Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. SBA2 Synthesis and Characterization
2.2. SBA3 Synthesis and Characterization
3. Results and Discussion
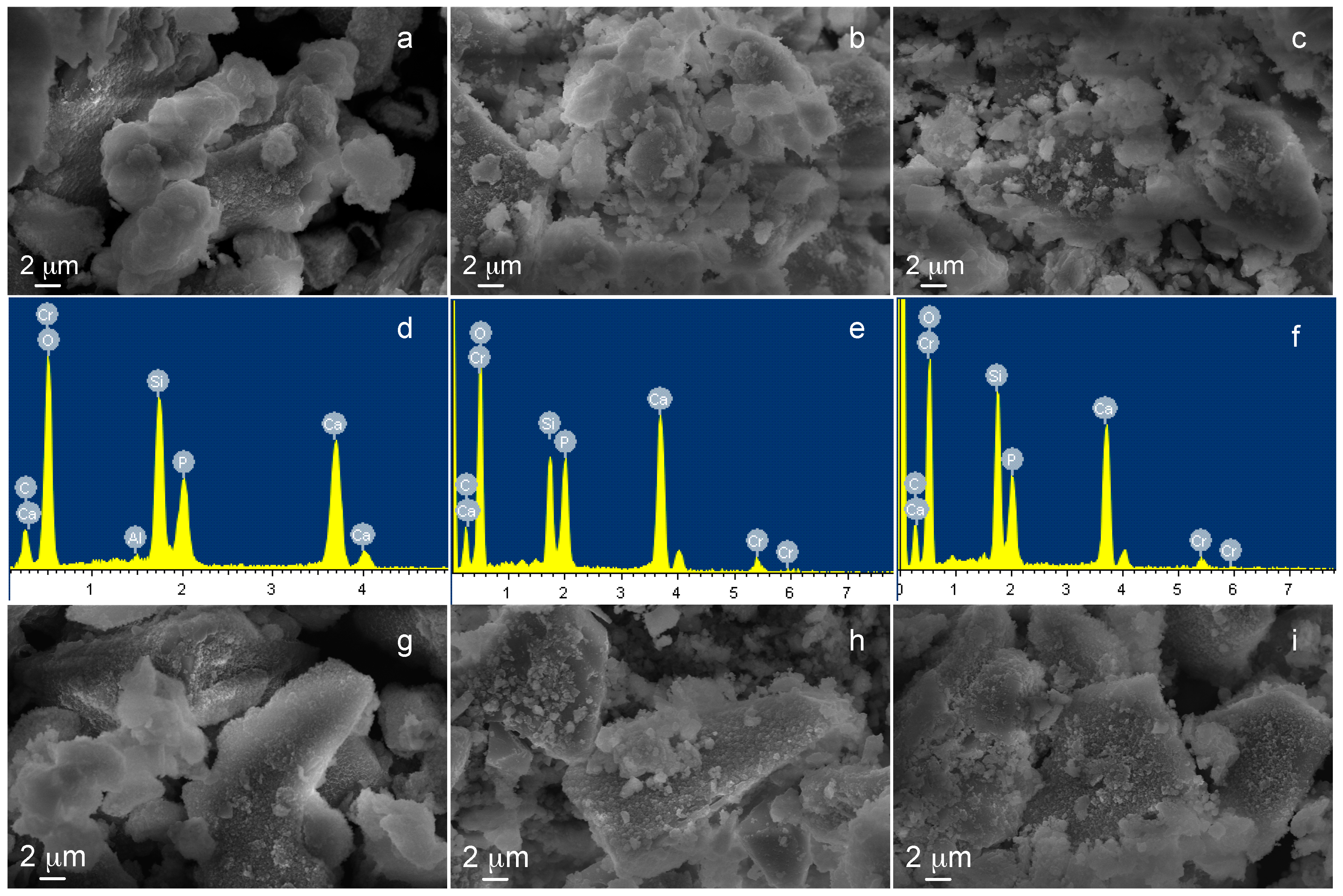
3.1. SBA2 Characterization
3.2. SBA3 Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1993, 81, 1705–1728. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Kaur, G.; Pickrell, G.; Sriranganathan, N.; Kumar, V.; Homa, D. Review and the state of the art: Sol-gel or melt quenched bioactive glasses for tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication, and apatite formation. J. Biomed. Mater. Res. Part A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, L.C.; Boccaccini, A.R. Bioactive Glass and Glass-Ceramic Scaffolds for Bone Tissue Engineering. Materials 2010, 33, 3867–3910. [Google Scholar] [CrossRef]
- Jones, J.R. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Verné, E. Bioactive Glass and Glass-Ceramic Coatings. In Bio-Glasses—An Introduction; Jones, R.J., Clare, A.G., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 107–119. [Google Scholar]
- Gerhardt, L.C.; Widdows, K.L.; Erol, M.M.; Burch, C.W.; Sanz-Herrera, J.A.; Ochoa, I.; Stämpfli, R.; Roqan, I.S.; Gabe, S.; Ansari, T.; et al. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials 2011, 32, 4096–4108. [Google Scholar] [CrossRef] [PubMed]
