Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion
Abstract
:1. Introduction
2. Rigid Particles
2.1. Surface Modification by Non-Covalent Adsorption
2.1.1. Small Molecules
2.1.2. Polymers
2.2. Surface Modification by Chemical Grafting
2.2.1. Small Molecules
2.2.2. Polymers
3. Soft Particles
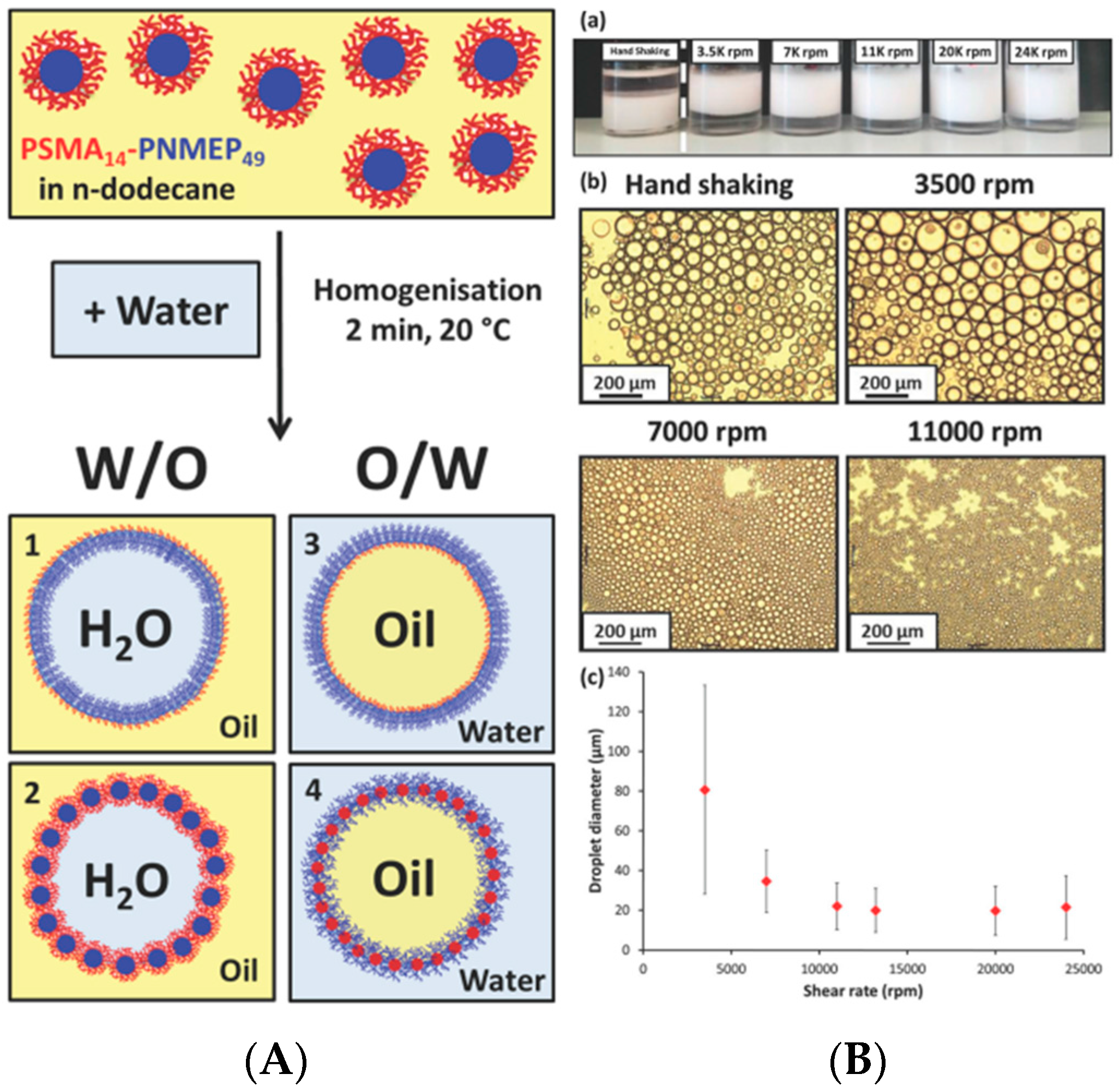
3.1. Self-Assembled Objects
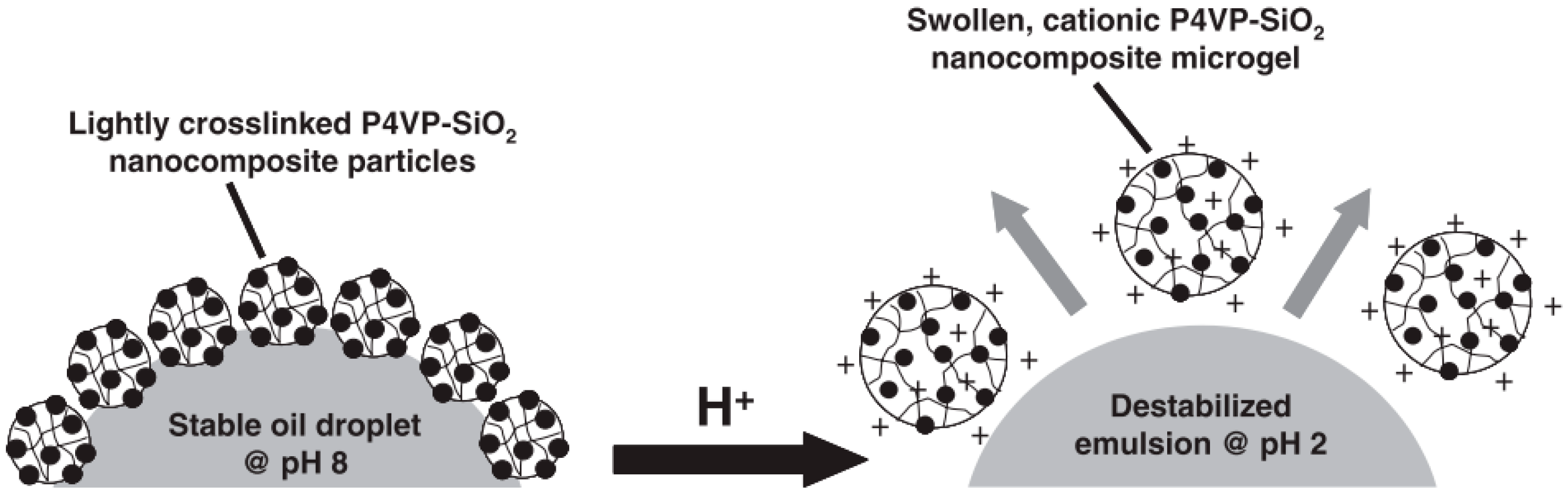
3.2. Microgels
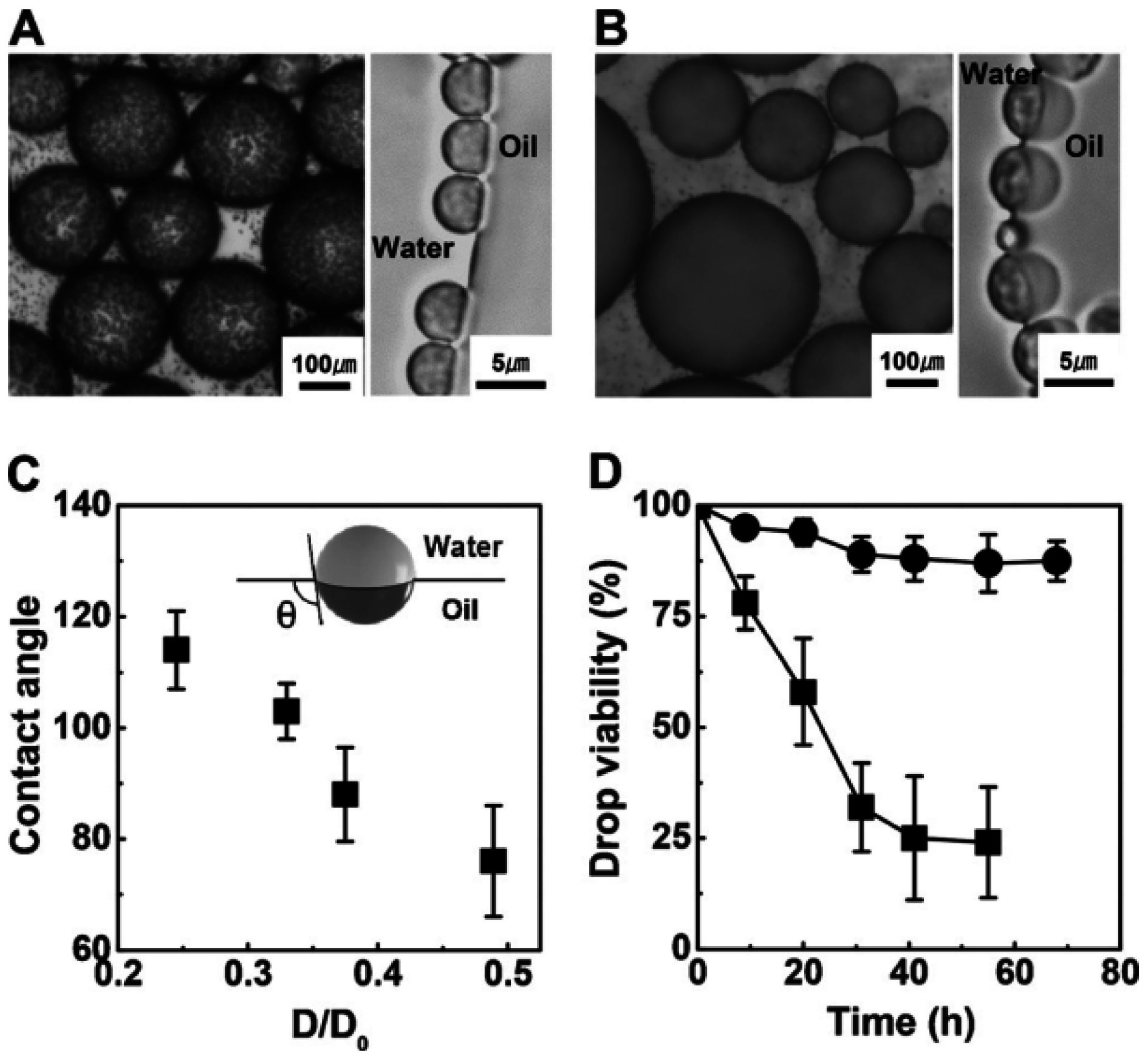
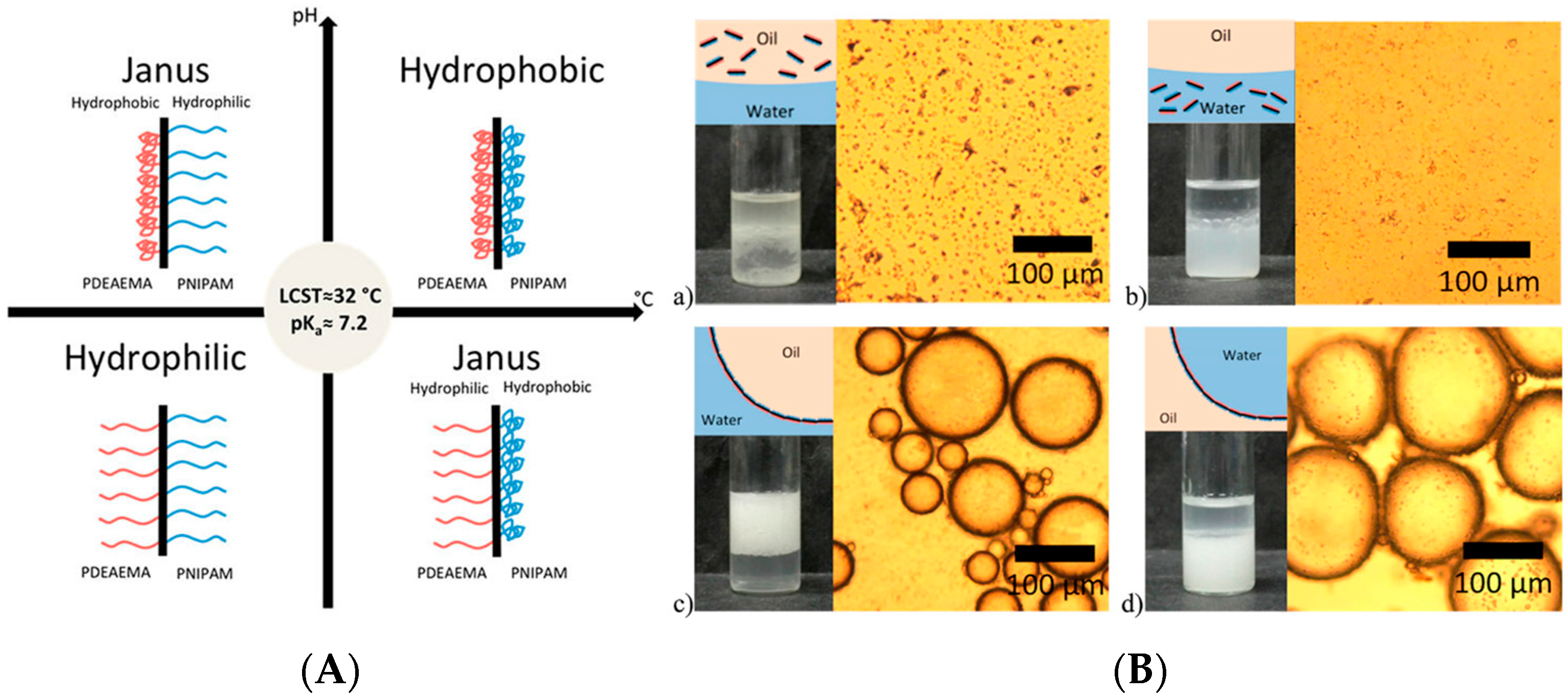
4. Janus Particles
4.1. Surface Chemistry
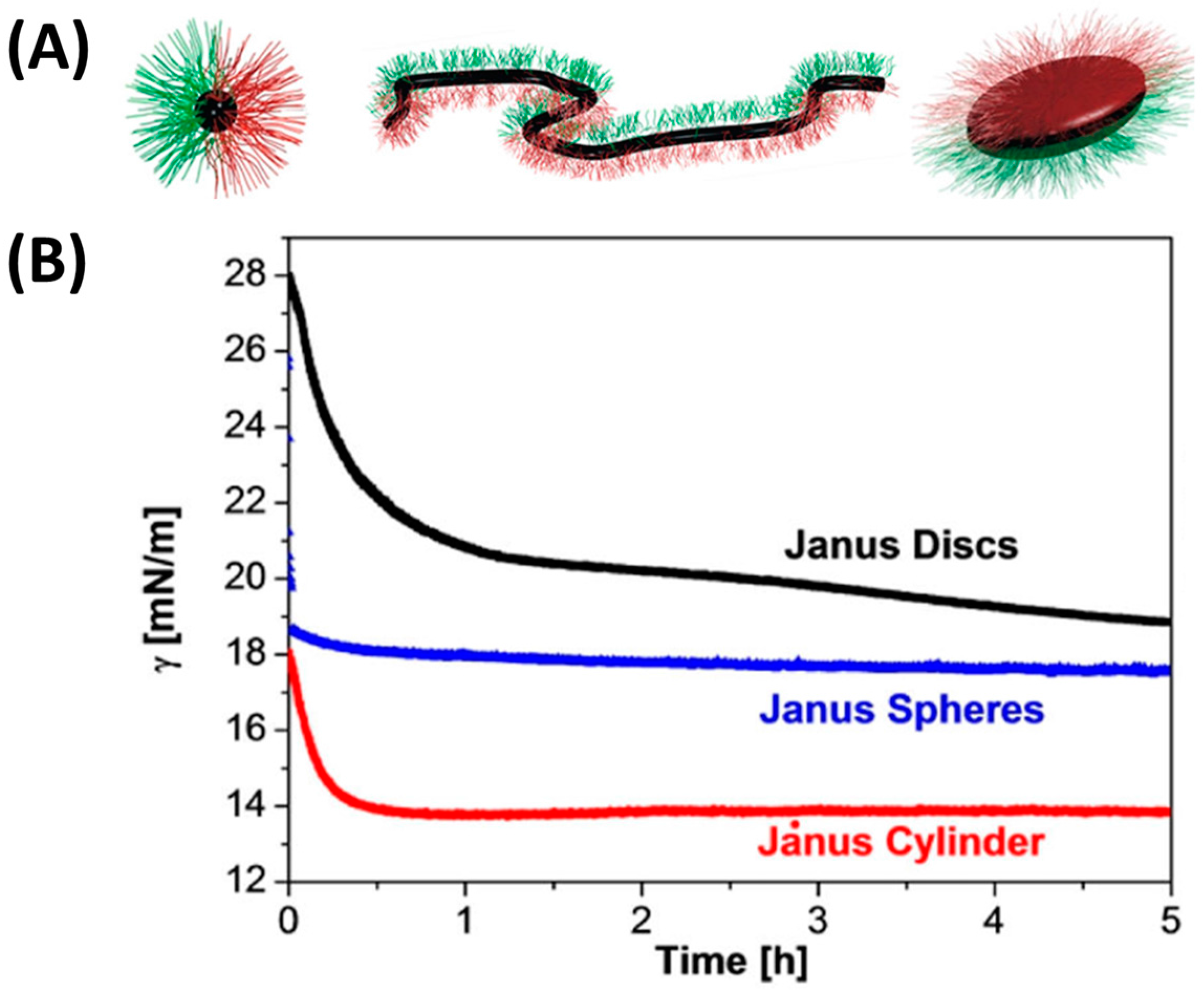
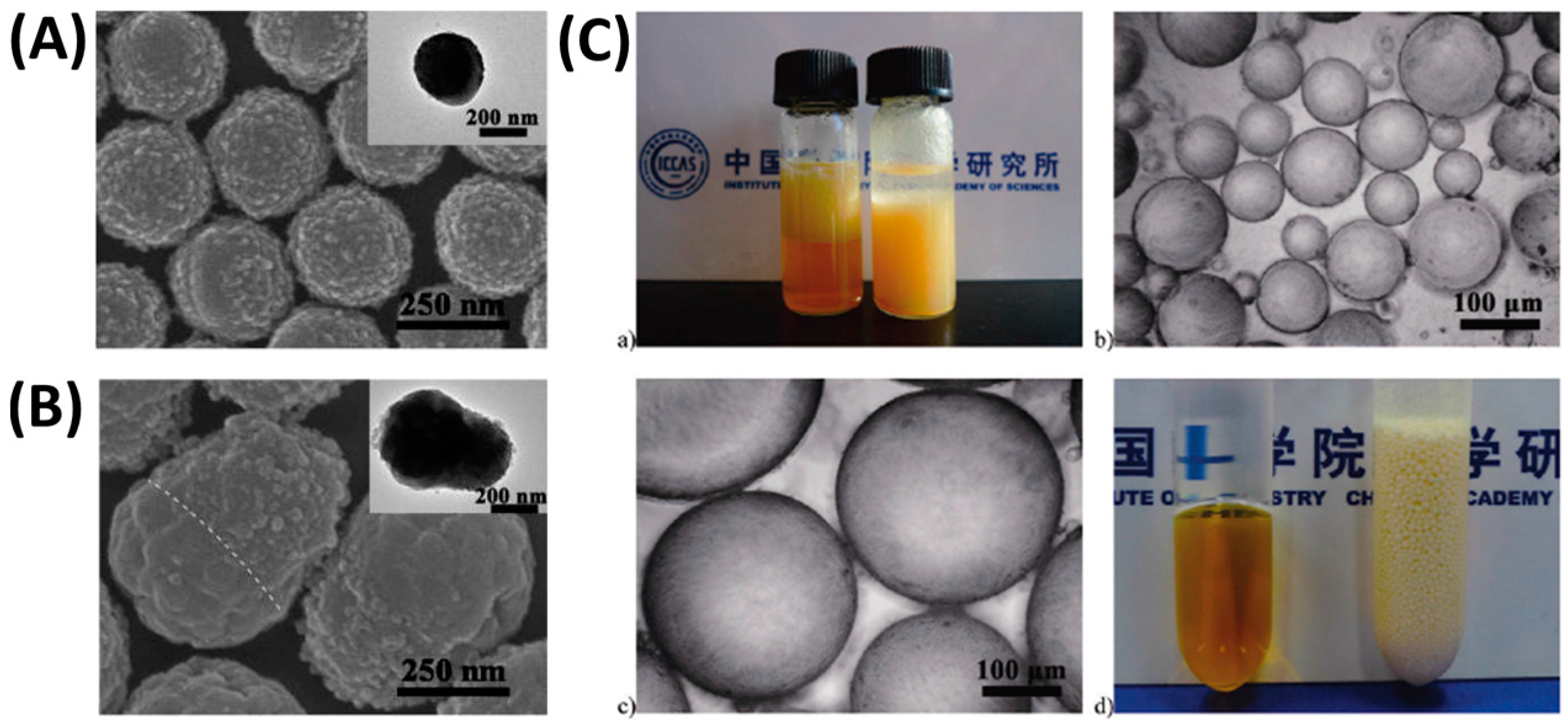
4.2. Shape
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, W.; Zhang, M.; Chu, L. Functional polymeric microparticles engineered from controllable microfluidic emulsions. Acc. Chem. Res. 2014, 47, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, Q.; Zhang, X.; Du, W.; Xu, H.; Wang, Y. Assembly of carbon nanotubes on polymer particles: Towards rapid shape change by near-infrared light. Part. Part. Syst. Charact. 2013, 30, 235–240. [Google Scholar] [CrossRef]
- Qian, Q.; Huang, X.; Zhang, X.; Xie, Z.; Wang, Y. One-step preparation of macroporous polymer particles with multiple interconnected chambers: A candidate for trapping biomacromolecules. Angew. Chem. Int. Ed. 2013, 52, 10625–10629. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, J.; Li, Y.; Yang, M.; Chang, Y.; Zhang, J.; Sun, Z.; Wang, Y. Enhanced light absorption in porous particles for ultra-NIR-sensitive biomaterials. ACS Macro Lett. 2015, 4, 392–397. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Z.; Liao, S.; Wang, J.; Wang, Y. A light-activated microheater for the remote control of enzymatic catalysis. Chem. Eur. J. 2016, 22, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; He, Y.; Wang, D.; Dong, L.; Du, W.; Wang, Y. Dynamic interfacial printing for monodisperse droplets and polymeric microparticles. Adv. Mater. Technol. 2016, 1, 1600021. [Google Scholar] [CrossRef]
- Kumar, A.; Li, S.; Cheng, C.-M.; Lee, D. Recent developments in phase inversion emulsification. Ind. Eng. Chem. Res. 2015, 54, 8375–8396. [Google Scholar] [CrossRef]
- Brown, P.; Butts, C.P.; Eastoe, J. Stimuli-responsive surfactants. Soft Matter 2013, 9, 2365–2374. [Google Scholar] [CrossRef]
- Liu, Y.; Jessop, P.G.; Cunningham, M.; Eckert, C.A.; Liotta, C.L. Switchable surfactants. Science 2006, 313, 958–960. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, M.; Rousseau, D. A one-step process for oil-in-water-in-oil double emulsion formation using a single surfactant. J. Colloid Interface Sci. 2012, 386, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qian, Q.; Wang, Y. Anisotropic particles from a one-pot double emulsion induced by partial wetting and their triggered release. Small 2014, 10, 1412–1420. [Google Scholar] [CrossRef] [PubMed]
- Macon, A.L.B.; Rehman, S.U.; Bell, R.V.; Weaver, J.V.M. Reversible assembly of pH responsive branched copolymer-stabilised emulsion via electrostatic forces. Chem. Commun. 2016, 52, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Ku, K.H.; Shin, J.M.; Klinger, D.; Jang, S.G.; Hayward, R.C.; Hawker, C.J.; Kim, B.J. Particles with tunable porosity and morphology by controlling interfacial instability in block copolymer emulsions. ACS Nano 2016, 10, 5243–5251. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.A.; Chang, C.B.; Graves, S.M.; Li, Z.B.; Mason, T.G.; Deming, T.J. Nanoscale double emulsions stabilized by single-component block copolypeptides. Nature 2008, 455, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.Z.; Sun, G.Q.; Cai, J.G.; Ngai, T. One-step formation of w/o/w multiple emulsions stabilized by single amphiphilic block copolymers. Langmuir 2012, 28, 2332–2336. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xiao, L.; Zhang, X.; Zhang, K.; Wang, Y. Emulsion templating cyclic polymers as microscopic particles with tunable porous morphology. Langmuir 2016, 32, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Velikov, K.P. Zein as a source of functional colloidal nano- and microstructures. Curr. Opin. Colloid Interface Sci. 2014, 19, 450–458. [Google Scholar] [CrossRef]
