Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Protein-Repellent Composite
- (1)
- 30% BT resin + 70% glass filler (referred to as “Composite with 0% MPC”);
- (2)
- 27% BT resin + 3% MPC + 70% glass filler (“Composite with 3% MPC”);
- (3)
- Commercial control composite Heliomolar (“Commercial composite control”);
- (4)
- Resin-modified glass ionomer Vitremer (“RMGI”);
- (5)
- Amalgam Coutour (“Amalgam”).
2.2. Mechanical Properties
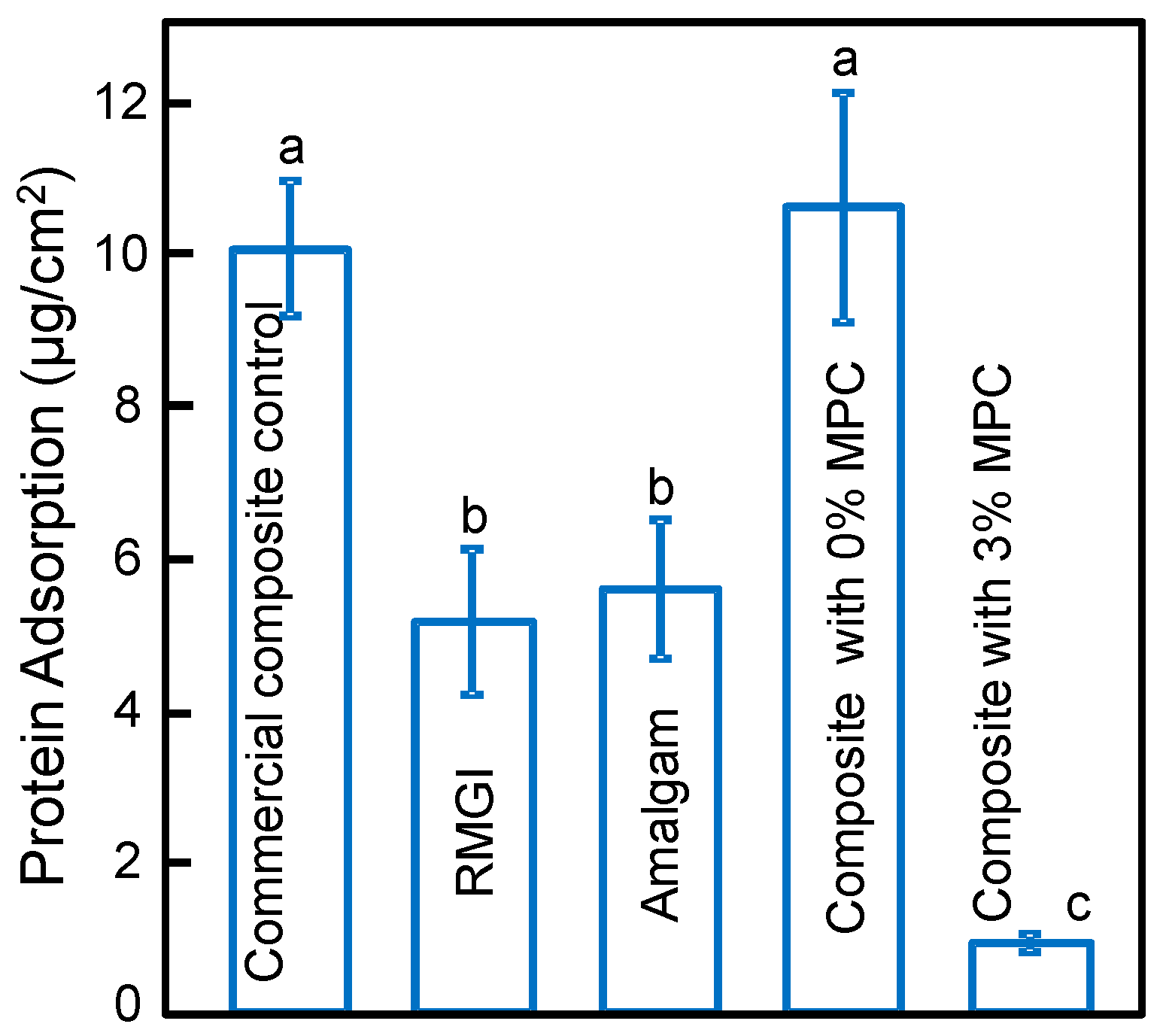
2.3. Characterization of Protein Adsorption
2.4. Saliva Collection for Biofilm Inoculum
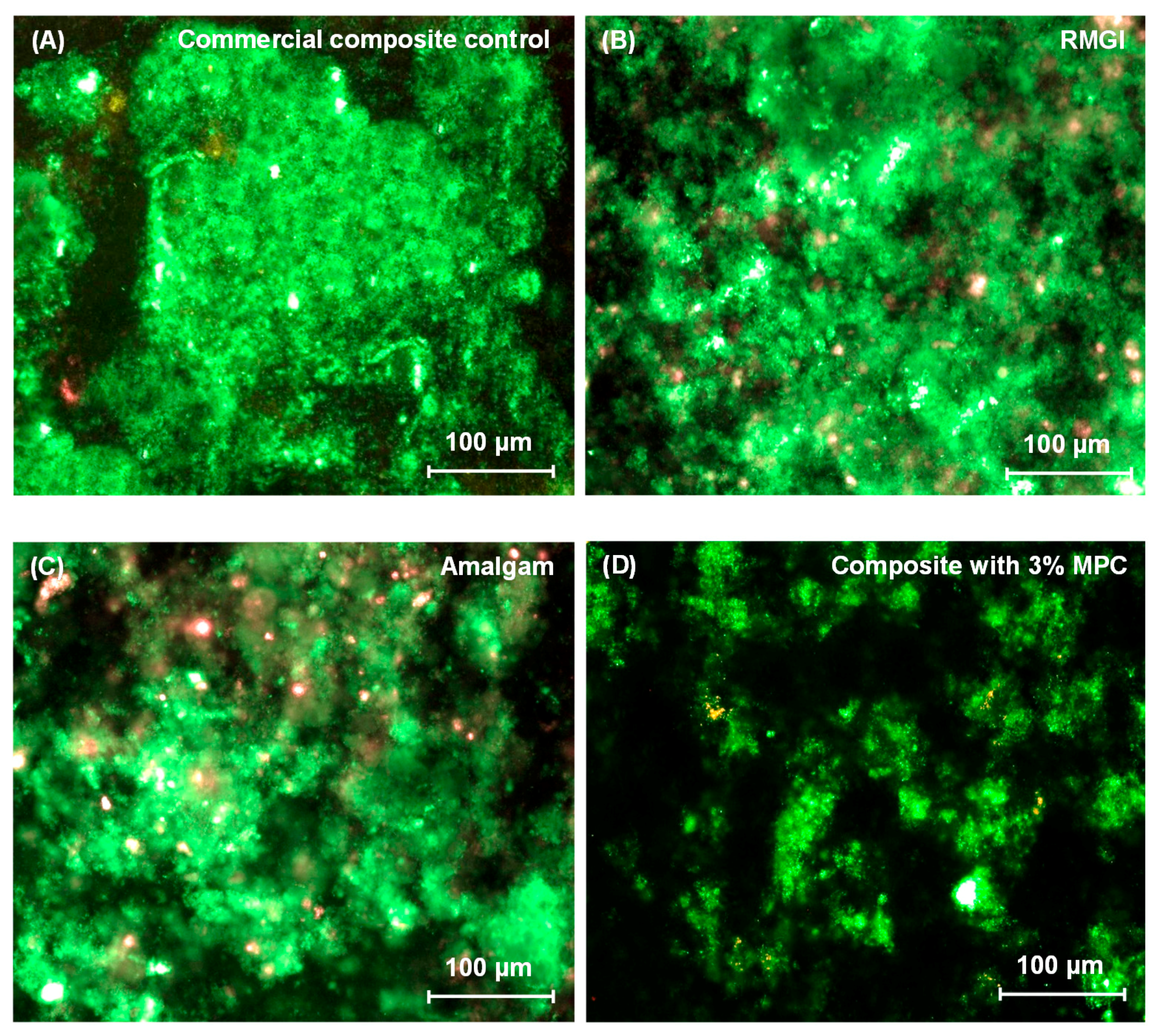
2.5. Dental Plaque Microcosm Biofilm Formation and Live/Dead Assay
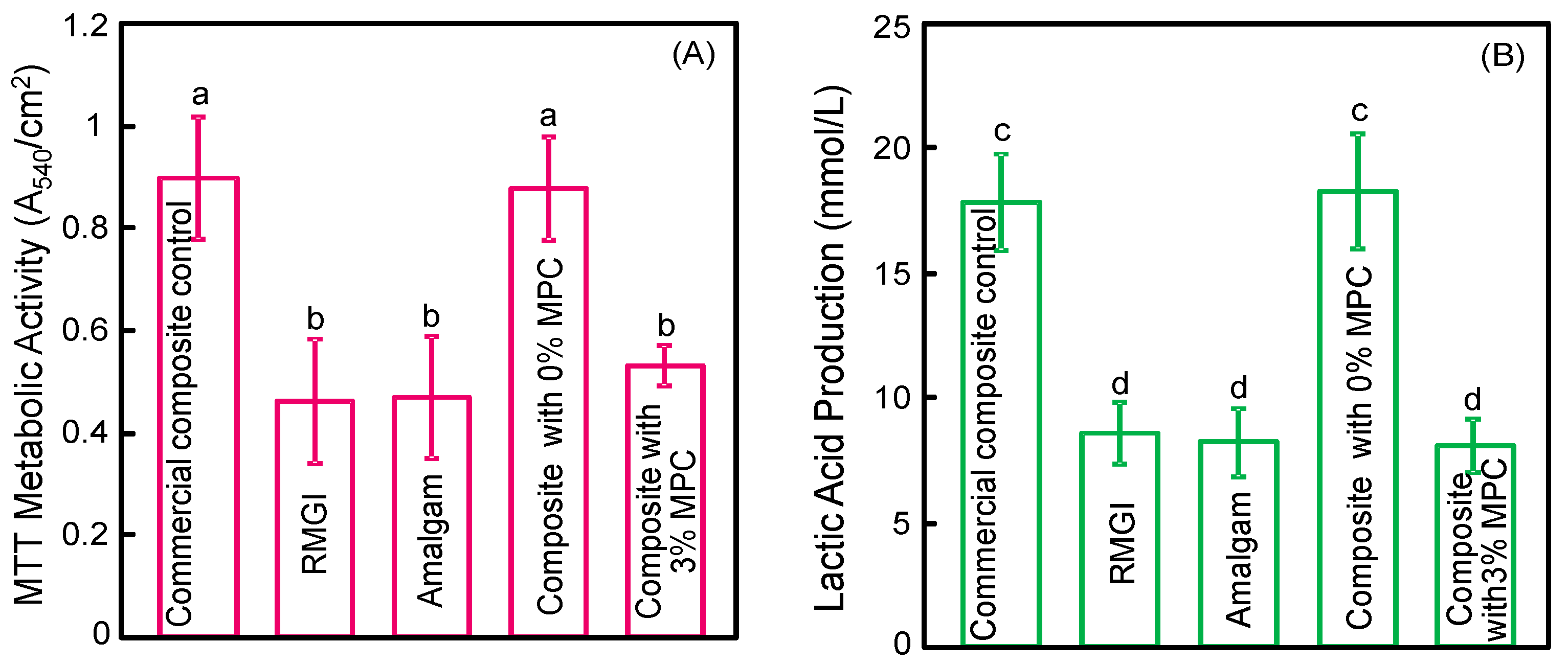
2.6. MTT Metabolic Assay
2.7. Lactic Acid Production
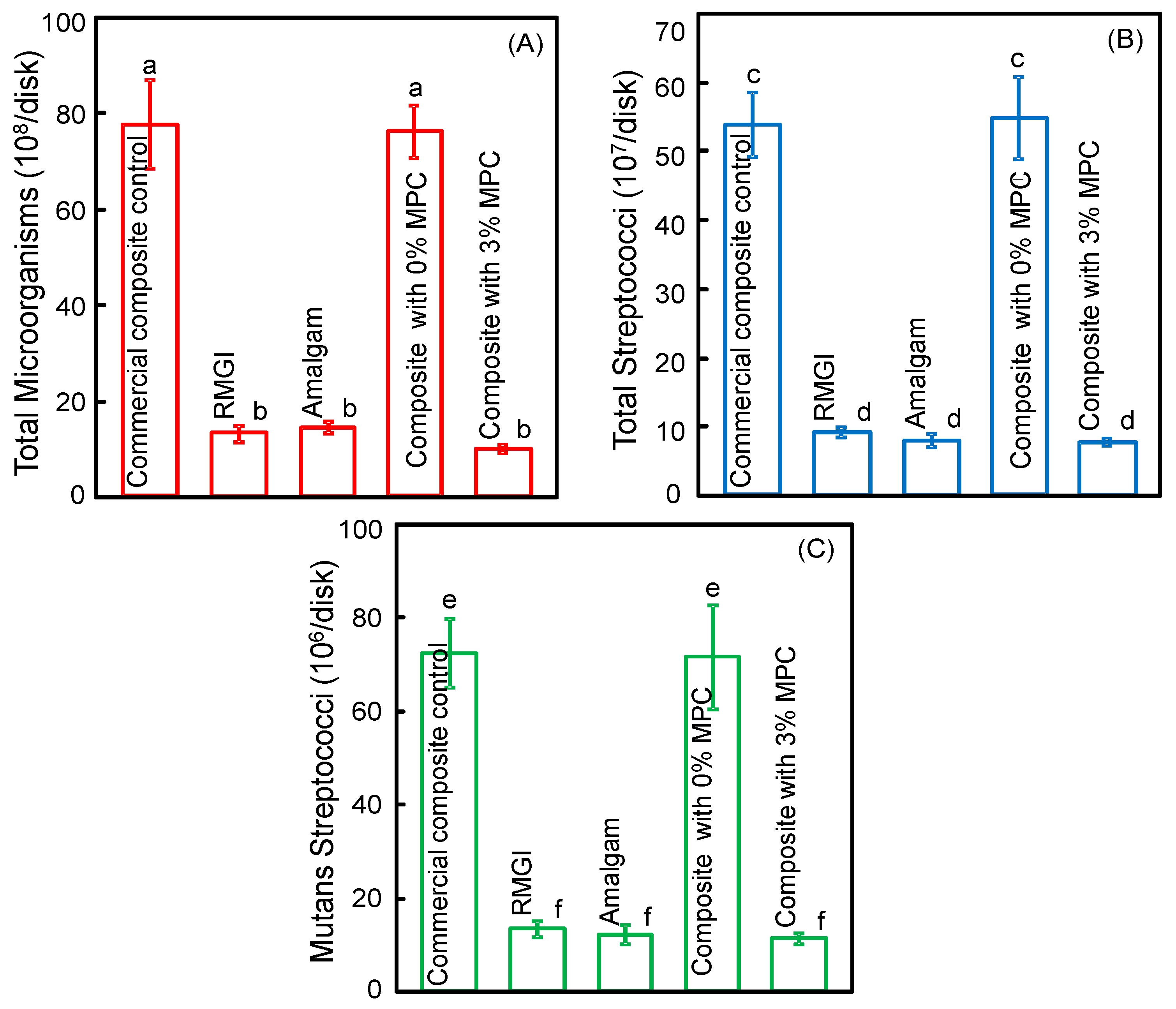
2.8. Biofilm Colony-Forming Unit (CFU) Counts
2.9. Statistical Analysis
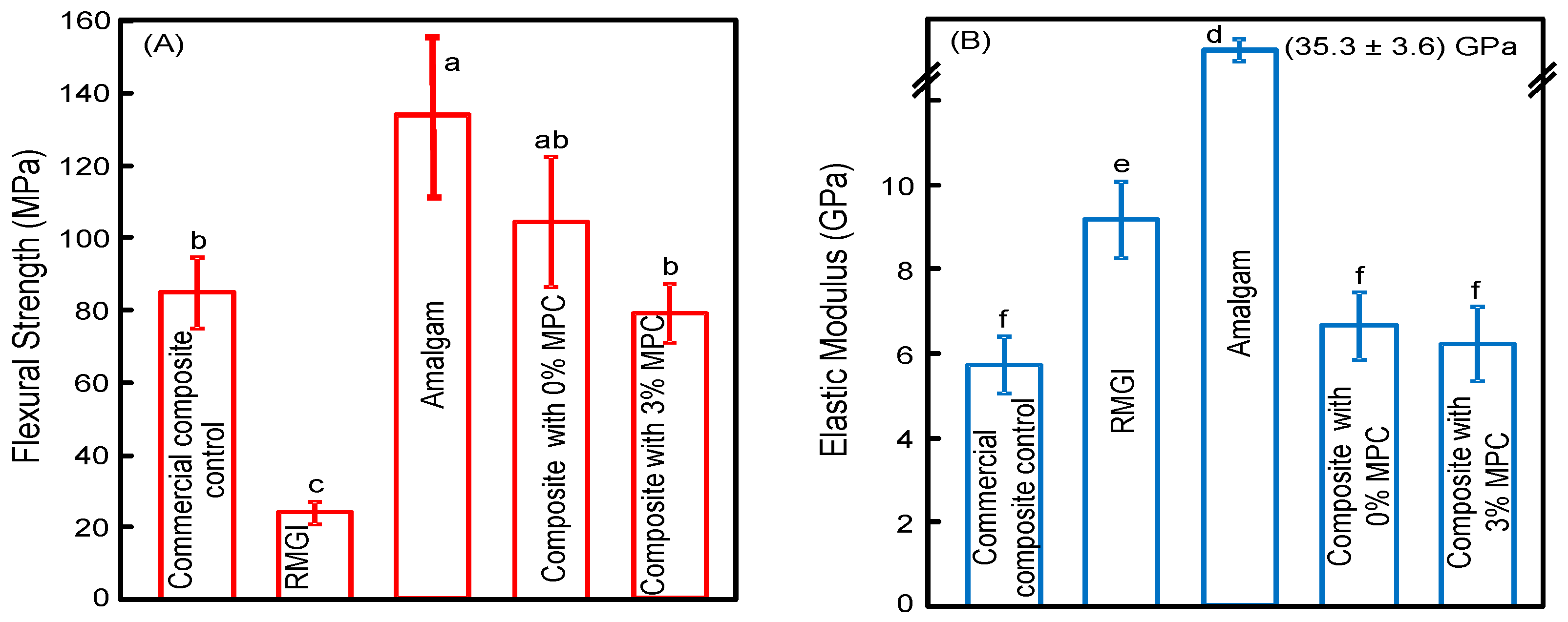
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C. Correlation of clinical performance with ‘in vitro tests’ of restorative dental materials that use polymer-based matrices. Dent. Mater. 2012, 28, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.C. Beginnings of the dental composite revolution. 1963. J. Am. Dent. Assoc. 2013, 144, S42–S46. [Google Scholar] [CrossRef]
- Lim, B.S.; Ferracane, J.L.; Sakaguchi, R.L.; Condon, J.R. Reduction of polymerization contraction stress for dental composites by two-step light-activation. Dent. Mater. 2002, 18, 436–444. [Google Scholar] [CrossRef]
- Watts, D.C.; Marouf, A.S.; Al-Hindi, A.M. Photo-polymerization shrinkage-stress kinetics in resin-composites: Methods development. Dent. Mater. 2003, 19, 1–11. [Google Scholar] [CrossRef]