- Mourino, V.; Cattalini, J.P.; Boccaccini, A.R. Metallic ions as therapeutic agents in tissue engineering scaffolds: An overview of their biological applications and strategies for new developments. J. R. Soc. Interface 2012, 9, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Miola, M.; Vitale Brovarone, C.; Maina, G.; Rossi, F.; Bergandi, L.; Ghigo, D.; Saracino, S.; Maggiora, M.; Canuto, R.A.; Muzio, G.; et al. In vitro study of manganese-doped bioactive glasses for bone regeneration. Mater. Sci. Eng. C 2014, 38, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Chrzanowski, W.; Pickup, D.M.; O’Dell, L.A.; Mordan, N.J.; Newport, R.J.; Smith, M.E.; Knowles, J.C. Structure and properties of strontium-doped phosphate-based glasses. J. R. Soc. Interface 2009, 6, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses beyond bone and teeth: Emerging applications in contact with soft tissues. Acta Biomater. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Miguez-Pacheco, V.; Greenspan, D.; Hench, L.L.; Boccaccini, A.R. Bioactive glasses in soft tissue repair. Am. Ceram. Soc. Bull. 2015, 94, 27–31. [Google Scholar]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Mouriño, V.; Boccaccini, A.R. Therapeutic inorganic ions in bioactive glasses to enhance bone formation and beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef]
- Rodríguez, J.P.; Ríos, S.; González, M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002, 85, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.; Vogt, S.; Fukai, T.; Glesne, D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009, 36, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.F. Copper stimulates proliferation of human endothelial cells under culture. J. Cell. Biochem. 1998, 69, 326–335. [Google Scholar] [CrossRef]
