart. Res. 2014, 16, 1–16. [Google Scholar] [CrossRef]
- De Faria, A.F.; Martinez, D.S.T.; Meira, S.M.M.; de Moraes, A.C.M.; Brandelli, A.; Filho, A.G.S.; Alves, O.L. Anti-adhesion and antibacterial activity of silver nanoparticles supported on graphene oxide sheets. Colloids Surf. B BioInterfaces 2014, 113, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Shi, X.; Cheng, L.; Luo, Y.; Dong, Z.; Gong, H.; Xu, L.; Zhong, Z.; Peng, R.; Liu, Z. Graphene-Based Nanocomposite As an Effective, Multifunctional, and Recyclable Antibacterial Agent. ACS Appl. Mater. Interfaces 2014, 6, 8542–8548. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Yu, J.C. Graphene-based photocatalytic composites. RSC Adv. 2011, 1, 1426. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Bao, Q.; Wu, J.; Wan, Y. Controlled drug release characteristics and enhanced antibacterial effect of graphene oxide-drug intercalated layered double hydroxide hybrid films. J. Mater. Chem. 2012, 22, 23106. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Khan, M.S.; Wu, H.F. Graphene oxide as a nanocarrier for gramicidin (GOGD) for high antibacterial performance. RSC Adv. 2014, 4, 50035–50046. [Google Scholar] [CrossRef]
- Pandey, H.; Parashar, V.; Parashar, R.; Prakash, R.; Ramteke, P.W.; Pandey, A.C. Controlled drug release characteristics and enhanced antibacterial effect of graphene nanosheets containing gentamicin sulfate. Nanoscale 2011, 3, 4104. [Google Scholar] [CrossRef] [PubMed]
- Niu, A.; Han, Y.; Wu, J.; Yu, N.; Xu, Q. Synthesis of One-Dimensional Carbon Nanomaterials Wrapped by Silver Nanoparticles and Their Antibacterial Behavior. J. Phys. Chem. C 2010, 114, 12728–12735. [Google Scholar] [CrossRef]
- Akhavan, O.; Abdolahad, M.; Abdi, Y.; Mohajerzadeh, S. Synthesis of titania/carbon nanotube heterojunction arrays for photoinactivation of E. coli in visible light irradiation. Carbon 2009, 47, 3280–3287. [Google Scholar] [CrossRef]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube–TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Joo, Y.T.; Jung, K.H.; Kim, M.J.; Kim, Y. Preparation of antibacterial PDMAEMA-functionalized multiwalled carbon nanotube via atom transfer radical polymerization. J. Appl. Polym. Sci. 2013, 127, 1508–1518. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, Y.; Lim, Y. Preparation of antibacterial polyester glass mat sheets containing pdmaema-functionalized mwnt nanocomposites. Int. J. Mod. Phys. B 2011, 25, 4311–4314. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zheng, X.; Chen, A.; Chen, Y.; He, G.; Chen, H. Hydroxyl functionalization of single-walled carbon nanotubes causes inhibition to the bacterial denitrification process. Chem. Eng. J. 2015, 279, 47–55. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Mashino, T.; Nishikawa, D.; Takahashi, K.; Usui, N.; Yamori, T.; Seki, M.; Endo, T.; Mochizuki, M. Antibacterial and antiproliferative activity of cationic fullerene derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 4395–4397. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Bianco, A.; Pellarini, F.; Tossi, A.; Giangaspero, A.; Zelezetsky, I.; Briand, J.P.; Prato, M. Solid-Phase Synthesis of Fullerene-peptides. J. Am. Chem. Soc. 2002, 124, 12543–12549. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.J.; Ashcroft, J.M.; Chen, D.; Min, G.; Kim, C.H.; Murkhejee, B.; Larabell, C.; Keasling, J.D.; Chen, F.F. Charge-Associated Effects of Fullerene Derivatives on Microbial Structural Integrity and Central Metabolism. Nano Lett. 2007, 7, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Fortner, J.D.; Guo, W.; Lyon, D.; Boyd, A.M.; Ausman, K.D.; Tao, Y.J.; Sitharaman, B.; Wilson, L.J.; Hughes, J.B.; et al. The Differential Cytotoxicity of Water-Soluble Fullerenes. Nano Lett. 2004, 4, 1881–1887. [Google Scholar] [CrossRef]