- Filippidi, E.; Patel, A.R.; Bouwens, E.C.M.; Voudouris, P.; Velikov, K.P. All-natural oil-filled microcapsules from water-insoluble proteins. Adv. Funct. Mater. 2014, 24, 5962–5968. [Google Scholar] [CrossRef]
- McClements, D.J. Protein-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 305–313. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface 2004, 108–109, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S. Protein stabilization of emulsions and foams. J. Food Sci. 2005, 70, R54–R66. [Google Scholar] [CrossRef]
- Dickinson, E. Flocculation of protein-stabilized oil-in-water emulsions. Colloids Surf. B Biointerfaces 2010, 81, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Boker, A.; He, J.; Emrick, T.; Russell, T.P. Self-assembly of nanoparticles at interfaces. Soft Matter 2007, 3, 1231–1248. [Google Scholar] [CrossRef]
- Kumar, A.; Park, B.J.; Tu, F.Q.; Lee, D. Amphiphilic Janus particles at fluid interfaces. Soft Matter 2013, 9, 6604–6617. [Google Scholar] [CrossRef]
- Tavernier, I.; Wijaya, W.; Van der Meeren, P.; Dewettinck, K.; Patel, A.R. Food-grade particles for emulsion stabilization. Trends Food Sci. Technol. 2016, 50, 159–174. [Google Scholar] [CrossRef]
- Lam, S.; Velikov, K.P.; Velev, O.D. Pickering stabilization of foams and emulsions with particles of biological origin. Curr. Opin. Colloid Interface Sci. 2014, 19, 490–500. [Google Scholar] [CrossRef]
- Chevalier, Y.; Bolzinger, M.A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surf. A 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Wang, X.F.; Shi, Y.; Graff, R.W.; Lee, D.; Gao, H.F. Developing recyclable pH-responsive magnetic nanoparticles for oil-water separation. Polymer 2015, 72, 361–367. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, Y.; Chen, S.; Jup, J.; Li, Y.; Wang, T.; Wang, Q. Stimuli-responsive composite particles as solid-stabilizers for effective oil harvesting. ACS Appl. Mater. Interfaces 2014, 6, 13334–13338. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Hoffmann, M.; Mueller, A.H.E. Emulsion polymerization using Janus particles as stabilizers. Angew. Chem. Int. Ed. 2008, 47, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Voorn, D.J.; Ming, W.; van Herk, A.M. Polymer-clay nanocomposite latex particles by inverse Pickering emulsion polymerization stabilized with hydrophobic montmorillonite platelets. Macromolecules 2006, 39, 2137–2143. [Google Scholar] [CrossRef]
- Tuncer, C.; Samav, Y.; Ulker, D.; Baker, S.B.; Butun, V. Multi-responsive microgel of a water-soluble monomer via emulsion polymerization. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Haaj, S.B.; Thielemans, W.; Magnin, A.; Boufi, S. Starch nanocrystal stabilized Pickering emulsion polymerization for nanocomposites with improved performance. ACS Appl. Mater. Interfaces 2014, 6, 8263–8273. [Google Scholar] [CrossRef] [PubMed]
- Pera-Titus, M.; Leclercq, L.; Clacens, J.-M.; De Campo, F.; Nardello-Rataj, V. Pickering interfacial catalysis for biphasic systems: From emulsion design to green reactions. Angew. Chem. Int. Ed. 2015, 54, 2006–2021. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, C.; Ju, E.; Ji, H.; Ren, J.; Binks, B.P.; Qu, X. Design of surface-active artificial enzyme particles to stabilize Pickering emulsions for high-performance biphasic biocatalysis. Adv. Mater. 2016, 28, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Cheng, F.; Binks, B.P.; Yang, H. pH-responsive gas-water-solid interface for multiphase catalysis. J. Am. Chem. Soc. 2015, 137, 15015–15025. [Google Scholar] [CrossRef] [PubMed]
- Whitby, C.P.; Wanless, E.J. Controlling Pickering emulsion destabilisation: A route to fabricating new materials by phase inversion. Materials 2016, 9, 626. [Google Scholar] [CrossRef]
