In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections
Abstract
:1. Introduction
2. Experimental Section
2.1. Deposition of Silver Nanoparticles on Cotton Gauzes
2.2. SEM Analysis on Silver Coated Gauzes
2.3. Thermo-Gravimetric Analysis TGA
2.4. Qualitative and Quantitative Evaluation of the Antibacterial Capability
2.5. Quantification of Bacterial Biofilm on Cotton Gauzes
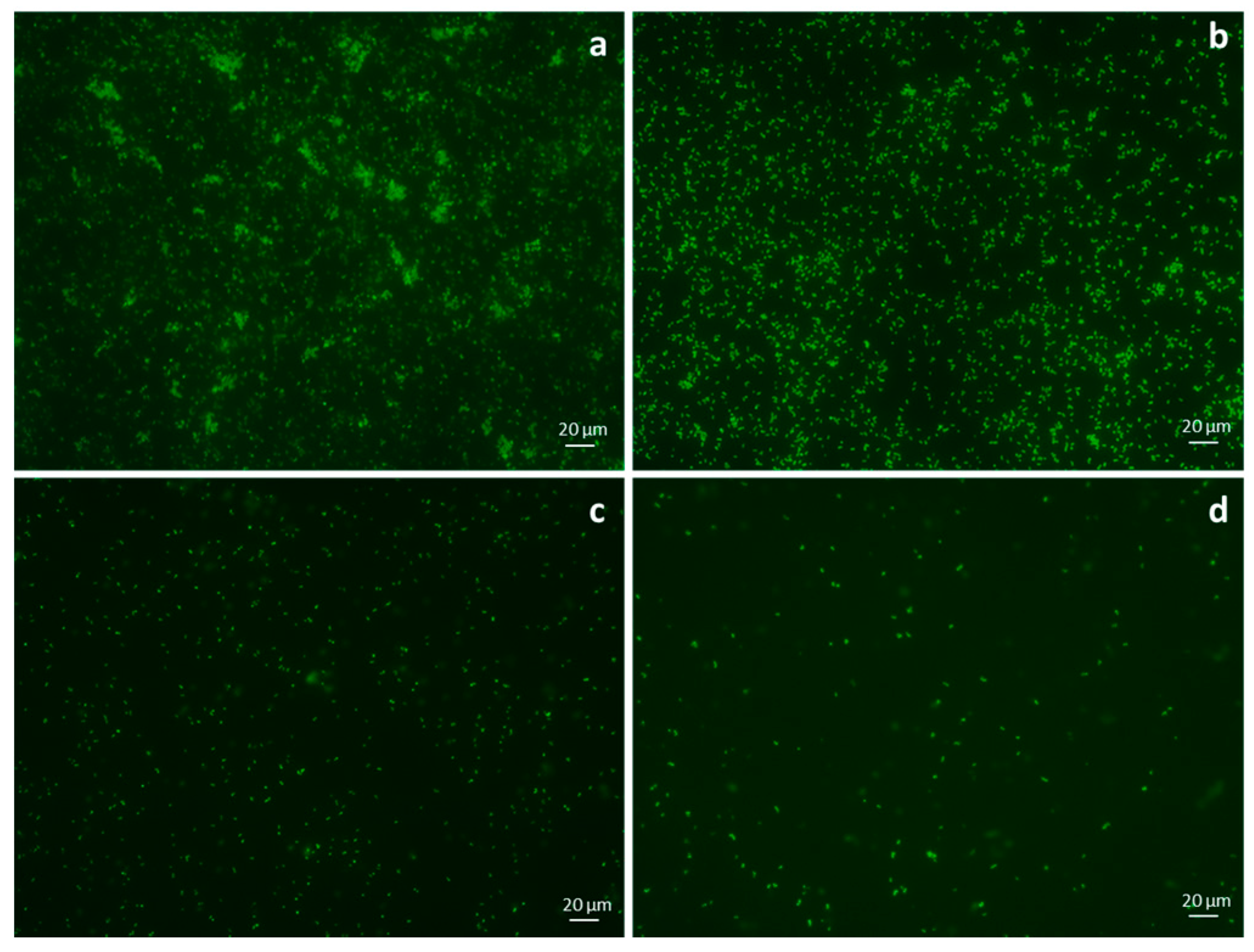
2.6. Fluorescence Microscopy on Adherent Bacteria
2.7. SEM Analysis of the Bacterial Adhesion on Cotton Gauzes
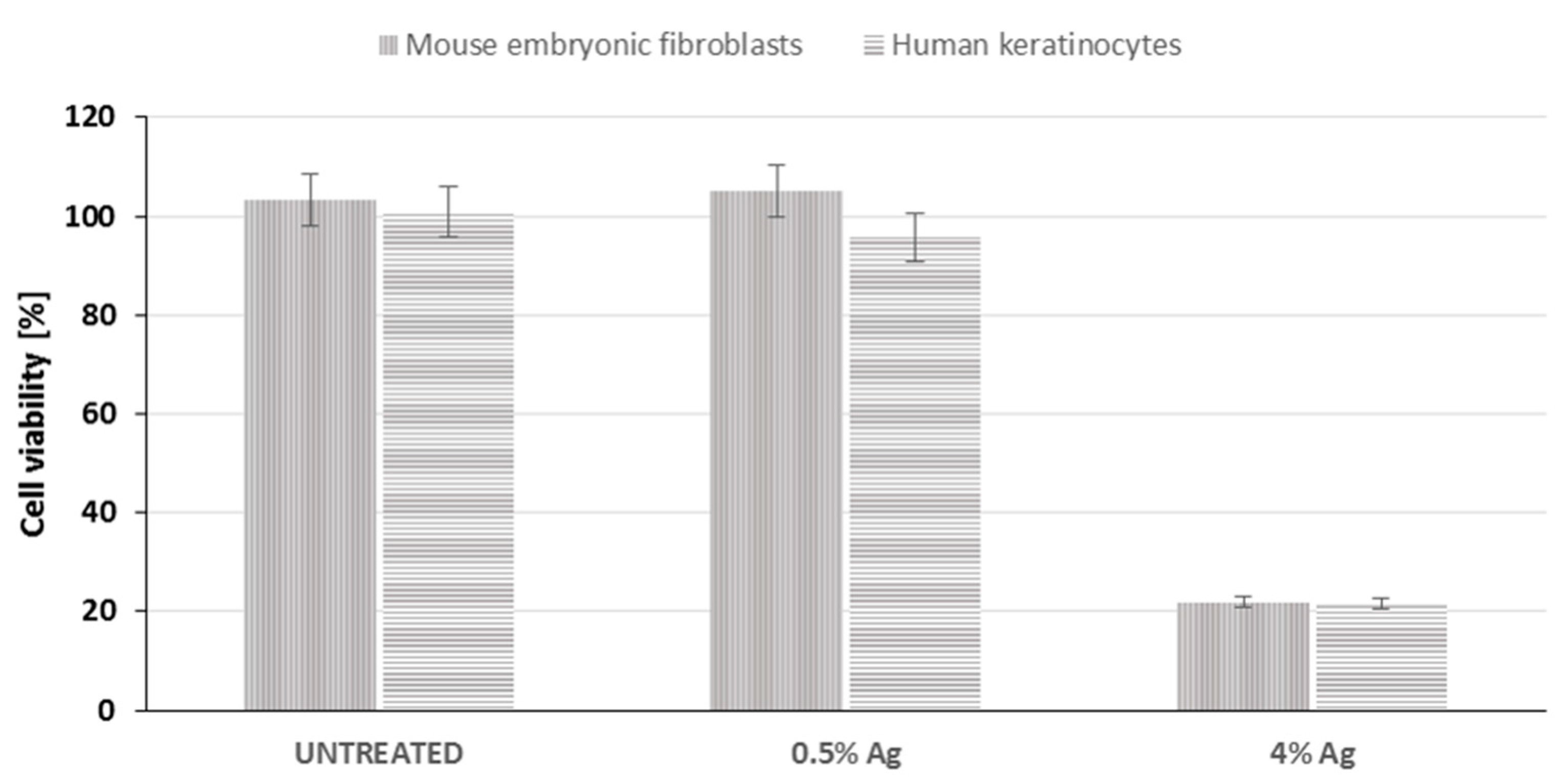
2.8. Cytotoxicity Test
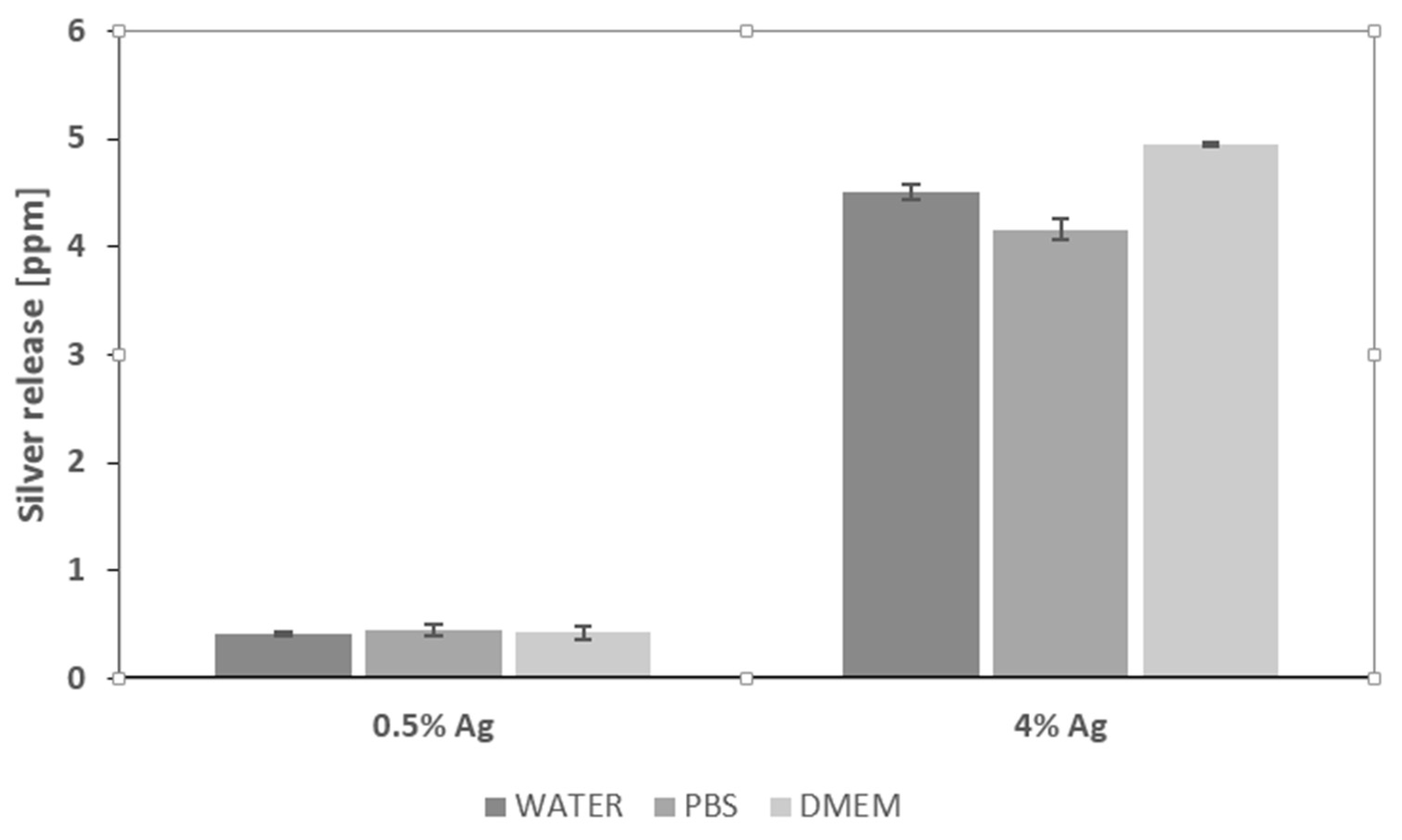
2.9. ICP-MS Analysis
3. Results
3.1. Deposition of Silver Nanoparticles on Cotton Gauzes
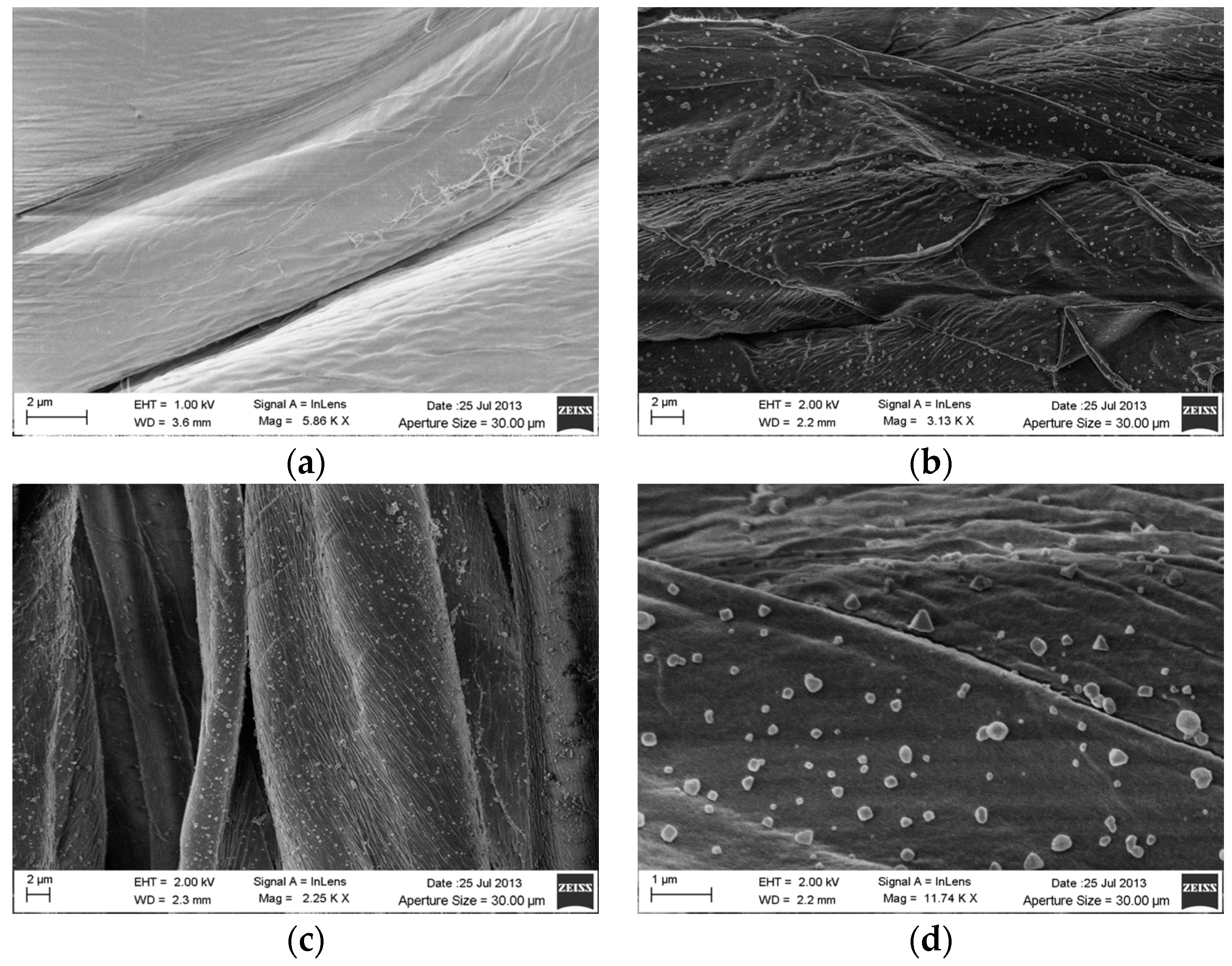
3.2. SEM Analysis on Silver Coated Gauzes
3.3. Thermo-Gravimetric Analysis TGA
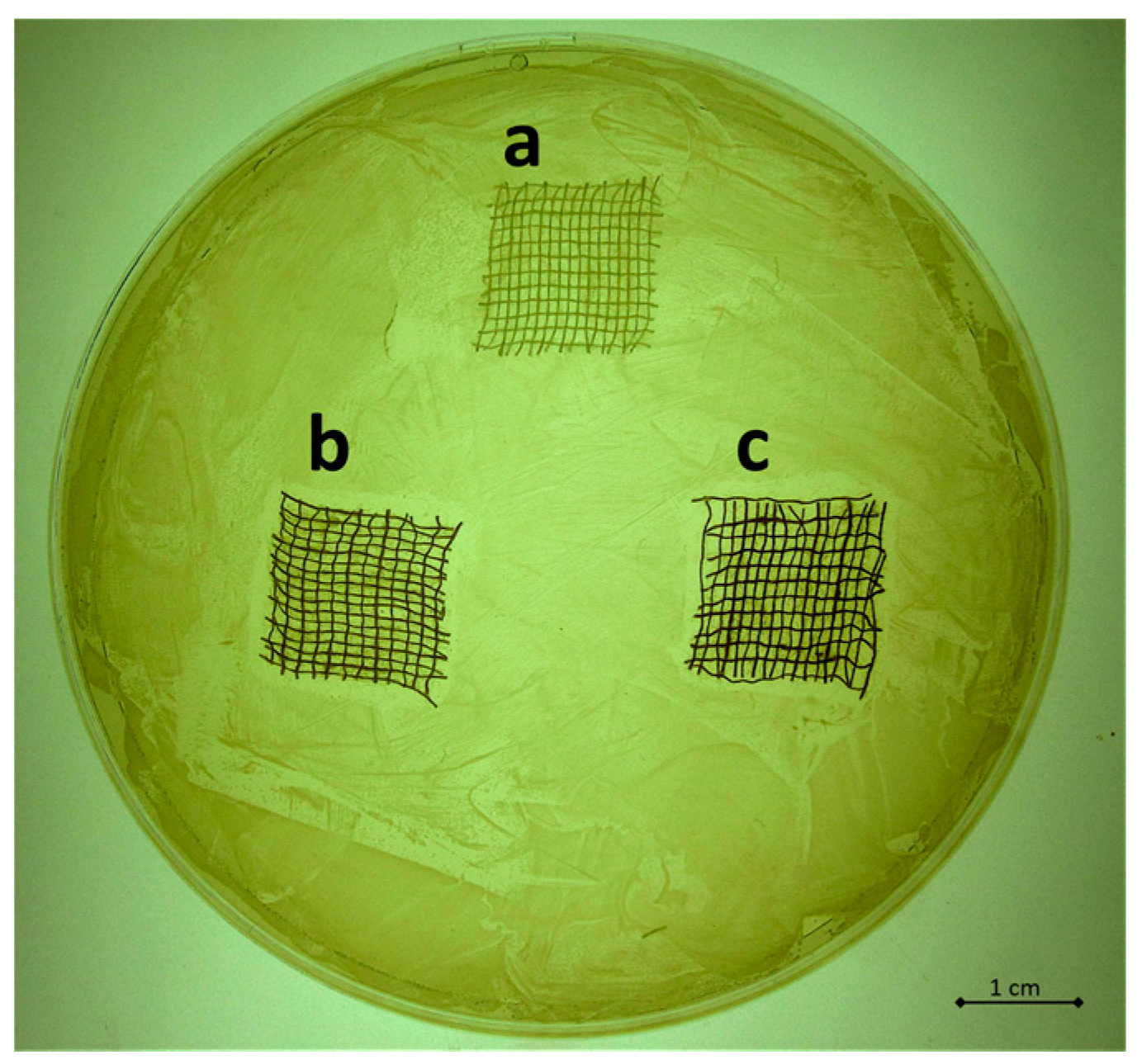
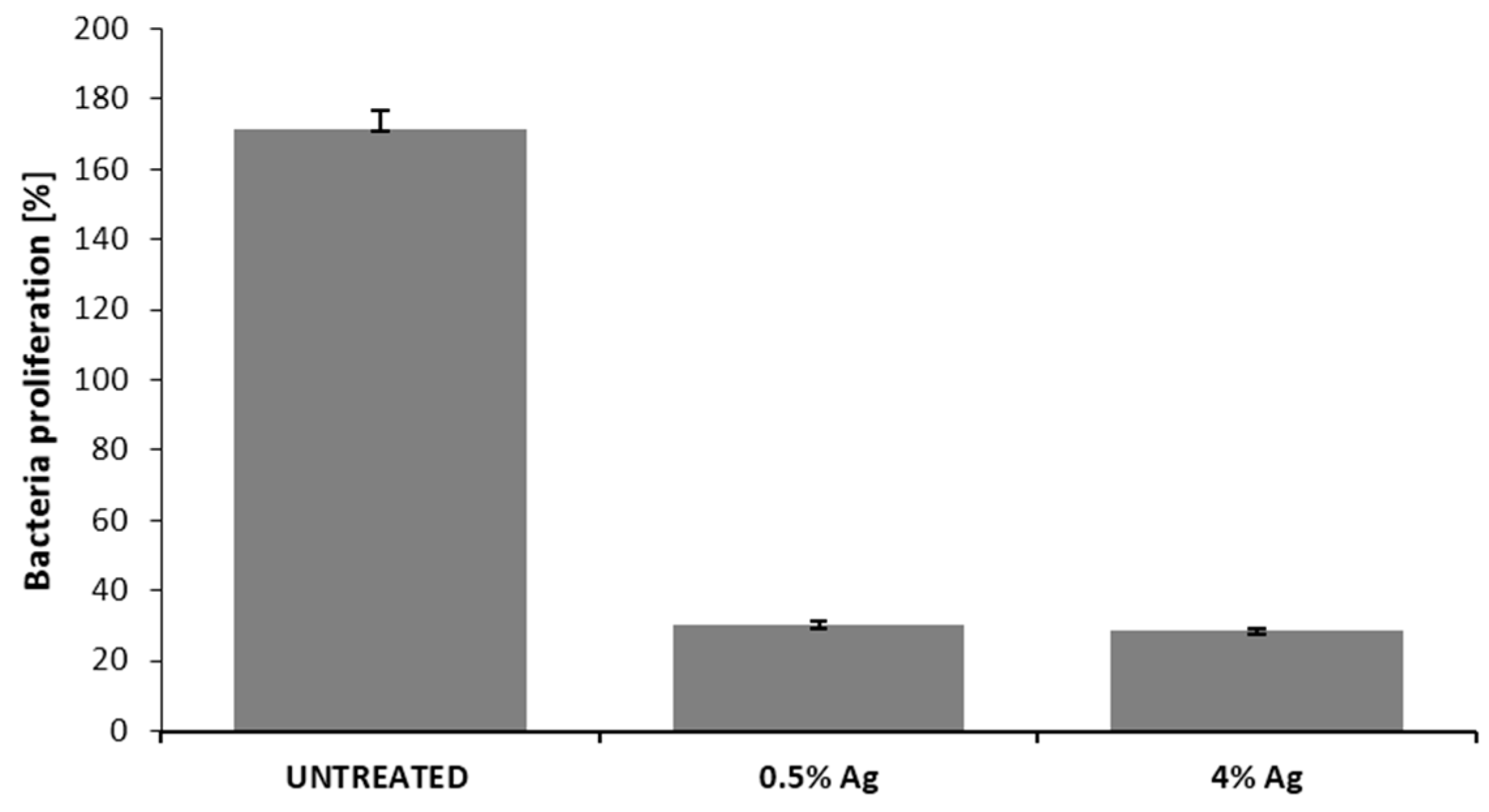
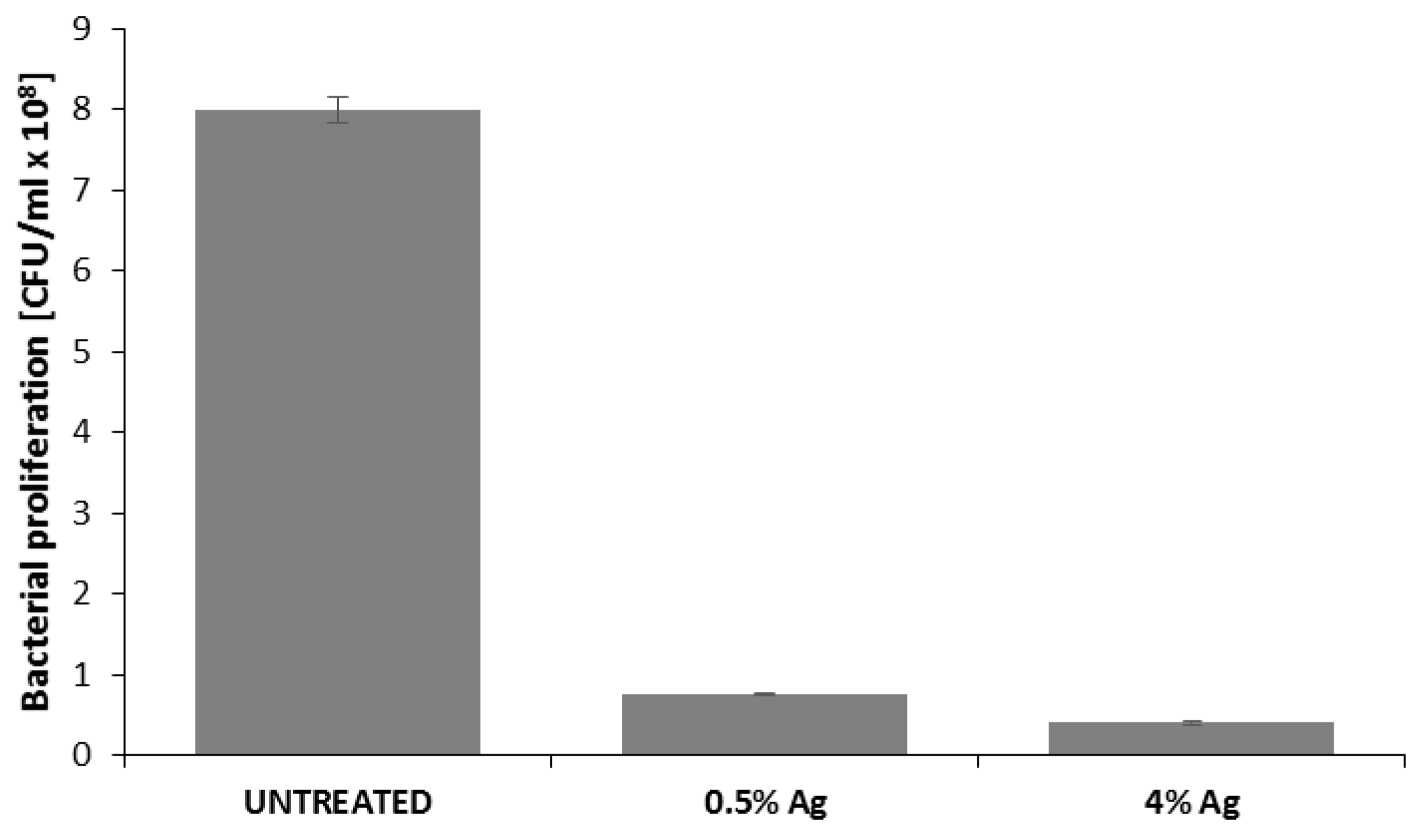
3.4. Qualitative and Quantitative Evaluation of the Antibacterial Capability
3.5. Quantification of Bacterial Biofilm on Cotton Gauzes
3.6. Fluorescence Microscopy on Adherent Bacteria
3.7. SEM Analysis of the Bacterial Adhesion on Cotton Gauzes
3.8. Cytotoxicity Test
3.9. ICP-MS Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SEM | Scanning Electron Microscopy |
| TGA | Thermo-Gravimetric Analysis |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide colorimetric assay |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
References