- Lu, H.; Stansbury, J.W.; Bowman, C.N. Impact of curing protocol on conversion and shrinkage stress. J. Dent. Res. 2005, 84, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.; Petersen, P.E.; Piper, D.; Schmalz, G.; Meyer, D. The challenge for innovation in direct restorative materials. Adv. Dent. Res. 2013, 25, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Pallesen, U.; van Dijken, J.W.; Halken, J.; Hallonsten, A.L.; Höigaard, R. A prospective 8-year follow-up of posterior resin composite restorations in permanent teeth of children and adolescents in Public Dental Health Service: Reasons for replacement. Clin. Oral Investig. 2014, 18, 819–827. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.; Luis, H.; Martin, M.D.; Leroux, B.G.; Rue, T.; Leitão, J. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. J. Am. Dent. Assoc. 2007, 138, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Kopperud, S.E.; Tveit, A.B.; Gaarden, T.; Sandvik, L.; Espelid, L. Longevity of posterior dental restorations and reasons for failure. Eur. J. Oral Sci. 2012, 120, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Delaviz, Y.; Finer, Y.; Santerre, J.P. Biodegradation of resin composites and adhesives by oral bacteria and saliva: A rationale for new material designs that consider the clinical environment and treatment challenges. Dent. Mater. 2014, 30, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Bourbia, M.; Ma, D.; Cvitkovitch, D.G.; Santerre, J.P.; Finer, Y. Cariogenic bacteria degrade dental resin composites and adhesives. J. Dent. Res. 2013, 92, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Svanberg, M.; Majör, I.A.; Ørstavik, D. Mutans streptococci in plaque from margins of amalgam, composite and glass ionomer restorations. J. Dent. Res. 1990, 69, 861–864. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Bahir, R.; Matalon, S.; Domb, A.J.; Weiss, E.I. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent. Mater. 2008, 24, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Khalichi, P.; Singh, J.; Cvitkovitch, D.G.; Santerre, J.P. The influence of triethylene glycol derived from dental composite resins on the regulation of Streptococcus mutans gene expression. Biomaterials 2009, 30, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kuper, N.K.; van de Sande, F.H.; Opdam, N.J.; Bronkhorst, E.M.; de Soet, J.J.; Cenci, M.S. Restoration materials and secondary caries using an in vitro biofilm model. J. Dent. Res. 2015, 94, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Lynch, C.D. Amalgam and minimal intervention: An incompatible relationship. Prim. Dent. J. 2013, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Devine, D.; Marsh, P.D. Oral biofilms: Molecular analysis, challenges, and future prospects in dental diagnostics. Clin. Cosmet. Investig. Dent. 2013, 5, 11–19. [Google Scholar] [PubMed]
- Lewis, A.L. Phosphorylcholine-based polymers and their use in the prevention of biofouling. Colloids Surf. B Biointerface 2000, 18, 261–275. [Google Scholar] [CrossRef]
- Zhang, N.; Chen, C.; Melo, M.A.; Bai, Y.X.; Cheng, L.; Xu, H.H. A novel protein-repellent dental composite containing 2-methacryloyloxyethyl phosphorylcholine. Int. J. Oral Sci. 2015, 7, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Ishihara, K.; Ueda, T.; Nakabayashi, N. Preparation of phospholipid polymers and their properties as polymer hydrogel membranes. Polym. J. 1990, 22, 355–360. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Iwasaki, Y. Copolymers of 2-methacryloyloxyethyl phosphorylcholine (MPC) as biomaterials. Biomed. Mater. Eng. 2004, 14, 345–354. [Google Scholar] [PubMed]