- Tcholakova, S.; Denkov, N.D.; Lips, A. Comparison of solid particles, globular proteins and surfactants as emulsifiers. Phys. Chem. Chem. Phys. 2008, 10, 1608–1627. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fang, R.; Wang, D.; Wang, J.; Xu, H.; Wang, Y.; Zhang, X. Tuning polymeric amphiphilicity via Se-N interactions: Towards one-step double emulsion for highly selective enzyme mimics. Small 2015, 11, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, Y.; Shi, J.; Ngo, H.T.; Shen, C.; Du, W.; Wang, Y. High-internal-phase emulsion tailoring polymer amphiphilicity towards an efficient NIR-sensitive bacteria filter. Small 2015, 11, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.; Wang, D.; Ma, N.; Li, C.; Wang, Y. Surfactant-free emulsions with erasable triggered phase inversions. Langmuir 2016, 32, 11039–11042. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Huang, X.; Wang, Y. Managing the phase separation in double emulsion by tuning amphiphilicity via a supramolecular route. Langmuir 2014, 30, 14460–14468. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as surfactants - similarities and differences. Curr. Opin. Colloid Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Leal-Calderon, F.; Schmitt, V. Solid-stabilized emulsions. Curr. Opin. Colloid Interface Sci. 2008, 13, 217–227. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J.; Liang, F.; Wang, Q.; Zhang, C.; Qu, X.; Li, J.; Qiu, D.; Yang, Z. Robust anisotropic composite particles with tunable Janus balance. Macromolecules 2012, 45, 5176–5184. [Google Scholar] [CrossRef]
- Jiang, S.; Granick, S. Controlling the geometry (Janus balance) of amphiphilic colloidal particles. Langmuir 2008, 24, 2438–2445. [Google Scholar] [CrossRef] [PubMed]
- Daware, S.V.; Basavaraj, M.G. Emulsions stabilized by silica rods via arrested demixing. Langmuir 2015, 31, 6649–6654. [Google Scholar] [CrossRef] [PubMed]
- Schoth, A.; Landfester, K.; Munoz-Espi, R. Surfactant-free polyurethane nanocapsules via inverse Pickering miniemulsion. Langmuir 2015, 31, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Ye, L.; Kong, M.; Yang, Q.; Li, G.; Huang, Y. Pickering emulsions stabilized by shape-controlled silica microrods. RSC Adv. 2016, 6, 24195–24202. [Google Scholar] [CrossRef]
- De Folter, J.W.J.; Hutter, E.M.; Castillo, S.I.R.; Klop, K.E.; Philipse, A.P.; Kegel, W.K. Particle shape anisotropy in Pickering emulsions: Cubes and peanuts. Langmuir 2014, 30, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Ahn, W.J.; Jung, H.S.; Choi, H.J. Pickering emulsion polymerized smart magnetic poly(methyl methacrylate)/Fe2O3 composite particles and their stimulus-response. RSC Adv. 2015, 5, 23094–23100. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Wang, J.; Xu, J.; Sun, D. Phase inversion of emulsions containing a lipophilic surfactant induced by clay concentration. Langmuir 2013, 29, 3889–3894. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Wu, H.; Wang, H.; Du, Q. Interconnectivity of macroporous hydrogels prepared via graphene oxide-stabilized Pickering high internal phase emulsions. Langmuir 2016, 32, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Creighton, M.A.; Zhu, W.; van Krieken, F.; Petteruti, R.A.; Gao, H.; Hurt, R.H. Three-dimensional graphene-based microbarriers for controlling release and reactivity in colloidal liquid phases. ACS Nano 2016, 10, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Creighton, M.A.; Ohata, Y.; Miyawaki, J.; Bose, A.; Hurt, R.H. Two-dimensional materials as emulsion stabilizers: Interfacial thermodynamics and molecular barrier properties. Langmuir 2014, 30, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- Avendano, C.; Brun, N.; Fontaine, O.; In, M.; Mehdi, A.; Stocco, A.; Vioux, A. Multiwalled carbon nanotube/cellulose composite: From aqueous dispersions to Pickering emulsions. Langmuir 2016, 32, 3907–3916. [Google Scholar] [CrossRef] [PubMed]
- Tasset, S.; Cathala, B.; Bizot, H.; Capron, I. Versatile cellular foams derived from CNC-stabilized Pickering emulsions. RSC Adv. 2014, 4, 893–898. [Google Scholar] [CrossRef]