- Percival, S.; Slone, W.; Linton, S.; Okel, T.; Corum, L.; Thomas, J.G. The antimicrobial efficacy of a silver alginate dressing against a broad spectrum of clinically relevant wound isolates. Int. Wound J. 2011, 8, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Bloemsma, G.C.; Dokter, J.; Boxma, H.; Oen, I.M. Mortality and causes of death in a burn center. Burns 2008, 34, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Abedini, F.; Ahmadi, A.; Yavari, A.; Hosseini, V.; Mousavi, S. Comparison of silver nylon wound dressing and silver sulfadiazine in partial burn wound therapy. Int. Wound J. 2013, 10, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.D.; Wolcott, R.D.; Percival, S.L. Biofilms in wounds: Management strategies. J. Wound Care 2008, 17, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Bowler, P.G.; Welsby, S.; Towers, V.; Booth, R.; Hogarth, A.; Rowlands, V.; Joseph, A.; Jones, S.A. Multidrug-resistant organisms, wounds and topical antimicrobial protection. Int. Wound J. 2012, 9, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.J.; Tingley-Kelley, K.; Warriner, R.A. Silver treatments and silver-impregnated dressings for the healing of leg wounds and ulcers: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2010, 63, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.; White, R.; Hoekstra, H. Topical silver-impregnated dressings and the importance of the dressing technology. Int. Wound J. 2009, 6, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Kirketerp-Moller, K.; Kristiansen, S.; Phipps, R.; Nielsen, A.K.; Jensen, P.O.; Hoiby, N.; Givskov, M. Silver against Pseudomonas aeruginosa biofilms. APMIS 2007, 115, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver I: Its antibacterial properties and mechanism of action. J. Wound Care 2002, 11, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Kostenko, V.; Lyczak, J.; Turner, K.; Martinuzzi, R.J. Impact of Silver-Containing Wound Dressings on Bacterial Biofilm Viability and Susceptibility to Antibiotics during Prolonged Treatment. Antimicrob. Agents Chemother. 2010, 54, 5120–5131. [Google Scholar] [CrossRef] [PubMed]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.J.; Shafii, S.M.; Ko, F.; Donate, G.; Wright, T.E.; Mannari, R.J.; Payne, W.G.; Smith, D.J.; Robson, M.C. Comparative evaluation of silver-containing antimicrobial dressings and drugs. Int. Wound J. 2007, 4, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, M.H.; Burrell, R.E.; Nadworny, P.L. Evaluating antimicrobial efficacy of new commercially available silver dressings. Int. Wound J. 2010, 7, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Thomas, J.G.; Slone, W.; Linton, S.; Corum, L.; Okel, T. The efficacy of silver dressings and antibiotics on MRSA and MSSA isolated from burn patients. Wound Repair Regen. 2011, 19, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Sannino, A.; Maffezzoli, A.; Licciulli, A. Antibacterial Surface Treatments Based on Silver Clusters Deposition. U.S. Patent 20090130181, 21 May 2009. [Google Scholar]