- Bosi, S.; da Ros, T.; Spalluto, G.; Prato, M. Fullerene derivatives: An attractive tool for biological applications. Eur. J. Med. Chem. 2003, 38, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Bosi, S.; da Ros, T.; Castellano, S.; Banfi, E.; Prato, M. Antimycobacterial activity of ionic fullerene derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 1043–1045. [Google Scholar] [CrossRef]
- Mashino, T.; Usui, N.; Okuda, K.; Hirota, T.; Mochizuki, M. Respiratory chain inhibition by fullerene derivatives: Hydrogen peroxide production caused by fullerene derivatives and a respiratory chain system. Bioorg. Med. Chem. 2003, 11, 1433–1438. [Google Scholar] [CrossRef]
- Lee, J.; Mackeyev, Y.; Cho, M.; Li, D.; Kim, J.H.; Wilson, L.J.; Alvarez, P.J.J. Photochemical and Antimicrobial Properties of Novel C60 Derivatives in Aqueous Systems. Environ. Sci. Technol. 2009, 43, 6604–6610. [Google Scholar] [CrossRef] [PubMed]
- Krueger, A. The structure and reactivity of nanoscale diamond. J. Mater. Chem. 2008, 18, 1485–1492. [Google Scholar] [CrossRef]
- Krueger, A.; Lang, D. Functionality is Key: Recent Progress in the Surface Modification of Nanodiamond. Adv. Funct. Mater. 2012, 22, 890–906. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Mogil’naya, O.A.; Bondar, V.S. Comparative study of antibacterial properties of Lysozyme upon its adsorption and covalent binding to nanodiamonds. Nanotechnol. Russ. 2012, 7, 658–665. [Google Scholar] [CrossRef]
- Mogilnaya, O.; Bondar, V. Antibacterial Properties of Lysozyme Immobilized on Nanodiamonds. Micro Nanosyst. 2012, 4, 41–47. [Google Scholar] [CrossRef]
- Nguyen, T.T.B.; Chang, H.C.; Wu, V.W.K. Adsorption and hydrolytic activity of lysozyme on diamond nanocrystallites. Diam. Relat. Mater. 2007, 16, 872–876. [Google Scholar] [CrossRef]
- Perevedentseva, E.; Cheng, C.Y.; Chung, P.H.; Tu, J.S.; Hsieh, Y.H.; Cheng, C.L. The interaction of the protein lysozyme with bacteria E. coli observed using nanodiamond labelling. Nanotechnology 2007, 18, 315102. [Google Scholar] [CrossRef]
- Szunerits, S.; Barras, A.; Boukherroub, R. Antibacterial Applications of Nanodiamonds. Int. J. Environ. Res. Public Health 2016, 13, 413. [Google Scholar] [CrossRef] [PubMed]
- Turcheniuk, V.; Raks, V.; Issa, R.; Cooper, I.R.; Cragg, P.J.; Jijie, R.; Dumitrascu, N.; Mikhalovska, L.I.; Barras, A.; Zaitsev, V.; et al. Antimicrobial activity of menthol modified nanodiamond particles. Diam. Relat. Mater. 2015, 57, 2–8. [Google Scholar] [CrossRef] [Green Version]
- Miksovsky, J.; Voss, A.; Kozarova, R.; Kocourek, T.; Pisarik, P.; Ceccone, G.; Kulisch, W.; Jelinek, M.; Apostolova, M.D.; Reithmaier, J.P.; et al. Cell adhesion and growth on ultrananocrystalline diamond and diamond-like carbon films after different surface modifications. Appl. Surf. Sci. 2014, 297, 95–102. [Google Scholar] [CrossRef]
- Shao, W.; Zhao, Q.; Abel, E.W.; Bendavid, A. Influence of interaction energy between Si-doped diamond-like carbon films and bacteria on bacterial adhesion under flow conditions. J. Biomed. Mater. Res. A 2010, 93A, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- Tong, W.; Fox, K.; Ganesan, K.; Turnley, A.M.; Shimoni, O.; Tran, P.A.; Lohrmann, A.; McFarlane, T.; Ahnood, A.; Garrett, D.J.; et al. Fabrication of planarised conductively patterned diamond for bio-applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, Q.; Liu, Y.; Wang, S.; Abel, E.W. Reduction of bacterial adhesion on modified DLC coatings. Colloids Surf. B BioInterfaces 2008, 61, 182–187. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. https://doi.org/10.3390/ma9080617
Maas M. Carbon Nanomaterials as Antibacterial Colloids. Materials. 2016; 9(8):617. https://doi.org/10.3390/ma9080617
Chicago/Turabian StyleMaas, Michael. 2016. "Carbon Nanomaterials as Antibacterial Colloids" Materials 9, no. 8: 617. https://doi.org/10.3390/ma9080617