- Godfrin, M.P.; Tiwari, A.; Bose, A.; Tripathi, A. Phase and steady shear behavior of dilute carbon black suspensions and carbon black stabilized emulsions. Langmuir 2014, 30, 15400–15407. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Marway, H.S.; Kasem, H.; Pelton, R.; Cranston, E.D. Dried and redispersible cellulose nanocrystal Pickering emulsions. ACS Macro Lett. 2016, 5, 185–189. [Google Scholar] [CrossRef]
- Cherhal, F.; Cousin, F.; Capron, I. Structural description of the interface of Pickering emulsions stabilized by cellulose nanocrystals. Biomacromolecules 2016, 17, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, N.; Kawamura, A.; Kohri, M.; Nakamura, Y.; Fujii, S. Polydopamine particle as a particulate emulsifier. Polymers 2016, 8, 62. [Google Scholar] [CrossRef]
- Qi, F.; Wu, J.; Sun, G.Q.; Nan, F.F.; Ngai, T.; Ma, G.H. Systematic studies of Pickering emulsions stabilized by uniform-sized PLGA particles: Preparation and stabilization mechanism. J. Mater. Chem. B 2014, 2, 7605–7611. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, Z.; Wang, C.; Tong, Z. Lignin-based Pickering HIPEs for macroporous foams and their enhanced adsorption of copper(II) ions. Chem. Commun. 2013, 49, 7144–7146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Z.; Bian, W.; Feng, L.; Wu, Z.; Wang, P.; Zeng, X.; Wu, T. Stabilizing oil-in-water emulsions with regenerated chitin nanofibers. Food Chem. 2015, 183, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Tzoumaki, M.V.; Moschakis, T.; Kiosseoglou, V.; Biliaderis, C.G. Oil-in-water emulsions stabilized by chitin nanocrystal particles. Food Hydrocoll. 2011, 25, 1521–1529. [Google Scholar] [CrossRef]
- Binks, B.P.; Isa, L.; Tyowua, A.T. Direct measurement of contact angles of silica particles in relation to double inversion of Pickering emulsions. Langmuir 2013, 29, 4923–4927. [Google Scholar] [CrossRef] [PubMed]
- Worthen, A.J.; Foster, L.M.; Dong, J.; Bollinger, J.A.; Peterman, A.H.; Pastora, L.E.; Bryant, S.L.; Truskett, T.M.; Bielawski, C.W.; Johnston, K.P. Synergistic formation and stabilization of oil-in-water emulsions by a weakly interacting mixture of zwitterionic surfactant and silica nanoparticles. Langmuir 2014, 30, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, L.; Liu, G.; Tan, J.; Liu, S.; Sun, D. Oil-in-water emulsions stabilized by Laponite particles modified with short-chain aliphatic amines. Colloids Surf. A 2012, 400, 44–51. [Google Scholar] [CrossRef]
- Li, W.; Zhao, C.; Tan, J.; Jiang, J.; Xu, J.; Sun, D. Roles of methyl orange in preparation of emulsions stabilized by layered double hydroxide particles. Colloids Surf. A 2013, 421, 173–180. [Google Scholar] [CrossRef]
- Santini, E.; Guzman, E.; Ferrari, M.; Liggieri, L. Emulsions stabilized by the interaction of silica nanoparticles and palmitic acid at the water-hexane interface. Colloids Surf. A 2014, 460, 333–341. [Google Scholar] [CrossRef]
- Sadeghpour, A.; Pirolt, F.; Glatter, O. Submicrometer-sized Pickering emulsions stabilized by silica nanoparticles with adsorbed oleic acid. Langmuir 2013, 29, 6004–6012. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, A.; Rodriguez-Abreu, C.; Menner, A.; Bismarck, A.; Esquena, J. Antagonistic effects between magnetite nanoparticles and a hydrophobic surfactant in highly concentrated Pickering emulsions. Langmuir 2014, 30, 5064–5074. [Google Scholar] [CrossRef] [PubMed]
- Sturzenegger, P.N.; Gonzenbach, U.T.; Koltzenburg, S.; Gauckler, L.J. Controlling the formation of particle-stabilized water-in-oil emulsions. Soft Matter 2012, 8, 7471–7479. [Google Scholar] [CrossRef]
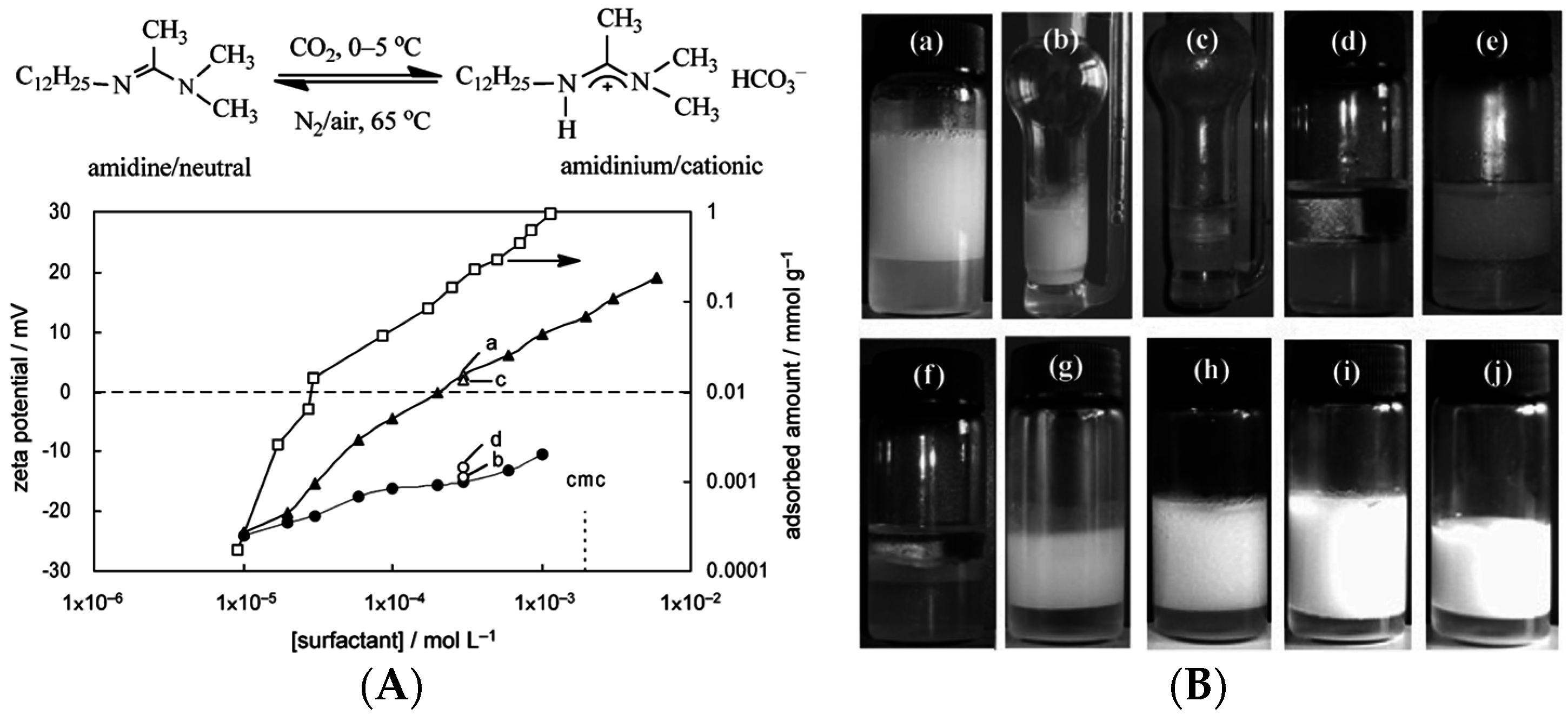
- Jiang, J.; Zhu, Y.; Cui, Z.; Binks, B.P. Switchable Pickering emulsions stabilized by silica nanoparticles hydrophobized in situ with a switchable surfactant. Angew. Chem. Int. Ed. 2013, 52, 12373–12376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jiang, J.; Liu, K.; Cui, Z.; Binks, B.P. Switchable Pickering emulsions stabilized by silica nanoparticles hydrophobized in situ with a conventional cationic surfactant. Langmuir 2015, 31, 3301–3307. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Yang, L.; Cui, Y.; Binks, B.P. Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant. Langmuir 2010, 26, 4717–4724. [Google Scholar] [CrossRef] [PubMed]