- Pollini, M.; Paladini, F.; Licciulli, A.; Maffezzoli, A.; Sannino, A.; Nicolais, L. Antibacterial natural leather for application in the public transport system. J. Coat. Technol. Res. 2013, 10, 239–245. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F.; Licciulli, A.; Maffezzoli, A.; Sannino, A. Engineering Nanostructured Silver Coatings for Antimicrobial Applications. In Nanoantimicrobials Progress and Prospects; Cioffi, N., Rai, M., Eds.; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA, 2012; pp. 313–336. [Google Scholar]
- Paladini, F.; Cooper, I.R.; Pollini, M. Development of antibacterial and antifungal silver-coated polyurethane foams as air filtration units for the prevention of respiratory diseases. J. Appl. Microbiol. 2014, 116, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Pollini, M.; Paladini, F.; Sannino, A.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Nitti, M.A.; Valentini, M.; Valentini, A. Nonconventional Routes to Silver Nanoantimicrobials: Technological Issues, Bioactivity and Applications. In Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases; Rai, M., Kon, K., Eds.; Elsevier: London, UK; San Diego, CA, USA; Waltham, MA, USA; Oxford, UK, 2015; pp. 87–105. [Google Scholar]
- Chang, Y.C.; Yang, C.Y.; Sun, R.L.; Cheng, Y.F.; Kao, W.C.; Yang, P.C. Rapid single cell detection of Staphylococcus aureus by aptamer-conjugated gold nanoparticles. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Napavichayanun, S.; Amornsudthiwat, P.; Pienpinijtham, P.; Aramwit, P. Interaction and effectiveness of antimicrobials along with healing-promoting agents in a novel biocellulose wound dressing. Mater. Sci. Eng. C 2015, 55, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Calamak, S.; Erdogdu, C.; Ozalp, M.; Ulubayram, K. Silk fibroin based antibacterial