- Monge, S.; Canniccioni, B.; Graillot, A.; Robin, J.J. Phosphorus-containing polymers: A great opportunity for the biomedical field. Biomacromolecules 2011, 13, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Melo, M.A.; Bai, Y.; Xu, H.H. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. J. Dent. 2014, 42, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, J.; Melo, M.A.; Weir, M.D.; Bai, Y.; Xu, H.H. Protein-repellent and antibacterial dental composite to inhibit biofilms and caries. J. Dent. 2015, 43, 225–234. [Google Scholar] [CrossRef] [PubMed]
- McBain, A.J.; Sissons, C.; Ledder, R.G.; Sreenivasan, P.K.; De Vizio, W.; Gilbert, P. Development and characterization of a simple perfused oral microcosm. J. Appl. Microbiol. 2005, 98, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.; Xu, H.H. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Salli, K.M.; Ouwehand, A.C. The use of in vitro model systems to study dental biofilms associated with caries: A short review. J. Oral Microbiol. 2015, 7, 26149. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X.D. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.P.; Zanin, I.C.; Lima, J.P.; Vasconcelos, S.M.; Melo, M.A.; Beltrão, H.C.; Rodrigues, L.K. In situ effects of restorative materials on dental biofilm and enamel demineralisation. J. Dent. 2009, 37, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.K.; Wang, S.; Zhou, X.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.D.; Cheng, L. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- De Fucio, S.B.; Puppin-Rontani, R.M.; de Carvalho, F.G.; Mattos-Graner Rde, O.; Correr-Sobrinho, L.; Garcia-Godoy, F. Analyses of biofilms accumulated on dental restorative materials. Am. J. Dent. 2009, 2, 131–136. [Google Scholar]
