Preparation and Electrocapacitive Properties of Hierarchical Porous Carbons Based on Loofah Sponge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Preparation of the Loofah Sponge-Based Porous Carbons (LSCs)
2.3. Characterization of the Porous Carbon
2.4. Electrochemical Performance
3. Results and Discussion
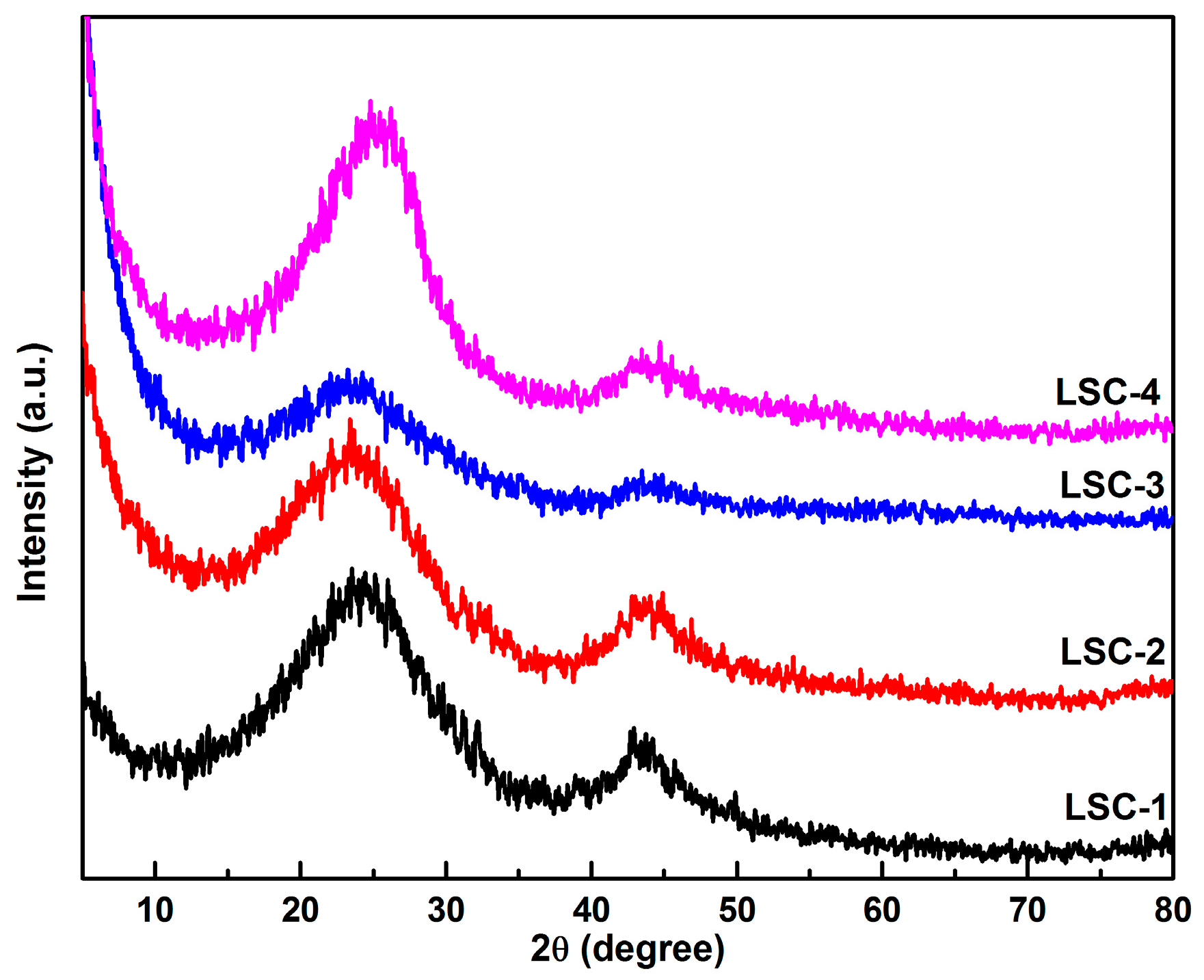
3.1. Morphology and Structure of Porous Carbons
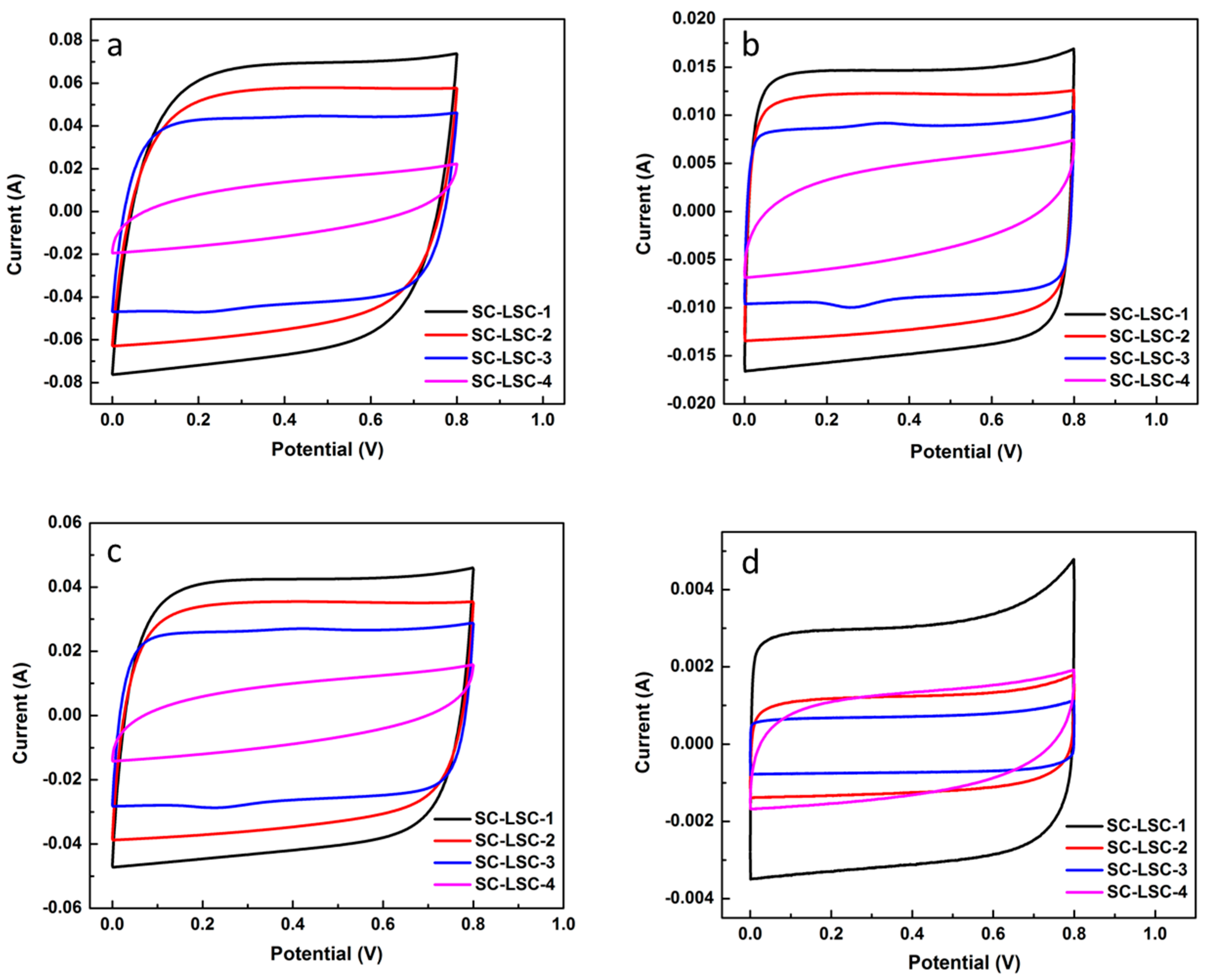
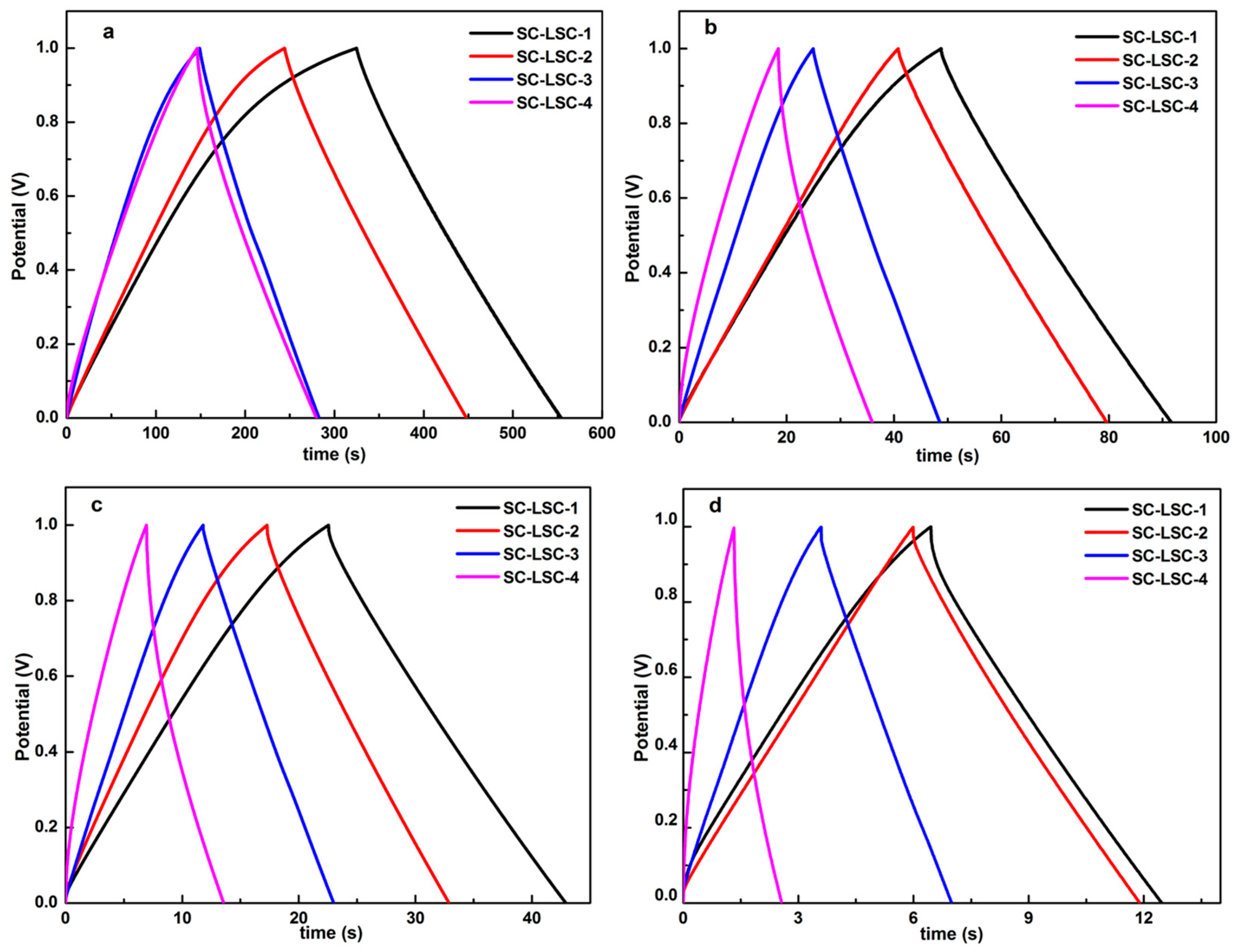
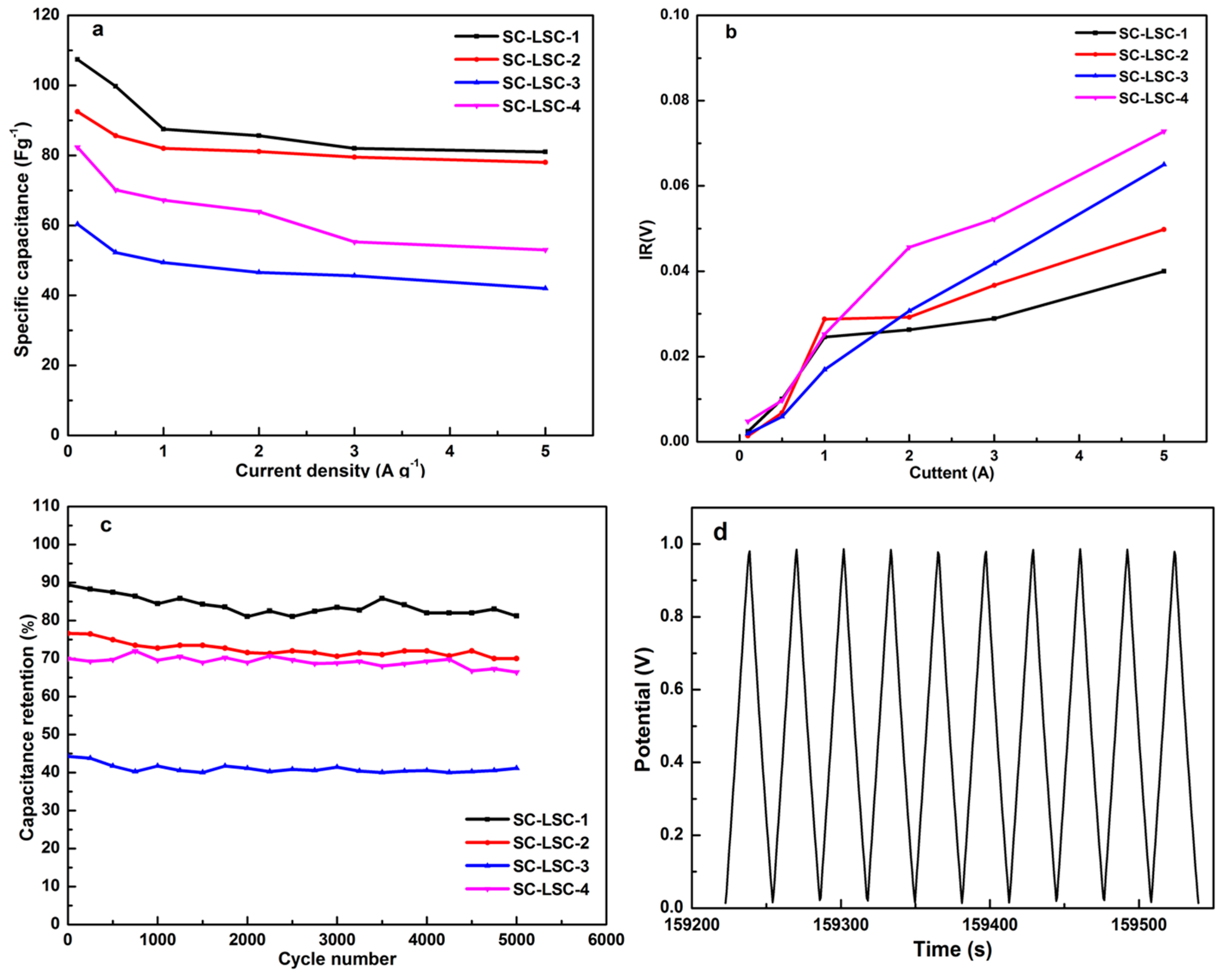
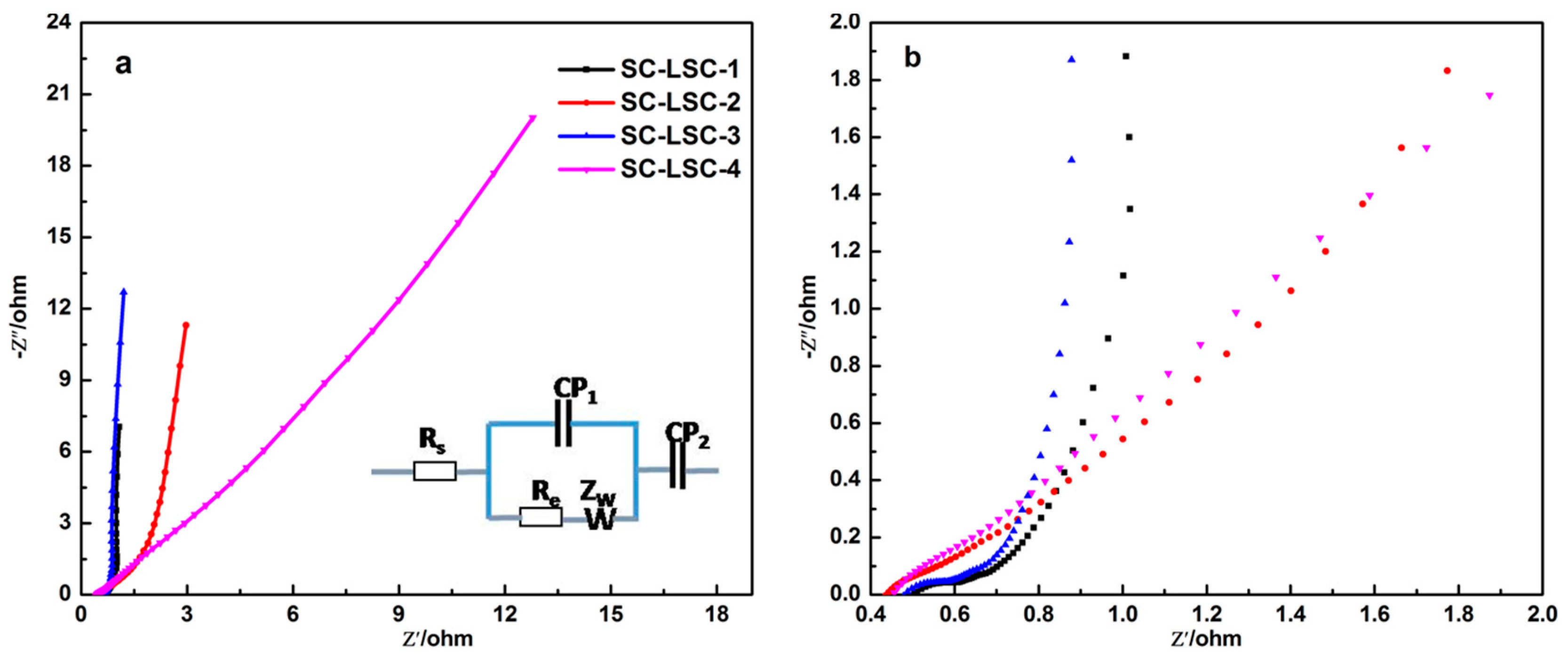
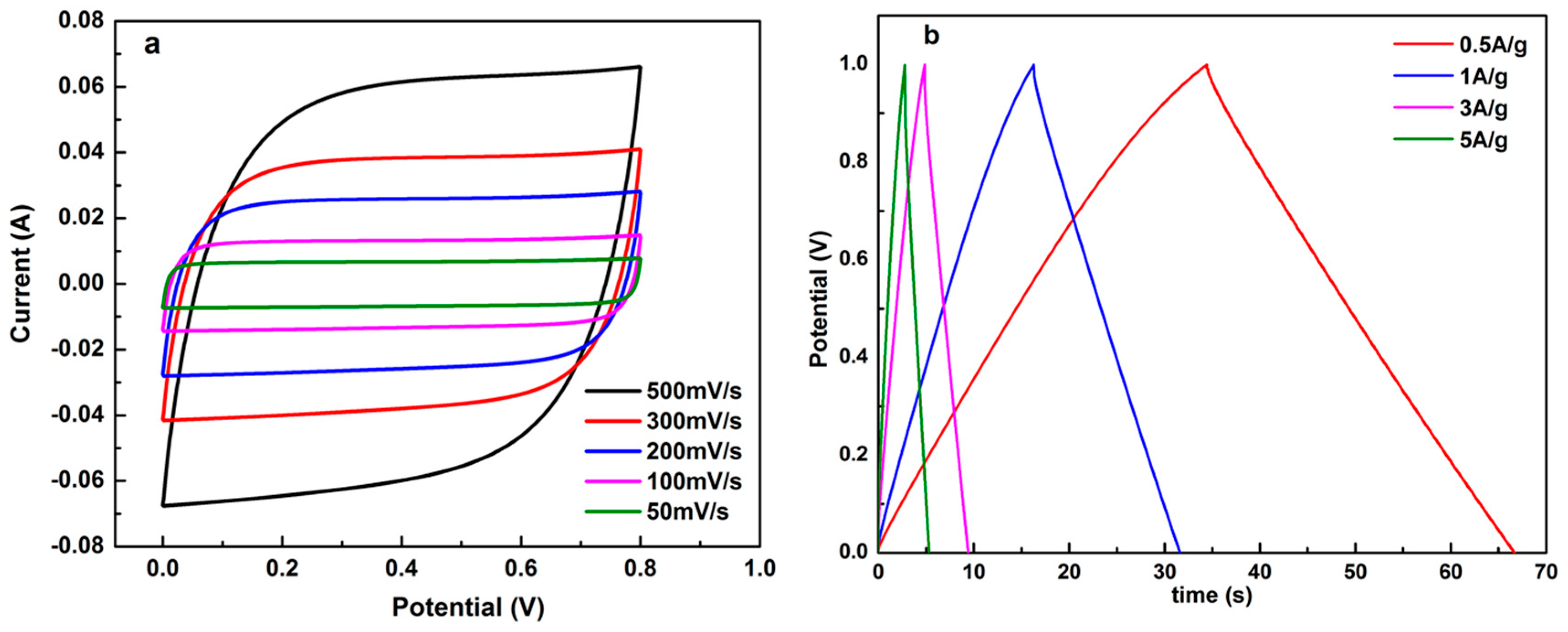
3.2. Electrochemical Characterization of LSC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Smalley, R.E. Future global energy prosperity: The terawatt challenge. Mrs Bull. 2005, 30, 412–417. [Google Scholar] [CrossRef]
- Armaroli, N.; Vincenzo, B. The future of energy supply: Challenges and opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef] [PubMed]
- Rolison, D.R.; Nazar, L.F. Electrochemical energy storage to power the 21st century. Mrs Bull. 2011, 36, 486–493. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, M.; Konno, H.; Tanaike, O. Carbon materials for electrochemical capacitors. J. Power Sources 2010, 195, 7880–7903. [Google Scholar] [CrossRef]