bionanotextiles as wound dressing materials. Mater. Sci. Eng. C 2014, 43, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Khundkar, R.; Malic, C.; Burge, T. Use of Acticoat™ dressing in burns: What is the evidence? Burns 2010, 36, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Verbelen, J.; Hoeksema, H.; Heyneman, A.; Pirayesh, A.; Monstrey, S. Aquacel® Ag dressing versus Acticoat™ dressing in partial thickness burns: A prospective, randomized, controlled study in 100 patients. Part 1: Burn wound healing. Burns 2013. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Dai, T.; Xuan, Y.; Tegos, G.P.; Hamblin, M.R. Synergistic combination of chitosan acetate with nanoparticle silver as a topical antimicrobial: Efficacy against bacterial burn infections. Antimicrob. Agents Chemother. 2011, 55, 3432–3438. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Rigo, C.; Munivrana, I.; Vindigni, V.; Azzena, B.; Barbante, C.; Fenzi, F.; Guerriero, P.; Cairns, W.R. Development and application of methods for the determination of silver in polymeric dressings used for the care of burns. Talanta 2013, 115, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Knetsch, M.L.W.; Koole, L.H. New Strategies in the Development of Antimicrobial Coatings: The Example of Increasing Usage of Silver and Silver Nanoparticles. Polymers 2011, 3, 340–366. [Google Scholar] [CrossRef]
- Abboud, E.C.; Legare, T.B.; Settle, J.C.; Boubekri, A.M.; Barillo, D.J.; Marcet, J.E.; Sanchez, J.E. Do silver-based wound dressings reduce pain? A prospective study and review of the literature. Burns 2014, 40. [Google Scholar] [CrossRef] [PubMed]