- Pendrys, D.G. Resin-modified glass-ionomer cement (RM-GIC) may provide greater caries preventive effect compared with composite resin, but high-quality studies are needed. J. Evid. Based. Dent. Pract. 2011, 11, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin-based composite performance: Are there some things we can’t predict? Dent. Mater. 2013, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Khalichi, P.; Cvitkovitch, D.G.; Santerre, J.P. Composite resin degradation products from BisGMA monomer modulate the expression of genes associated with biofilm formation and other virulence factors in Streptococcus mutans. J. Biomed. Mater. Res. A 2009, 88, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Misra, A.; Goncalves, S.E.; Laurence, J.S. Proteins, pathogens, and failure at the composite-tooth interface. J. Dent. Res. 2014, 93, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, L.; Campoccia, D.; Rizzi, S.; Donati, M.E.; Breschi, L.; Prati, C. Evaluation of bacterial adhesion of Streptococcus mutans on dental restorative materials. Biomaterials 2004, 25, 4457–4463. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, L.M.; Ribeiro, C.C.; Gonçalves, N.C.; Del Bel Cury, A.A.; Aires, C.P.; Tengan, C. The short-term in situ model to evaluate the anticariogenic potential of ionomeric materials. J. Dent. 2005, 33, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.P.; Pereira-Cenci, T.; Silva, W.M.; Coelho-de-Souza, F.H.; Demarco, F.F.; Cenci, M.S. Effect of cariogenic biofilm challenge on the surface hardness of direct restorative materials in situ. J. Dent. 