- Giavaresi, G.; Torricelli, P.; Fornasari, P.M.; Giardino, R.; Barbucci, R.; Leone, G. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials 2005, 26, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Kang, Y.J. Role of copper in angiogenesis and its medicinal implications. Curr. Med. Chem. 2009, 16, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Gérard, C.; Bordeleau, L.J.; Barralet, J.; Doillon, C.J. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials 2010, 31, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Choi, Y.; Kim, K.M.; Choi, S.Y. Evaluation of copper ion of antibacterial effect on Pseudomonas aeruginosa, Salmonella typhimurium and Helicobacter pylori and optical, mechanical properties. Appl. Surf. Sci. 2012, 258, 3823–3828. [Google Scholar] [CrossRef]
- Raffi, M.; Mehrwan, S.; Bhatti, T.M.; Akhter, J.I.; Hameed, A.; Yawar, W.; Hasan, M.M. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann. Microbiol. 2010, 60, 75–80. [Google Scholar] [CrossRef]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol-gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef] [PubMed]
- Bonici, A.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Piva, A. Synthesis and characterization of bioactive glasses functionalized with Cu nanoparticles and organic molecules. J. Eur. Ceram. Soc. 2012, 32, 2777–2783. [Google Scholar] [CrossRef]
- Goh, Y.F.; Alshemary, A.M.; Akram, M.; Rafiq, M.; Kadir, A.; Hussa, R. Bioactive glass: An in-vitro comparative study of doping with nanoscale copper and silver particles. Appl. Glass Sci. 2014, 5, 255–266. [Google Scholar] [CrossRef]
- Aina, V.; Cerrato, G.; Martra, G.; Malavasi, G.; Lusvardi, G.; Menabue, L. Towards the controlled release of metal nanoparticles frombiomaterials: Physico-chemical, morphological and bioactivityfeatures of Cu-containing sol-gel glasses. Appl. Surf. Sci. 2013, 283, 240–248. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Pyare, R. Characterization of CuO substituted 45S5 Bioactive Glasses and Glass—Ceramics. Int. J. Sci. Technol. Res. 2012, 1, 28–41. [Google Scholar]
- Abou Neel, E.A.; Ahmed, I.; Pratten, J.; Nazhat, S.N.; Knowles, J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, A.; Wilson, M.; Knowles, J.C. The Effect of increasing copper content in phosphate-based glasses on biofilms of Streptococcus sanguis. Biomaterials 2003, 24, 1797–1807. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Borsella, E.; Dal Vecchio, A.; Garcıa, M.A.; Sada, C.; Gonella, F.; Polloni, R.; Quaranta, A.; van Wilderen, L.J.G.W. Copper doping of silicate glasses by the ion-exchange technique: A photoluminescence spectroscopy study. J. Appl. Phys. 2002, 91. [Google Scholar] [CrossRef]