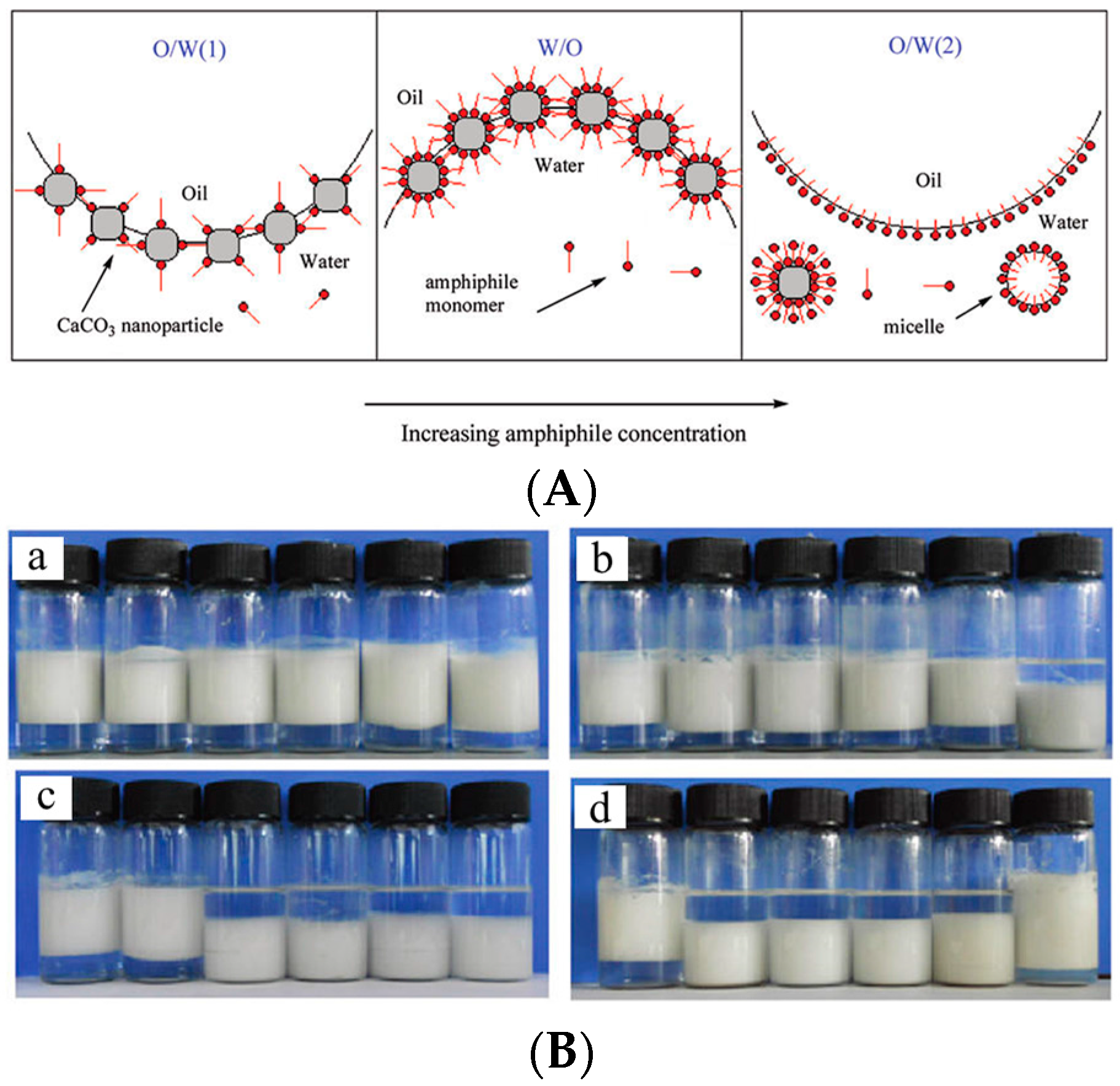
- Cui, Z.; Cui, C.; Zhu, Y.; Binks, B.P. Multiple phase inversion of emulsions stabilized by in situ surface activation of CaCO3 nanoparticles via adsorption of fatty acids. Langmuir 2012, 28, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Schrade, A.; Landfester, K.; Ziener, U. Pickering-type stabilized nanoparticles by heterophase polymerization. Chem. Soc. Rev. 2013, 42, 6823–6839. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Armes, S.P.; Verstraete, P.; Smets, J. Double emulsions and colloidosomes-in-colloidosomes using silica-based Pickering emulsifiers. Langmuir 2014, 30, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Patten, T.; Pelton, R.; Cranston, E.D. Synergistic stabilization of emulsions and emulsion gels with water-soluble polymers and cellulose nanocrystals. ACS Sustain. Chem. Eng. 2015, 3, 1023–1031. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Wang, L.; Li, C.; Xu, J.; Sun, D. Synergistic stabilization of emulsions by poly(oxypropylene)diamine and Laponite particles. Colloids Surf. A 2010, 353, 117–124. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S. Temperature-induced inversion of nanoparticle-stabilized emulsions. Angew. Chem. Int. Ed. 2005, 44, 4795–4798. [Google Scholar] [CrossRef] [PubMed]
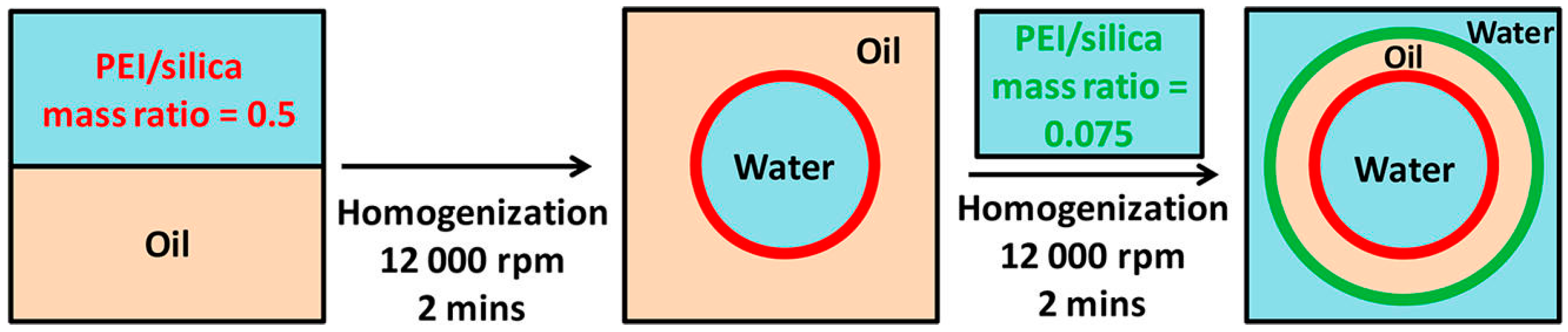
- Yoon, K.Y.; Li, Z.; Neilson, B.M.; Lee, W.; Huh, C.; Bryant, S.L.; Bielawski, C.W.; Johnston, K.P. Effect of adsorbed amphiphilic copolymers on the interfacial activity of superparamagnetic nanoclusters and the emulsification of oil in water. Macromolecules 2012, 45, 5157–5166. [Google Scholar] [CrossRef]
- Sun, G.; Li, Z.; Ngai, T. Inversion of particle-stabilized emulsions to form high-internal-phase emulsions. Angew. Chem. Int. Ed. 2010, 49, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, T.; Zhang, W. A strategy for separating and recycling solid catalysts based on the pH-triggered Pickering-emulsion inversion. Angew. Chem. Int. Ed. 2013, 52, 7455–7459. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, H. A pH-switched Pickering emulsion catalytic system: High reaction efficiency and facile catalyst recycling. Chem. Commun. 2015, 51, 7333–7336. [Google Scholar] [CrossRef] [PubMed]
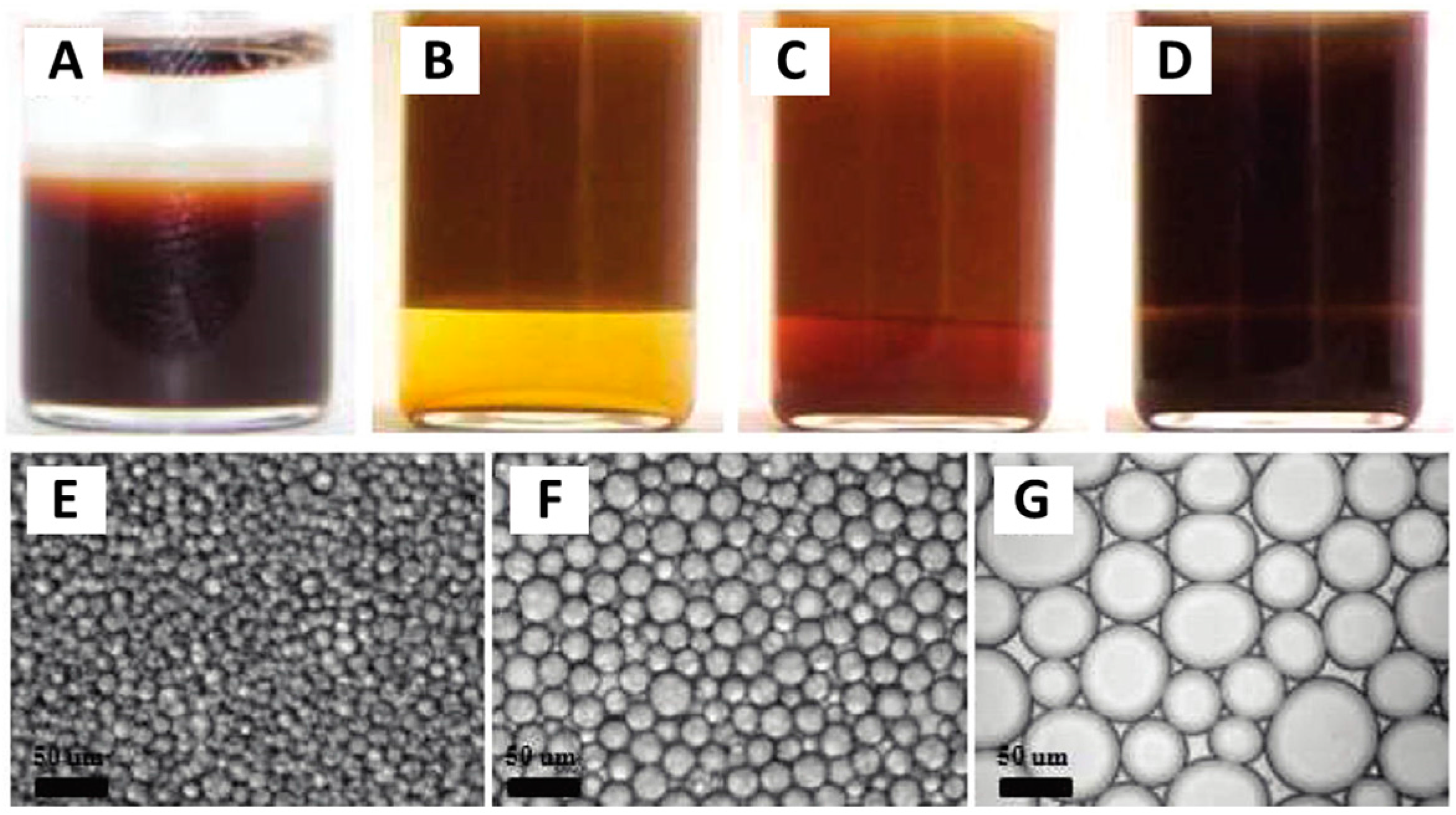
- Zhao, C.; Tan, J.; Li, W.; Tong, K.; Xu, J.; Sun, D. Ca2+ ion responsive Pickering emulsions stabilized by PSSMA nanoaggregates. Langmuir 2013, 29, 14421–14428. [Google Scholar] [CrossRef] [PubMed]
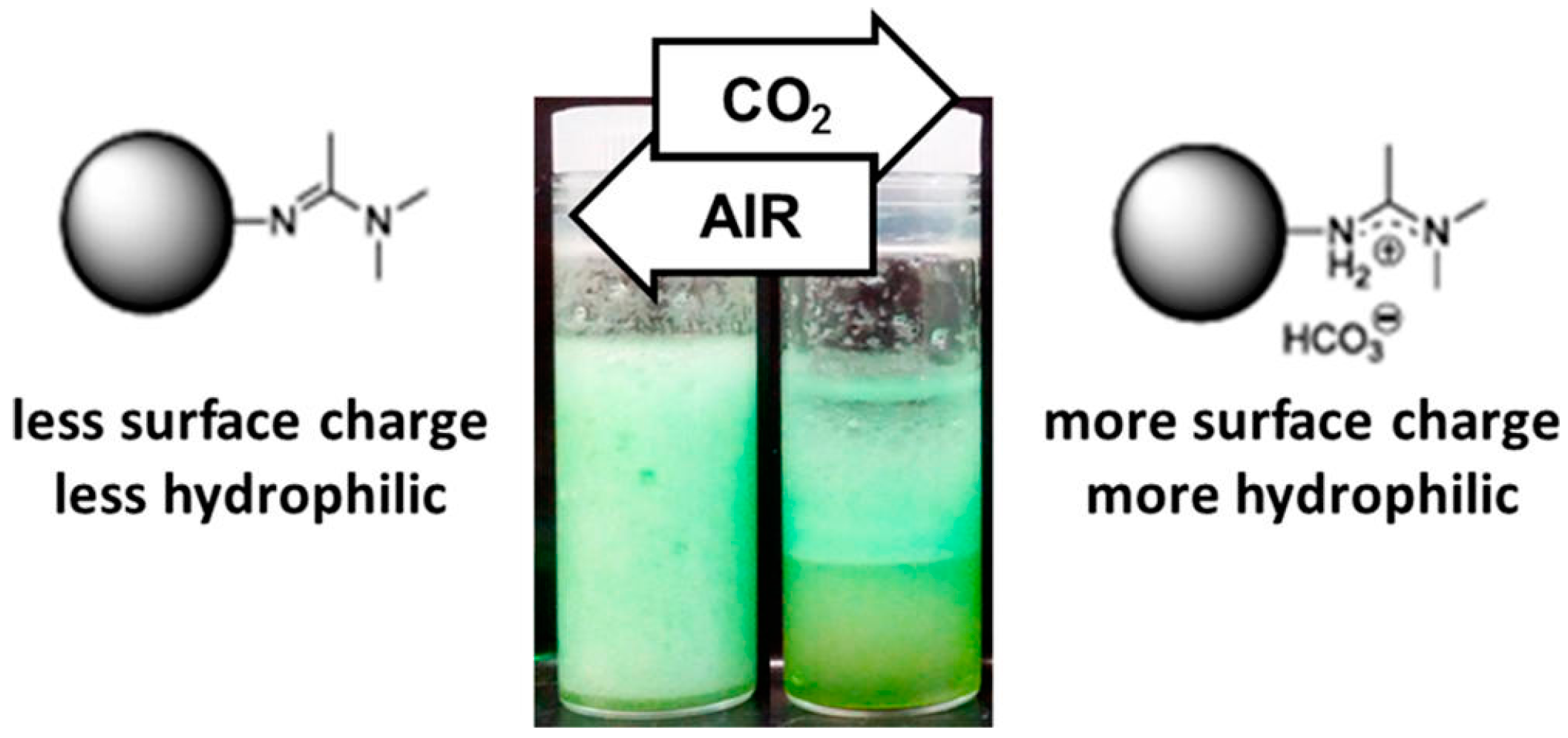
- Lin, S.; Theato, P. CO2-responsive polymers. Macromol. Rapid Commun. 2013, 34, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Liu, Q.; Xu, Z. Surfactant-free switchable emulsions using CO2-responsive particles. ACS Appl. Mater. Interfaces 2014, 6, 6898–6904. [Google Scholar] [CrossRef] [PubMed]
- Fameau, A.L.; Lam, S.; Velev, O.D. Multi-stimuli responsive foams combining particles and self-assembling fatty acids. Chem. Sci. 2013, 4, 3874–3881. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Bing, W.; Zhang, Z.; Li, Z.; Ren, J.; Qu, X. Light controlled reversible inversion of nanophosphor-stabilized Pickering emulsions for biphasic enantioselective biocatalysis. J. Am. Chem. Soc. 2014, 136, 7498–7504. [Google Scholar] [CrossRef] [PubMed]
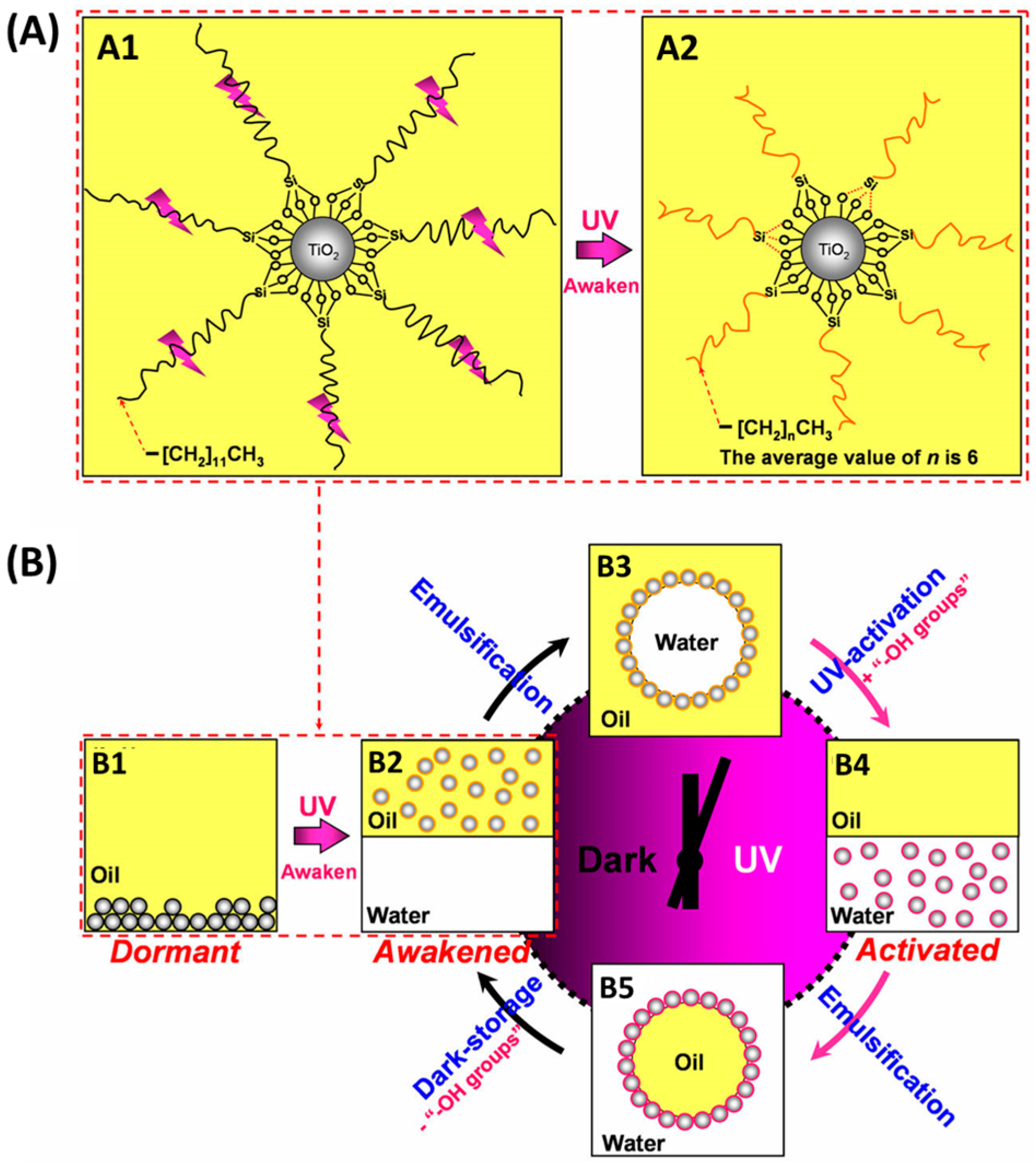
- Zhang, Q.; Bai, R.-X.; Guo, T.; Meng, T. Switchable Pickering emulsions stabilized by awakened TiO2 nanoparticle emulsifiers using UV/dark actuation. ACS Appl. Mater. Interfaces 2015, 7, 18240–18246. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhang, Q.; Qiu, X.; Zhu, S. CO2-responsive diethylaminoethyl-modified lignin nanoparticles and their application as surfactants for CO2/N2-switchable Pickering emulsions. Green Chem. 2014, 16, 4963–4968. [Google Scholar] [CrossRef]