2012, 40, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Sheng, J.; Nguyen, P.T.; Marquis, R.E. Cooperative inhibition by fluoride and zinc of glucosyl transferase production and polysaccharide synthesis by mutans streptococci in suspenysion cultures and biofilms. FEMS Microbiol. Lett. 2006, 254, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Gama-Teixeira, A.; Simionato, M.R.; Elian, S.N.; Sobral, M.A.; Luz, M.A. Streptococcus mutans-induced secondary caries adjacent to glass ionomer cement, composite resin and amalgam restorations in vitro. Braz. Oral Res. 2007, 21, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Silver, S. Bacterial silver resistance: Molecular biology and uses and misuses of silver compounds. FEMS Microbiol. Rev. 2003, 27, 341–353. [Google Scholar] [CrossRef]
- Magne, P. Composite resins and bonded porcelain: The post-amalgam era? J. Calif. Dent. Assoc. 2006, 34, 135–147. [Google Scholar] [PubMed]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Nomura, H.; Mihara, T.; Kurita, K.; Iwasaki, Y.; Nakabayashi, N. Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 1998, 39, 323–330. [Google Scholar] [CrossRef]
- Lewis, A.L.; Tolhurst, L.A.; Stratford, P.W. Analysis of a phosphorylcholine-based polymer coating on a coronary stent pre-and post-implantation. Biomaterials 2002, 23, 1697–1706. [Google Scholar] [CrossRef]
- Sibarani, J.; Takai, M.; Ishihara, K. Surface modification on microfluidic devices with 2-methacryloyloxyethyl phosphorylcholine polymers for reducing unfavorable protein adsorption. Colloids Surf. B Biointerfaces 2007, 54, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Kawaguchi, H.; Ishihara, K.; Kyomoto, M.; Karita, T.; Ito, H. Wear resistance of artificial hip