- Gonella, F.; Quaranta, A.; Padovani, S.; Sada, C.; D’Acapito, F.; Maurizio, C.; Battaglin, G.; Cattaruzza, E. Copper diffusion in ion-exchanged soda-lime glass. Appl. Phys. 2005, 81, 1065–1071. [Google Scholar] [CrossRef]
- Gonella, F.; Quaranta, A.; Cattaruzza, E.; Padovani, S.; Sada, C.; D’Acapito, F.; Maurizio, C. Cu-alkali ion exchange in glass: A model for the copper diffusion based on XAFS experiments. Comput. Mater. Sci. 2005, 33, 31–36. [Google Scholar] [CrossRef]
- Sakka, K.; Kamiya, A.; Kato, K. Incorporation of Copper into Glass by the Cu-Na Ion Exchange. J. Non-Cryst. Solids 1982, 5, 77–90. [Google Scholar] [CrossRef]
- Dimitrova, M.; Ivanova, Y.; Dimitriev, Y. Ion-exchange colouring of float glasses in vapours and melts of copper-containing salt. J. Univ. Chem. Technol. Metall. 2012, 47, 409–414. [Google Scholar]
- National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard M2-A9, 9th ed.; NCCLS: Villanova, PA, USA, 2003. [Google Scholar]
- Capoccia, D.; Montanaro, L.; Speziale, P.; Arciola, C.R. Antibiotic-loaded biomaterials and the risks for the spread of antibiotic resistance following their prophylactic and therapeutic clinical use. Biomaterials 2010, 31, 6363–6377. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Björkvik, L.; Wang, X.; Hupa, L. Dissolution of bioactive glasses in acidic solutions with the focus on lactic acid. Int. J. Appl. Glass Sci. 2016. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Mačković, M.; Hoppe, A.; Detsch, R.; Mohn, D.; Stark, W.J.; Spiecker, E.; Boccaccini, A.R. Bioactive glass (type 45S5) nanoparticles: In vitro reactivity on nanoscale and biocompatibility. J. Nanopart. Res. 2012, 14. [Google Scholar] [CrossRef] [Green Version]
- Di Nunzio, S.; Vitale Brovaronea, C.; Spriano, S.; Milanese, D.; Verné, E.; Bergo, V.; Maina, G.; Spinelli, P. Silver containing bioactive glasses prepared by molten salt ion-exchange. J. Eur. Ceram. Soc. 2004, 24, 2935–2942. [Google Scholar] [CrossRef]
- Verne, E.; Ferraris, S.; Miola, M.; Fucale, G.; Maina, G.; Martinasso, G.; Canuto, R.A.; di Nunzio, S.; Vitale-Brovarone, C. Synthesis and characterisation of bioactive and antibacterial glass-ceramic Part 1—Microstructure, properties and biological behaviour. Adv. Appl. Ceram. 2008, 107, 234–244. [Google Scholar] [CrossRef]
- Perera, G.; Doremus, R.H.; Lanford, W. Dissolution Rates of Silicate Glasses in Water at pH 7. J. Am. Ceram. Soc. 1991, 74, 1269–1274. [Google Scholar] [CrossRef]
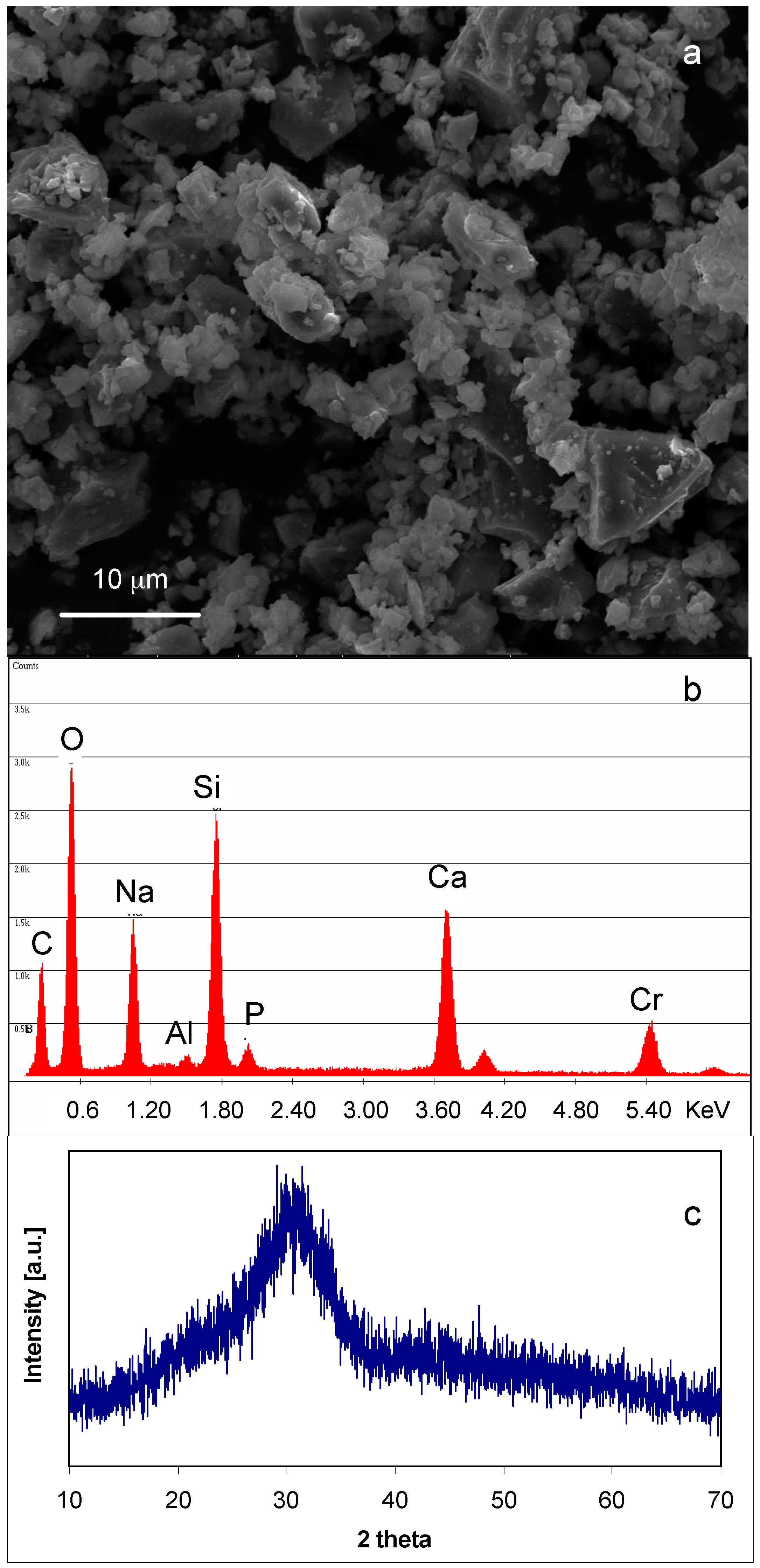
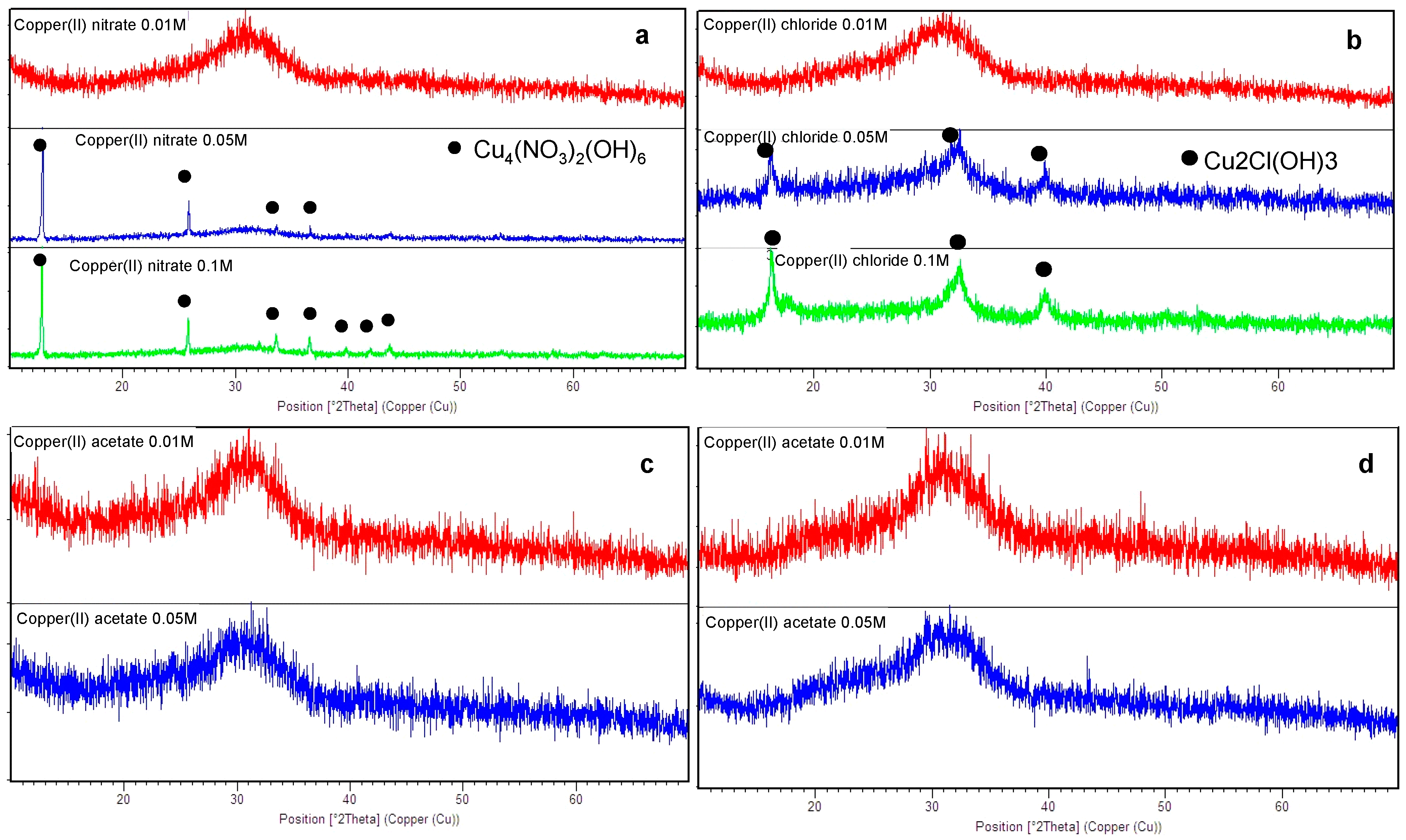




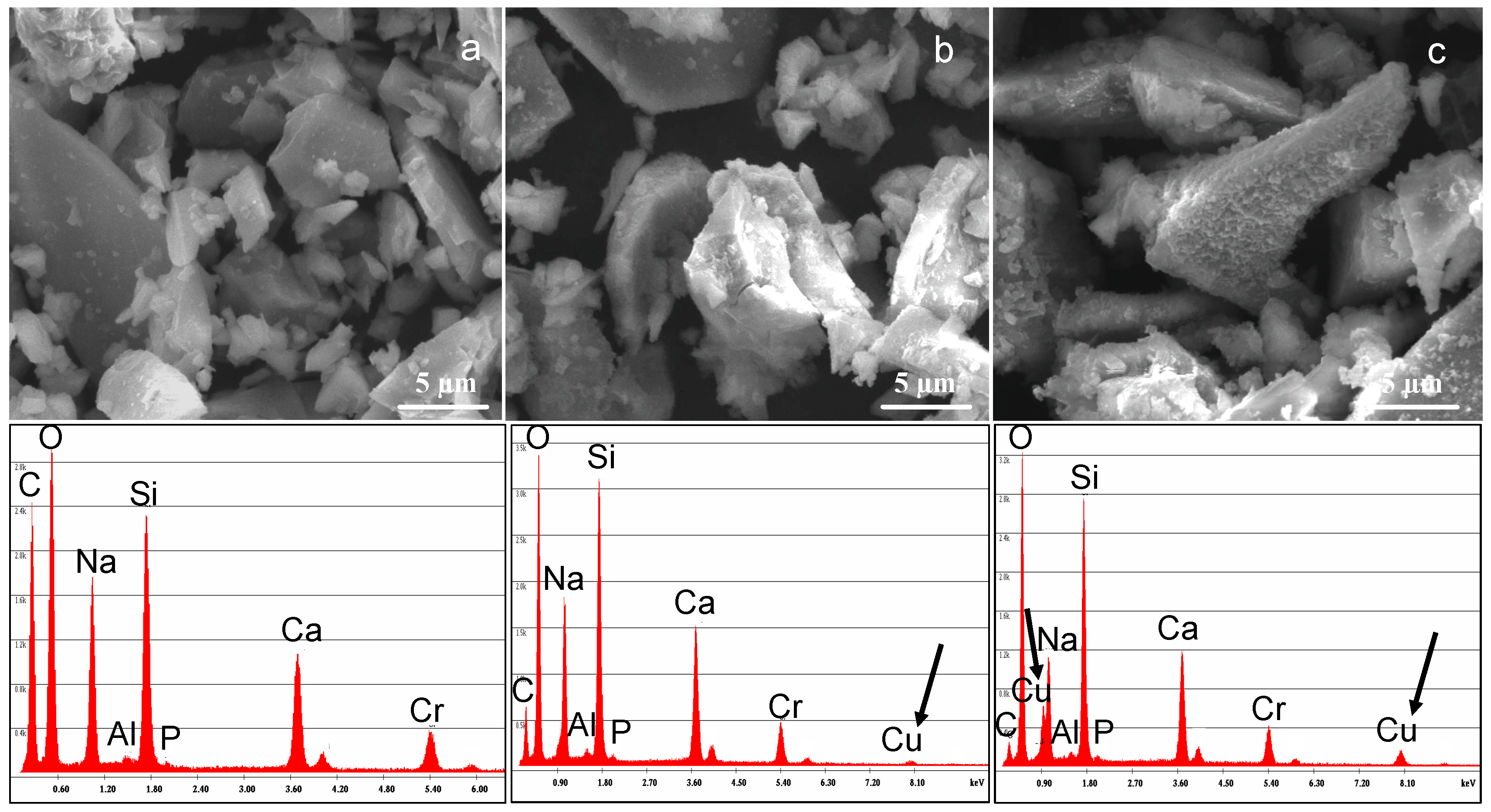

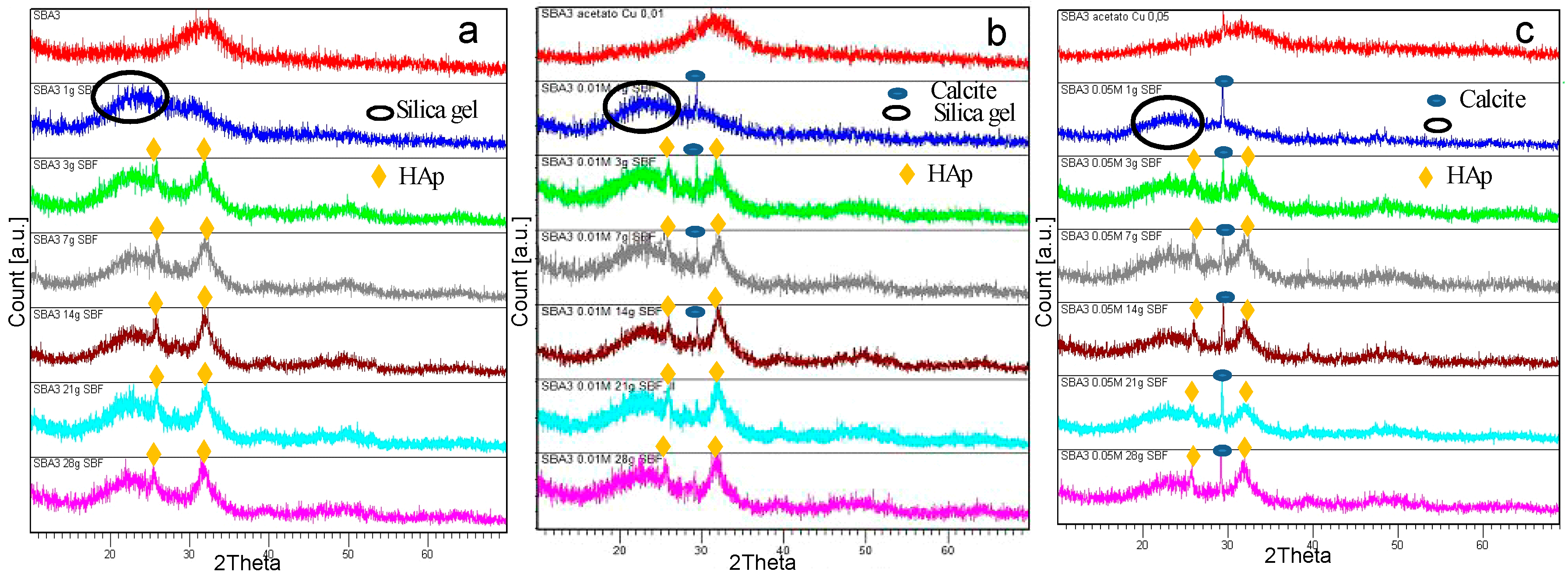

| Copper Salts | 0.01 M | 0.05 M | 0.1 M |
|---|---|---|---|
| Copper(II) nitrate Cu(NO3)2·3H2O | x | x | x |
| Copper(II) chloride CuCl2·2H2O | x | x | x |
| Copper(II) acetate Cu(CH3COO)2·H2O | x | x | |
| Copper(II) sulphate CuSO4 | x | x |
| Copper Solutions | at % Cu | Crystalline Phase |