- Tang, J.; Lee, M.F.X.; Zhang, W.; Zhao, B.; Berry, R.M.; Tam, K.C. Dual responsive Pickering emulsion stabilized by poly 2-(dimethylamino)ethyl methacrylate grafted cellulose nanocrystals. Biomacromolecules 2014, 15, 3052–3060. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Berry, R.M.; Tam, K.C. Stimuli-responsive cellulose nanocrystals for surfactant-free oil harvesting. Biomacromolecules 2016, 17, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.L.; Mable, C.J.; Lane, J.A.; Derry, M.J.; Fielding, L.A.; Armes, S.P. Preparation of Pickering double emulsions using block copolymer worms. Langmuir 2015, 31, 4137–4144. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Sun, J.; Zhao, D.; Hu, Q.; Liu, X.; Jiang, M. Influence of photo-cross-linking on emulsifying performance of the self-assemblies of poly(7-(4-vinylbenzyloxyl)-4-methylcoumarin-co-acrylic acid). Langmuir 2014, 30, 6669–6677. [Google Scholar] [CrossRef] [PubMed]
- Mable, C.J.; Warren, N.J.; Thompson, K.L.; Mykhaylyk, O.O.; Armes, S.P. Framboidal ABC triblock copolymer vesicles: A new class of efficient Pickering emulsifier. Chem. Sci. 2015, 6, 6179–6188. [Google Scholar] [CrossRef]
- Wei, W.; Wang, T.; Yi, C.; Liu, J.; Liu, X. Self-assembled micelles based on branched poly(styrene-alt-maleic anhydride) as particulate emulsifiers. RSC Adv. 2015, 5, 1564–1570. [Google Scholar] [CrossRef]
- Yi, C.; Liu, N.; Zheng, J.; Jiang, J.; Liu, X. Dual-responsive poly(styrene-alt-maleic acid)-graft-poly(n-isopropyl acrylamide) micelles as switchable emulsifiers. J. Colloid Interface Sci. 2012, 380, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Geng, Z.; Sun, M.; Liu, X.; Chen, S. Pickering emulsion stabilized by self-assembled micelles of amphiphilic random copolymer p(St-co-DM). J. Dispers. Sci. Technol. 2014, 35, 757–764. [Google Scholar] [CrossRef]
- Fujii, S.; Cai, Y.L.; Weaver, J.V.M.; Armes, S.P. Syntheses of shell cross-linked micelles using acidic abc triblock copolymers and their application as pH-responsive particulate emulsifiers. J. Am. Chem. Soc. 2005, 127, 7304–7305. [Google Scholar] [CrossRef] [PubMed]
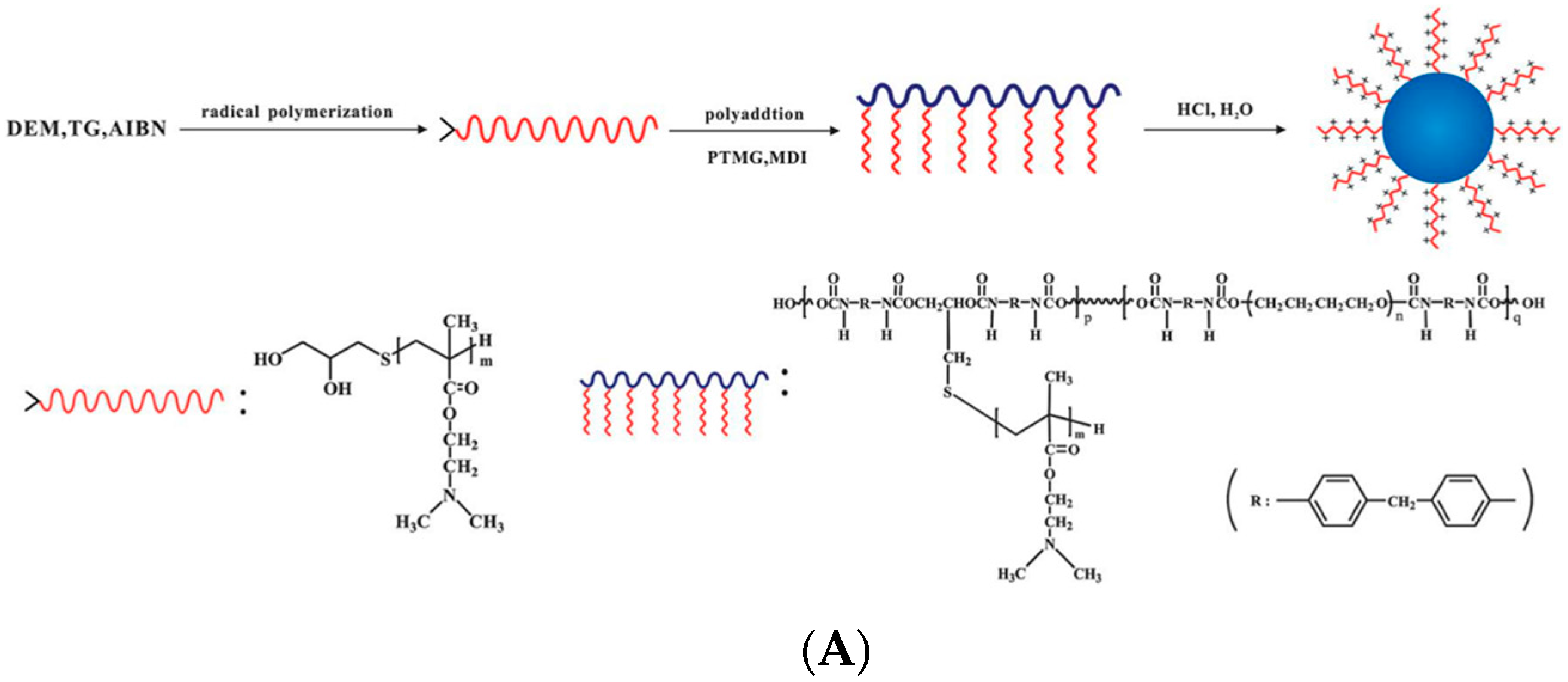
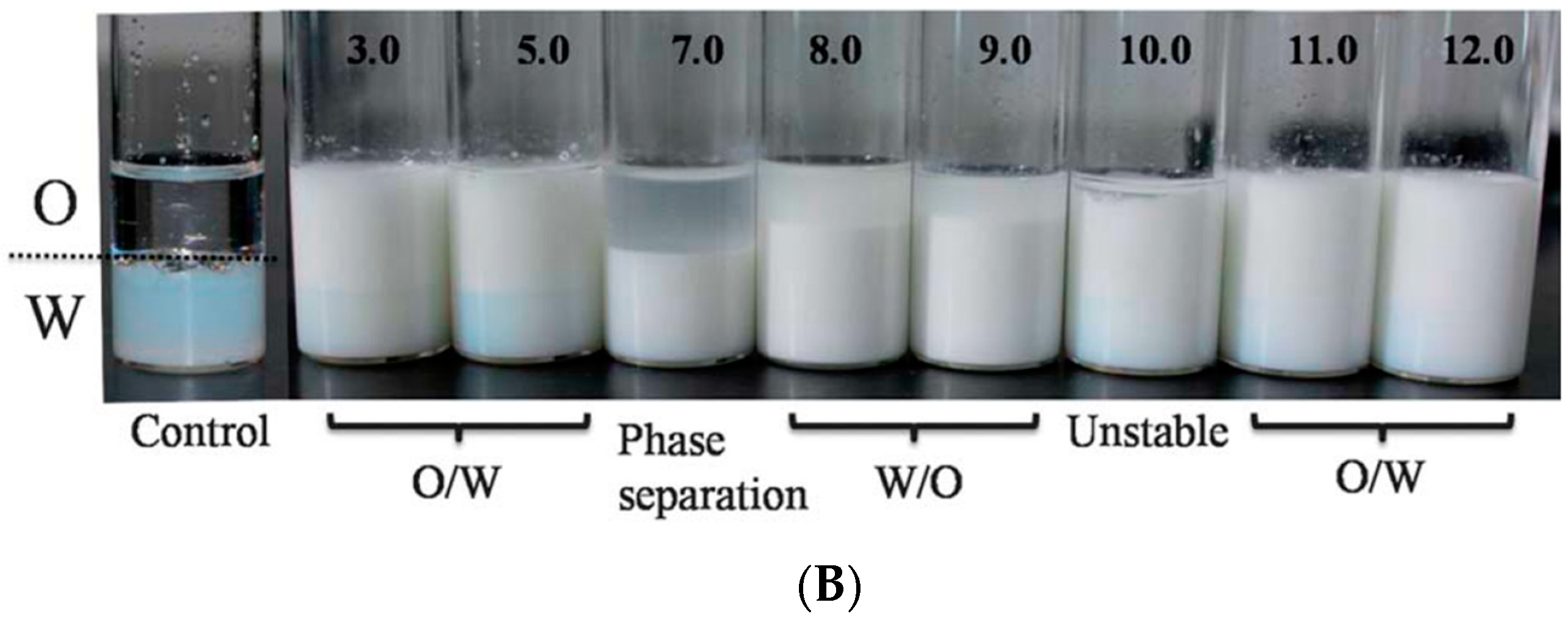
- Ma, C.; Bi, X.; Ngai, T.; Zhang, G. Polyurethane-based nanoparticles as stabilizers for oil-in-water or water-in-oil Pickering emulsions. J. Mater. Chem. A 2013, 1, 5353–5360. [Google Scholar] [CrossRef]
- Cunningham, V.J.; Armes, S.P.; Musa, O.M. Synthesis, characterisation and Pickering emulsifier performance of poly(stearyl methacrylate)-poly(n-2-(methacryloyloxy)ethyl pyrrolidone) diblock copolymer nano-objects via RAFT dispersion polymerisation in n-dodecane. Polym. Chem. 2016, 7, 1882–1891. [Google Scholar] [CrossRef]
- Richtering, W. Responsive emulsions stabilized by stimuli-sensitive microgels: Emulsions with special non-pickering properties. Langmuir 2012, 28, 17218–17229. [Google Scholar] [CrossRef] [PubMed]
- Destribats, M.; Eyharts, M.; Lapeyre, V.; Sellier, E.; Varga, I.; Ravaine, V.; Schmitt, V. Impact of PNIPAM microgel size on its ability to stabilize Pickering emulsions. Langmuir 2014, 30, 1768–1777. [Google Scholar] [CrossRef] [PubMed]
- Morse, A.J.; Madsen, J.; Growney, D.J.; Armes, S.P.; Mills, P.; Swart, R. Microgel colloidosomes based on pH-responsive poly(tert-butylaminoethyl methacrylate) latexes. Langmuir 2014, 30, 12509–12519. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Milani, A.H.; Carney, L.; Yan, J.; Cui, Z.; Thaiboonrod, S.; Saunders, B.R. Doubly crosslinked microgel-colloidosomes: A versatile method for pH-responsive capsule assembly using microgels as macro-crosslinkers. Chem. Commun. 2015, 51, 3854–3857. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ming, T.; Wang, J.; Ngai, T. High internal phase emulsions stabilized solely by microgel particles. Angew. Chem. Int. Ed. 2009, 48, 8490–8493. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wei, X.; Ngai, T. Controlled production of polymer microspheres from microgel-stabilized high internal phase emulsions. Chem. Commun. 2011, 47, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ngai, T. Stimuli-responsive gel emulsions stabilized by microgel particles. Colloid Polym. Sci. 2011, 289, 489–496. [Google Scholar] [CrossRef]