- Frackowiak, E.; Béguin, F. Carbon materials for the electrochemical storage of energy in capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Kim, C.; Ngoc, B.T.N.; Yang, K.S.; Kojima, M.; Kim, Y.A.; Kim, Y.J.; Endo, M.; Yang, S.C. Self-sustained thin webs consisting of porous carbon nanofibers for supercapacitors via the electrospinning of polyacrylonitrile solutions containing zinc chloride. Adv. Mater. 2007, 19, 2341–2346. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, H.H.; Jin, H.J. Pseudocapacitive effects of n-doped carbon nanotube electrodes in supercapacitors. Materials 2012, 5, 1258–1266. [Google Scholar] [CrossRef]
- Li, M.; Ding, J.; Xue, J. Mesoporous carbon decorated graphene as an efficient electrode material for supercapacitors. J. Mater. Chem. A 2013, 1, 7469–7476. [Google Scholar] [CrossRef]
- Zhao, Y.Q.; Lu, M.; Tao, P.Y.; Zhang, Y.J.; Gong, X.T.; Yang, Z.; Zhang, G.Q.; Li, H.L. Hierarchically porous and heteroatom doped carbon derived from tobacco rods for supercapacitors. J. Power Sources 2016, 307, 391–400. [Google Scholar] [CrossRef]