- Fong, J.; Wood, F. Nanocrystalline silver dressings in wound management: A review. Int. J. Nanomed. 2006, 1, 441–449. [Google Scholar] [CrossRef]
- Lin, Y.H.; Hsu, W.S.; Chung, W.Y.; Ko, T.H.; Lin, J.H. Silver-based wound dressings reduce bacterial burden and promote wound healing. Int. Wound J. 2015. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J. Silver dressings: Their role in wound management. Int. Wound J. 2006, 3, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Ip, M.; Lui, S.L.; Poon, V.K.; Lung, I.; Burd, A. Antimicrobial activities of silver dressings: An in vitro comparison. J. Med. Microbiol. 2006, 55, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Moiemen, N.S.; Shale, E.; Drysdale, K.J.; Smith, G.; Wilson, Y.T.; Papini, R. Acticoat dressings and major burns: Systemic silver absorption. Burns 2011, 37, 27–35. [Google Scholar] [CrossRef] [PubMed]
- El-Rafie, M.H.; Ahmed, H.B.; Zahran, M.K. Characterization of nanosilver coated cotton fabrics and evaluation of its antibacterial efficacy. Carbohydr. Polym. 2014, 107, 74–181. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Caschera, D.; Padeletti, G.; Ingo, G.M.; Gigli, G. A brief review of surface-functionalized cotton fabrics. Surf. Innov. 2013, 1, 140–156. [Google Scholar] [CrossRef]
- Barnea, Y.; Weiss, J.; Gur, E. A review of the applications of the hydrofiber dressing with silver (Aquacel Ag) in wound care. Ther. Clin. Risk Manag. 