|---|---|---|
| Copper(II) nitrate 0.1 M | 9.2 ± 0.3 | Yes |
| Copper(II) nitrate 0.05 M | 4.5 ± 0.2 | Yes |
| Copper(II) nitrate 0.01 M | 3.7 ± 0.3 | No |
| Copper(II) chloride 0.1 M | 26.5 ± 0.6 | Yes |
| Copper(II) chloride 0.05 M | 9.9 ± 0.5 | Yes |
| Copper(II) chloride 0.01 M | 3.3 ± 0.5 | No |
| Copper(II) acetate 0.05 M | 7.4 ± 0.2 | No |
| Copper(II) acetate 0.01 M | 3.6 ± 0.5 | No |
| Copper(II) sulphate 0.05 M | 6.9 ± 0.1 | No |
| Copper(II) sulphate 0.01 M | 2.9 ± 0.1 | No |
| EDS | SBA3 | SBA3-Cu0.01 | SBA3-Cu0.05 |
|---|---|---|---|
| Elements | at % | at % | at % |
| Na | 32.1 | 27.7 | 22.7 |
| Al | 1.6 | 1.1 | 2 |
| Si | 38.9 | 39.5 | 37.9 |
| P | 0.6 | 0.7 | 0.7 |
| Ca | 26.9 | 26.9 | 22.5 |
| Cu | 0 | 4.2 | 14.1 |
| XPS-Elements | SBA3 | SBA3-Cu0.01 | SBA3-Cu0.05 |
|---|---|---|---|
| Na | 56.9 | 38.2 | 29.4 |
| Si | 25.8 | 43.3 | 46.6 |
| Ca | 17.3 | 11.6 | 7.0 |
| Cu | 0.00 | 6.9 | 17.0 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miola, M.; Verné, E. Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions. Materials 2016, 9, 405. https://doi.org/10.3390/ma9060405
Miola M, Verné E. Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions. Materials. 2016; 9(6):405. https://doi.org/10.3390/ma9060405
Chicago/Turabian StyleMiola, Marta, and Enrica Verné. 2016. "Bioactive and Antibacterial Glass Powders Doped with Copper by Ion-Exchange in Aqueous Solutions" Materials 9, no. 6: 405. https://doi.org/10.3390/ma9060405