- Endo, M.; Kim, Y.J.; Takeda, T.; Hayashi, T.; Koshiba, K.; Hara, H.; Dresselhaus, M.S. Poly-vinylidene chloride (PVDC)-based carbon as an electrode material for high power capacitors with an aqueous electrolyte. J. Electrochem. Soc. 2001, 148, A1135–A1140. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Dou, Y.; Zhao, D.; Fulvio, P.F.; Mayes, R.T.; Dai, S. Cheminform abstract: Carbon materials for chemical capacitive energy storage. Adv. Mater. 2011, 23, 4828–4850. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Capacitive energy storage in nanostructured carbon-electrolyte systems. Acc. Chem. Res. 2013, 46, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Bhaumik, A.; Wu, K.C.W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dubey, M.; Kharel, P.; Gu, Z.; Qi, H.F. Biochar activated by oxygen plasma for supercapacitors. J. Power Sources 2015, 274, 1300–1305. [Google Scholar] [CrossRef]
- Wei, L.; Sevilla, M.; Fuertes, A.B.; Mokaya, R.; Yushin, G. Hydrothermal carbonization of abundant renewable natural organic chemicals for high-performance supercapacitor electrodes. Adv. Energy Mater. 2011, 1, 356–361. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, M.H.; Hong, S.J.; Lee, M.E.; Park, Y.W.; Jin, H.-J. Hierarchically porous carbon nanosheets from waste coffee grounds for supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 3684–3690. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Cho, S.Y.; Shim, J.; Kim, B.H.; Chang, S.J.; Baek, S.J.; Huh, Y.S.; Tak, Y.; Park, Y.W.; Park, S.; et al. Microporous carbon nanoplates from regenerated silk proteins for supercapacitors. Adv. Mater. 2013, 25, 1993–1998. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.L.; Wen, T.; Guo, H.L.; Yang, S.; Wang, X.; Xu, A.W. Biomass-derived sponge-like carbonaceous hydrogels and aerogels for supercapacitors. J. Mater. Chem. A 2013, 2, 3589–3597. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Gan, L.; Liu, M.; Xiong, W.; Xu, Z.; Zhu, D.; Wright, D.S. A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes. J. Power Sources 2012, 209, 152–157. [Google Scholar] [CrossRef]
- Cui, Y.; Cheng, L.; Wen, C.; Sang, Y.; Guo, P.; Zhao, X.S. Capacitive behavior of chestnut shell-based porous carbon electrode in ionic liquid electrolytes. Colloids Surf. A 2016, 508, 173–177. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.G.; Li, M.T.; Meng, X.Y.; Wang, L.; Wang, R.H.; Yin, J.; Fu, H.G. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Guo, P.Z.; Ji, Q.Q.; Zhang, L.L.; Zhao, S.Y.; Zhao, X.S. Preparation and characterization of peanut shell-based microporous carbons as electrode materials for supercapacitors. Acta Phys. Chim. Sin. 2011, 27, 2836–2840. [Google Scholar]