2010, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Harding, K.; Gottrup, F.; Jawień, A.; Mikosiński, J.; Twardowska-Saucha, K.; Kaczmarek, S.; Sopata, M.; Shearman, C.; Pieronne, A.; Kommala, D. A prospective, multi-centre, randomised, open label, parallel, comparative study to evaluate effects of AQUACEL® Ag and Urgotul® Silver dressing on healing of chronic venous leg ulcers. Int. Wound J. 2012, 9, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Kaur, J.; Sun, L.; Wang, X. Multifunctionalization of cotton through in situ green synthesis of silver nanoparticles. Cellulose 2013, 20, 3053–3065. [Google Scholar] [CrossRef]
- Basri, D.F.; Xian, L.W.; Shukor, N.I.A.S.; Latip, J. Bacteriostatic Antimicrobial Combination: Antagonistic Interaction between Epsilon-Viniferin and Vancomycin against Methicillin-Resistant Staphylococcus aureus. Biomed. Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C.; Burgess, D.S. Polymyxins and Doripenem Combination Against KPC-Producing Klebsiella pneumonia. J. Clin. Med. Res. 2013, 5, 97–100. [Google Scholar] [PubMed]
- Paladini, F.; De Simone, S.; Sannino, A.; Pollini, M. Antibacterial and Antifungal Dressings Obtained by Photochemical Deposition of Silver Nanoparticles. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Paladini, F.; Picca, R.A.; Sportelli, M.C.; Cioffi, N.; Sannino, A.; Pollini, M. Surface chemical and biological characterization of flax fabrics modified with silver nanoparticles for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 52, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Sannino, A.; Pollini, M. In vivo testing of silver treated fibers for the evaluation of skin irritation effect and hypoallergenicity. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Paladini, F.; Pollini, M.; Sannino, A.; Ambrosio, L. Metal-Based Antibacterial Substrates for Biomedical Applications. Biomacromolecules 2015, 16, 1873–1885. [Google Scholar] [CrossRef] [PubMed]
- Schierholz, J.M.; Lucasj, L.J.; Rump, A.; Pulverer, G. Efficacy of silver-coated medical devices. J. Hosp. Infect. 1998, 40, 257–262. [Google Scholar] [CrossRef]
- Damm, C.; Munstedt, H.; Rosch, A. The antimicrobial efficacy of polyamide 6/silver-nano- and microcomposites. Mater. Chem. Phys. 2008, 108, 61–66. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Koller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]

| Sample | CFU/mL | Log Reduction |
|---|---|---|
| Untreated | 1.62 × 106 | – |
| 0.5% Ag | 5.27 × 102 | 3.49 |
| 4% Ag | 5.00 × 10 | 4.51 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paladini, F.; Di Franco, C.; Panico, A.; Scamarcio, G.; Sannino, A.; Pollini, M. In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections. Materials 2016, 9, 411. https://doi.org/10.3390/ma9060411
Paladini F, Di Franco C, Panico A, Scamarcio G, Sannino A, Pollini M. In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections. Materials. 2016; 9(6):411. https://doi.org/10.3390/ma9060411
Chicago/Turabian StylePaladini, Federica, Cinzia Di Franco, Angelica Panico, Gaetano Scamarcio, Alessandro Sannino, and Mauro Pollini. 2016. "In Vitro Assessment of the Antibacterial Potential of Silver Nano-Coatings on Cotton Gauzes for Prevention of Wound Infections" Materials 9, no. 6: 411. https://doi.org/10.3390/ma9060411