- Fujii, S.; Read, E.S.; Binks, B.P.; Armes, S.P. Stimulus-responsive emulsifiers based on nanocomposite microgel particles. Adv. Mater. 2005, 17, 1014–1018. [Google Scholar] [CrossRef]
- Binks, B.P.; Murakami, R.; Armes, S.P.; Fujii, S. Effects of pH and salt concentration on oil-in-water emulsions stabilized solely by nanocomposite microgel particles. Langmuir 2006, 22, 2050–2057. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.; Behrens, S.H.; Auweter, H. Novel emulsions stabilized by pH and temperature sensitive microgels. Chem. Commun. 2005, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Brugger, B.; Richtering, W. Emulsions stabilized by stimuli-sensitive poly(N-isopropylacrylamide)-co-methacrylic acid polymers: Microgels versus low molecular weight polymers. Langmuir 2008, 24, 7769–7777. [Google Scholar] [CrossRef] [PubMed]
- Brugger, B.; Rosen, B.A.; Richtering, W. Microgels as stimuli-responsive stabilizers for emulsions. Langmuir 2008, 24, 12202–12208. [Google Scholar] [CrossRef] [PubMed]
- de Folter, J.W.J.; van Ruijven, M.W.M.; Velikov, K.P. Oil-in-water Pickering emulsions stabilized by colloidal particles from the water-insoluble protein zein. Soft Matter 2012, 8, 6807–6815. [Google Scholar] [CrossRef]
- Fujii, S.; Aichi, A.; Muraoka, M.; Kishimoto, N.; Iwahori, K.; Nakamura, Y.; Yamashita, I. Ferritin as a bionano-particulate emulsifier. J. Colloid Interface Sci. 2009, 338, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiao, M.; Wang, J.; Ngai, T. Pure protein scaffolds from Pickering high internal phase emulsion template. Macromol. Rapid Commun. 2013, 34, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Lu, X.; Huang, Q. Double emulsion derived from kafirin nanoparticles stabilized Pickering emulsion: Fabrication, microstructure, stability and in vitro digestion profile. Food Hydrocoll. 2017, 62, 230–238. [Google Scholar] [CrossRef]
- Xiao, J.; Li, C.; Huang, Q. Kafirin nanoparticle-stabilized Pickering emulsions as oral delivery vehicles: Physicochemical stability and in vitro digestion profile. J. Agric. Food Chem. 2015, 63, 10263–10270. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, C.-H. Soy protein nanoparticle aggregates as Pickering stabilizers for oil-in-water emulsions. J. Agric. Food Chem. 2013, 61, 8888–8898. [Google Scholar] [CrossRef] [PubMed]
- Shimoni, G.; Shani Levi, C.; Levi Tal, S.; Lesmes, U. Emulsions stabilization by lactoferrin nano-particles under in vitro digestion conditions. Food Hydrocoll. 2013, 33, 264–272. [Google Scholar] [CrossRef]
- Zou, Y.; Guo, J.; Yin, S.-W.; Wang, J.-M.; Yang, X.-Q. Pickering emulsion gels prepared by hydrogen-bonded zein/tannic acid complex colloidal particles. J. Agric. Food Chem. 2015, 63, 7405–7414. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.M.; Yang, X.Q.; Wu, N.N.; Wang, L.J.; Wang, J.M.; Guo, J.; Yin, S.W. Protein-based Pickering emulsion and oil gel prepared by complexes of zein colloidal particles and stearate. J. Agric. Food Chem. 2014, 62, 2672–2678. [Google Scholar] [CrossRef] [PubMed]
- Juttulapa, M.; Piriyaprasarth, S.; Sriamornsak, P. Effect of pH on stability of oil-in-water emulsions stabilized by pectin-zein complexes. In Multi-Functional Materials and Structures IV; Sombatsompop, N., Bhattacharyya, D., Cheung, K.H.Y., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; Volume 747, pp. 127–130. [Google Scholar]
- Destribats, M.; Rouvet, M.; Gehin-Delval, C.; Schmitt, C.; Binks, B.P. Emulsions stabilised by whey protein microgel particles: Towards food-grade Pickering emulsions. Soft Matter 2014, 10, 6941–6954. [Google Scholar] [CrossRef] [PubMed]