- Balathanigaimani, M.S.; Shim, W.G.; Lee, M.J.; Chan, K.; Lee, J.W.; Moon, H. Highly porous electrodes from novel corn grains-based activated carbons for electrical double layer capacitors. Electrochem. Commun. 2008, 10, 868–871. [Google Scholar] [CrossRef]
- Kuratani, K.; Okuno, K.; Iwaki, T.; Kato, M.; Takeichi, N.; Miyuki, T.; Awazu, T.; Majima, M.; Sakai, T. Converting rice husk activated carbon into active material for capacitor using three-dimensional porous current collector. J. Power Sources 2011, 196, 10788–10790. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Cadek, M.; Wachtler, M.; Béguin, F. Carbon nanotubes as nanotexturing agents for high power supercapacitors based on seaweed carbons. ChemSusChem 2011, 4, 943–949. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Yang, F.; Yang, X. Promising carbons for supercapacitors derived from fungi. Adv. Mater. 2011, 23, 2745–2748. [Google Scholar] [CrossRef] [PubMed]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Zhu, Z.; Lu, G.Q. Nanoporous carbon electrode from waste coffee beans for high performance supercapacitors. Electrochem. Commun. 2008, 10, 1594–1597. [Google Scholar] [CrossRef]
- Sha, Y.; Lou, J.; Bai, S.; Wu, D.; Liu, B.; Ling, Y. Facile preparation of nitrogen-doped porous carbon from waste tobacco by a simple pre-treatment process and their application in electrochemical capacitor and CO2 capture. Mater. Res. Bull. 2015, 64, 327–332. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Huang, Y.; Wang, W. A fish scale based hierarchical lamellar porous carbon material obtained using a natural template for high performance electrochemical capacitors. J. Mater. Chem. 2010, 20, 4773–4775. [Google Scholar] [CrossRef]
- Luan, Y.T.; Wang, L.; Guo, S.N.; Jiang, B.J.; Zhao, D.D.; Yan, H.J.; Tian, C.G.; Fu, H.G. A hierarchical porous carbon material from a loofah sponge network for high performance supercapacitors. RSC Adv. 2015, 5, 42430–42437. [Google Scholar] [CrossRef]
- Li, J.P.; Ren, Z.G.; Ren, Y.Q.; Zhao, L.; Wang, S.G.; Yu, J. Activated carbon with micrometer-scale channels prepared from luffa sponge fibers and their application for supercapacitors. RSC Adv. 2014, 4, 35789–35796. [Google Scholar] [CrossRef]
- Chen, L.; Yan, C.J.; Luo, W.J.; Li, X.J.; Ge, W.; Zhou, S. Simple preparation and enhanced adsorption properties of loofah fiber adsorbent by ultraviolet radiation graft. Mater. Lett. 2015, 157, 303–306. [Google Scholar]