joints with poly(2-methacryloyloxyethyl phosphorylcholine) grafted polyethylene: Comparisons with the effect of polyethylene cross-linking and ceramic femoral heads. Biomaterials 2009, 30, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Schätzle, M.; Lang, N.P.; Ånerud, Å.; Boysen, H.; Bürgin, W.; Löe, H. The influence of margins of restorations on the periodontal tissues over 26 years. J. Clin. Periodontol. 2000, 27, 57–64. [Google Scholar] [CrossRef]
- Paolantonio, M.; D’Ercole, S.; Perinetti, G.; Tripodi, D.; Catamo, G.; Serra1, E.; Brue, C.; Piccolomini, R. Clinical and microbiological effects of different restorative materials on the periodontal tissues adjacent to subgingival class V restorations. 1-year results. J. Clin. Periodontol. 2004, 31, 200–207. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Dental and Craniofacial Research (NIDCR). RFA-DE-16-007. Novel or Enhanced Dental Restorative Materials for Class V Lesions. 2015. Available online: http://grants.nih.gov/grants/guide/rfa-files/RFA-DE-16–007.html (accessed on 1 September 2016). [Google Scholar]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Melo, M.A.S.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials? Materials 2016, 9, 888. https://doi.org/10.3390/ma9110888
Zhang N, Melo MAS, Weir MD, Reynolds MA, Bai Y, Xu HHK. Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials? Materials. 2016; 9(11):888. https://doi.org/10.3390/ma9110888
Chicago/Turabian StyleZhang, Ning, Mary A.S. Melo, Michael D. Weir, Mark A. Reynolds, Yuxing Bai, and Hockin H.K. Xu. 2016. "Do Dental Resin Composites Accumulate More Oral Biofilms and Plaque than Amalgam and Glass Ionomer Materials?" Materials 9, no. 11: 888. https://doi.org/10.3390/ma9110888