- Meshulam, D.; Lesmes, U. Responsiveness of emulsions stabilized by lactoferrin nano-particles to simulated intestinal conditions. Food Funct. 2014, 5, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jordan, A.; Nicolai, T.; Benyahia, L. Influence of the protein particle morphology and partitioning on the behavior of particle-stabilized water-in-water emulsions. Langmuir 2016, 32, 7189–7197. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P.; Fletcher, P.D.I. Particles adsorbed at the oil-water interface: A theoretical comparison between spheres of uniform wettability and “Janus” particles. Langmuir 2001, 17, 4708–4710. [Google Scholar] [CrossRef]
- Tu, F.; Lee, D. One-step encapsulation and triggered release based on Janus particle-stabilized multiple emulsions. Chem. Commun. 2014, 50, 15549–15552. [Google Scholar] [CrossRef] [PubMed]
- Mejia, A.F.; Diaz, A.; Pullela, S.; Chang, Y.-W.; Simonetty, M.; Carpenter, C.; Batteas, J.D.; Mannan, M.S.; Clearfield, A.; Cheng, Z. Pickering emulsions stabilized by amphiphilic nano-sheets. Soft Matter 2012, 8, 10245–10253. [Google Scholar] [CrossRef]
- Cao, Z.; Wang, G.; Chen, Y.; Lang, F.; Yang, Z. Light-triggered responsive Janus composite nanosheets. Macromolecules 2015, 48, 7256–7261. [Google Scholar] [CrossRef]
- Kim, J.W.; Cho, J.; Cho, J.; Park, B.J.; Kim, Y.-J.; Choi, K.-H.; Kim, J.W. Synthesis of monodisperse bi-compartmentalized amphiphilic Janus microparticles for tailored assembly at the oil-water interface. Angew. Chem. Int. Ed. 2016, 55, 4509–4513. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fang, Z.; Yang, D.; Gao, Y.; Li, H.; Chen, D. Water in oil emulsion stabilized by tadpole-like single chain polymer nanoparticles and its application in biphase reaction. ACS Appl. Mater. Interfaces 2014, 6, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Yi, F.; Xu, F.; Gao, Y.; Li, H.; Chen, D. Macrocellular polymer foams from water in oil high internal phase emulsion stabilized solely by polymer Janus nanoparticles: Preparation and their application as support for Pd catalyst. RSC Adv. 2015, 5, 40227–40235. [Google Scholar] [CrossRef]
- Chen, Y.; Liang, F.; Yang, H.; Zhang, C.; Wang, Q.; Qu, X.; Li, J.; Cai, Y.; Qiu, D.; Yang, Z. Janus nanosheets of polymer-inorganic layered composites. Macromolecules 2012, 45, 1460–1467. [Google Scholar] [CrossRef]
- Fujii, S.; Yokoyama, Y.; Miyanari, Y.; Shiono, T.; Ito, M.; Yusa, S.-I.; Nakamura, Y. Micrometer-sized gold–silica Janus particles as particulate emulsifiers. Langmuir 2013, 29, 5457–5465. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liang, F.; Zhang, G.; Ji, X.; Wang, Q.; Qu, X.; Song, X.; Yang, Z. Dually responsive Janus composite nanosheets. Macromolecules 2015, 48, 3598–3603. [Google Scholar] [CrossRef]
- Ruhland, T.M.; Groschel, A.H.; Ballard, N.; Skelhon, T.S.; Walther, A.; Muller, A.H.E.; Bon, S.A.F. Influence of Janus particle shape on their interfacial behavior at liquid-liquid interfaces. Langmuir 2013, 29, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Lee, D.; Shum, H.C.; Weitz, D.A. Colloid surfactants for emulsion stabilization. Adv. Mater. 2008, 20, 3239–3243. [Google Scholar] [CrossRef]
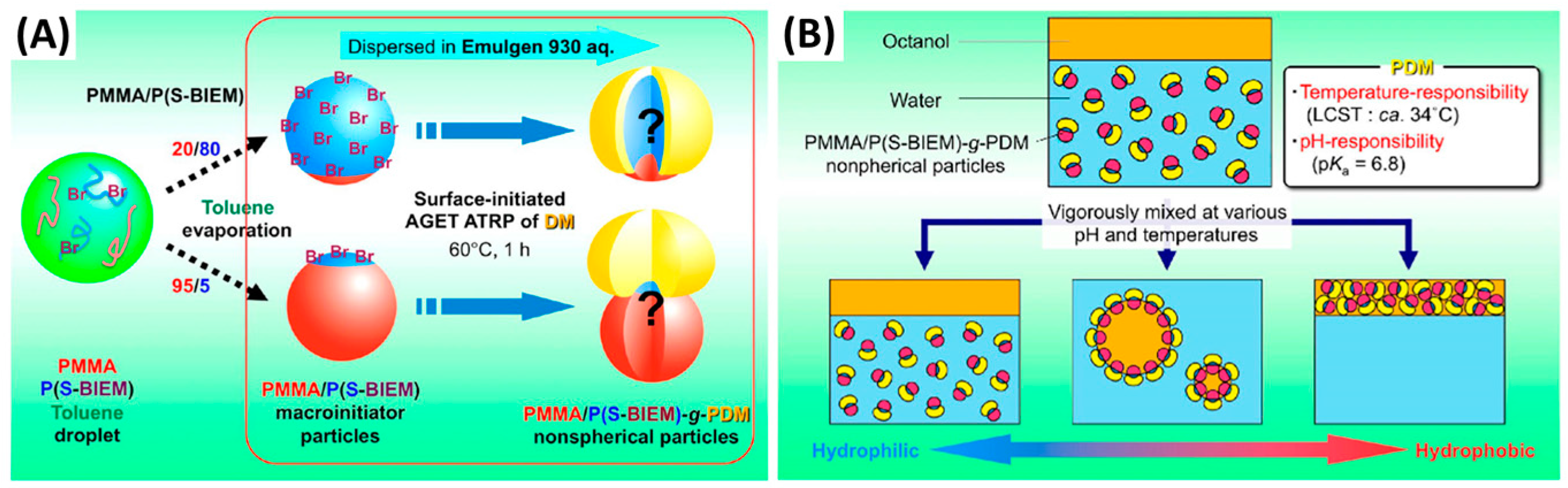
- Yamagami, T.; Kitayama, Y.; Okubo, M. Preparation of stimuli-responsive “mushroom-like” Janus polymer particles as particulate surfactant by site-selective surface-initiated AGET ATRP in aqueous dispersed systems. Langmuir 2014, 30, 7823–7832. [Google Scholar] [CrossRef] [PubMed]
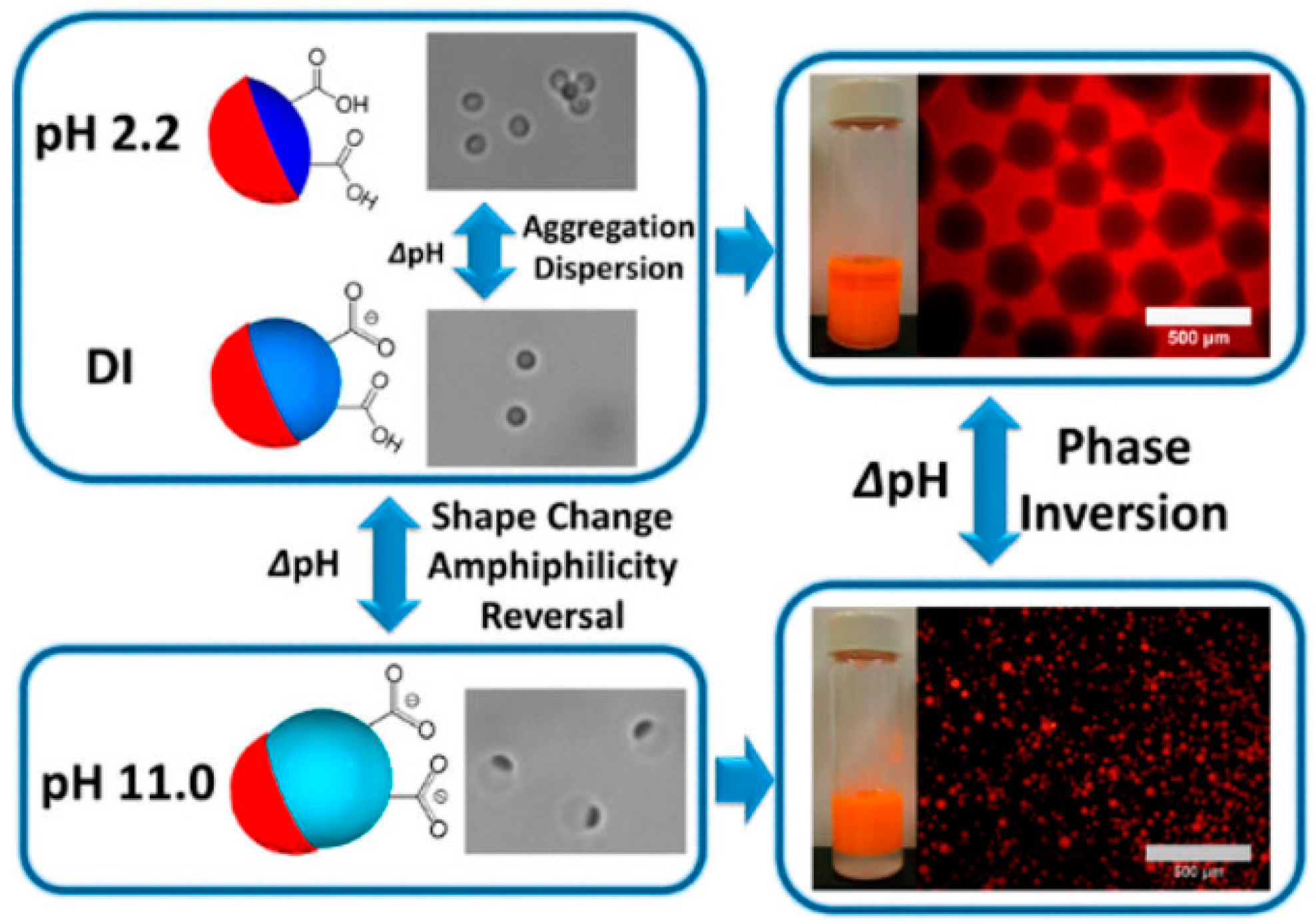
- Tu, F.; Lee, D. Shape-changing and amphiphilicity-reversing Janus particles with pH-responsive surfactant properties. J. Am. Chem. Soc. 2014, 136, 9999–10006. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, Y. Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion. Materials 2016, 9, 903. https://doi.org/10.3390/ma9110903
Wang Z, Wang Y. Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion. Materials. 2016; 9(11):903. https://doi.org/10.3390/ma9110903
Chicago/Turabian StyleWang, Zhen, and Yapei Wang. 2016. "Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion" Materials 9, no. 11: 903. https://doi.org/10.3390/ma9110903
APA StyleWang, Z., & Wang, Y. (2016). Tuning Amphiphilicity of Particles for Controllable Pickering Emulsion. Materials, 9(11), 903. https://doi.org/10.3390/ma9110903