- Roble, N.; Ogbonna, J.; Tanaka, H. A novel circulating loop bioreactor with cells immobilized in loofa (Luffa cylindrica) sponge for the bioconversion of raw cassava starch to ethanol. Appl. Microbiol. Biot. 2003, 60, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Boynard, C.A.; D’Almeida, J.R.M. Morphological characterization and mechanical behavior of sponge gourd (Luffa cylindrica)-polyester composite materials. Polym. Plast. Technol. 2010, 39, 489–499. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Cheng, L.; Li, H.; Wang, B.; Zhao, X.S.; Guo, P. Electrochemical properties of manganese ferrite-based supercapacitors in aqueous electrolyte: The effect of ionic radius. Colloids Surf. A 2014, 457, 94–99. [Google Scholar] [CrossRef]
- He, X.J.; Lei, J.W.; Geng, Y.J.; Zhang, X.Y.; Wu, M.B.; Zheng, M.D. Preparation of microporous activated carbon and its electrochemical performance for electricdouble layer capacitor. J. Phys. Chem. Solids 2009, 70, 738–744. [Google Scholar] [CrossRef]
- Sun, K.; Feng, E.; Peng, H.; Ma, G.; Wu, Y.; Wang, H.; Lei, Z. A simple and high-performance supercapacitor based on nitrogen-doped porous carbon in redox-mediated sodium molybdate electrolyte. Electrochim. Acta 2015, 158, 361–367. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Zhu, Y.Q.; Chen, X.Y.; Cao, Y. Pronounced improvement of supercapacitor capacitance by using redox active electrolyte of p-phenylenediamine. Electrochim. Acta 2015, 176, 941–948. [Google Scholar] [CrossRef]
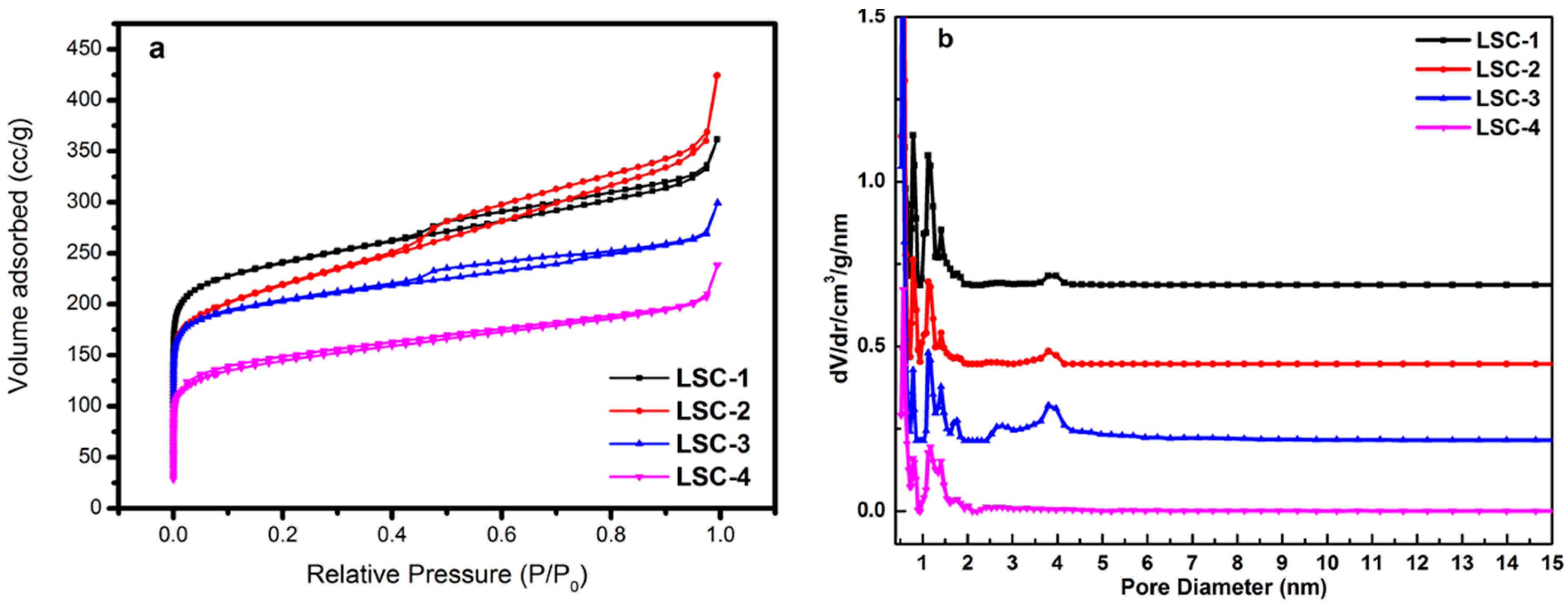
| Sample | Preparation Method | SBET (m2·g−1) | Micropore Area (m2·g−1) | MicroporeVolume (cm−3·g−1) | Vpore (cm−3·g−1) | Pore Width (nm) |
|---|---|---|---|---|---|---|
| LSC-1 | ZnCl2 activation | 1007 | 753 | 0.273 | 0.438 | 0.545 |
| LSC-2 | DMF soaking | 799 | 459 | 0.191 | 0.444 | 0.548 |
| LSC-3 | DMF solvothermal treatment | 773 | 608 | 0.243 | 0.328 | 0.573 |
| LSC-4 | Melamine hydrothermal treatment | 538 | 382 | 0.156 | 0.258 | 0.578 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhai, K.; Wang, G.; Li, Q.; Guo, P. Preparation and Electrocapacitive Properties of Hierarchical Porous Carbons Based on Loofah Sponge. Materials 2016, 9, 912. https://doi.org/10.3390/ma9110912
Li Z, Zhai K, Wang G, Li Q, Guo P. Preparation and Electrocapacitive Properties of Hierarchical Porous Carbons Based on Loofah Sponge. Materials. 2016; 9(11):912. https://doi.org/10.3390/ma9110912
Chicago/Turabian StyleLi, Zichao, Kuilu Zhai, Guoqiang Wang, Qun Li, and Peizhi Guo. 2016. "Preparation and Electrocapacitive Properties of Hierarchical Porous Carbons Based on Loofah Sponge" Materials 9, no. 11: 912. https://doi.org/10.3390/ma9110912







