Fabrication of Glass Fiber Reinforced Composites Based on Bio-Oil Phenol Formaldehyde Resin
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of BPF Resin
- At first, phenol and 70% of the total NaOH solution (30 wt %) were mixed in a three-necked flask. The mixture was heated to 70 °C in 30 min.
- Then, 80% of the total paraformaldehyde was added in batches, avoiding boiling. The mixture was kept at 70 °C–75 °C until completely depolymerized.
- Next, the remaining 30% of the total NaOH solution (30 wt %) was added. The mixture was heated to 80 °C in 30 min and then kept for 15 min.
- After the temperature was dropped to 70 °C, the remaining 20% of the total paraformaldehyde and bio-oil was added in batches.
- Finally, the mixture was heated to 85 °C–90 °C and held for 45 min. When the reaction was complete, it was rapidly cooled to 40 °C to yield the BPF resin.
2.3. Preparation of GF/BPF Composites
- The GF cloth with the size of 150 × 150 × 0.6 mm3 was immersed in ethanol solution for half an hour and then dried at 60 °C for 2 h.
- After surface clearing and drying, the mold which was made of acrylic resin in the size of 200 × 200 × 1 mm3, was coated with solid paraffin as the release agent.
- Next, the exact amount of acidic curing agent and silane coupling agent were gradually added to BPF resin and the mixture resin was blended for 10 min.
- Then, one piece of treated GF cloth was laid on the mold and coated with the same weight of mixture resin. Next, another piece of cloth was laid on the previous one and coated with mixture resin. The above operation was repeated until the composites achieved the ideal thickness, and held for 24 h at 25 °C to cure and mold. Usually, 6 pieces of GF cloth were needed to achieve the ideal thickness and the dial thickness gauges were used to measure the thickness.
- After demolding and further curing at 70 °C for 2 h, the composites was finally prepared.
2.4. Characterization of BPF and GF/BPF Composites
3. Results and Discussion
3.1. Characterization of BPF Resins and GF/BPF Composites
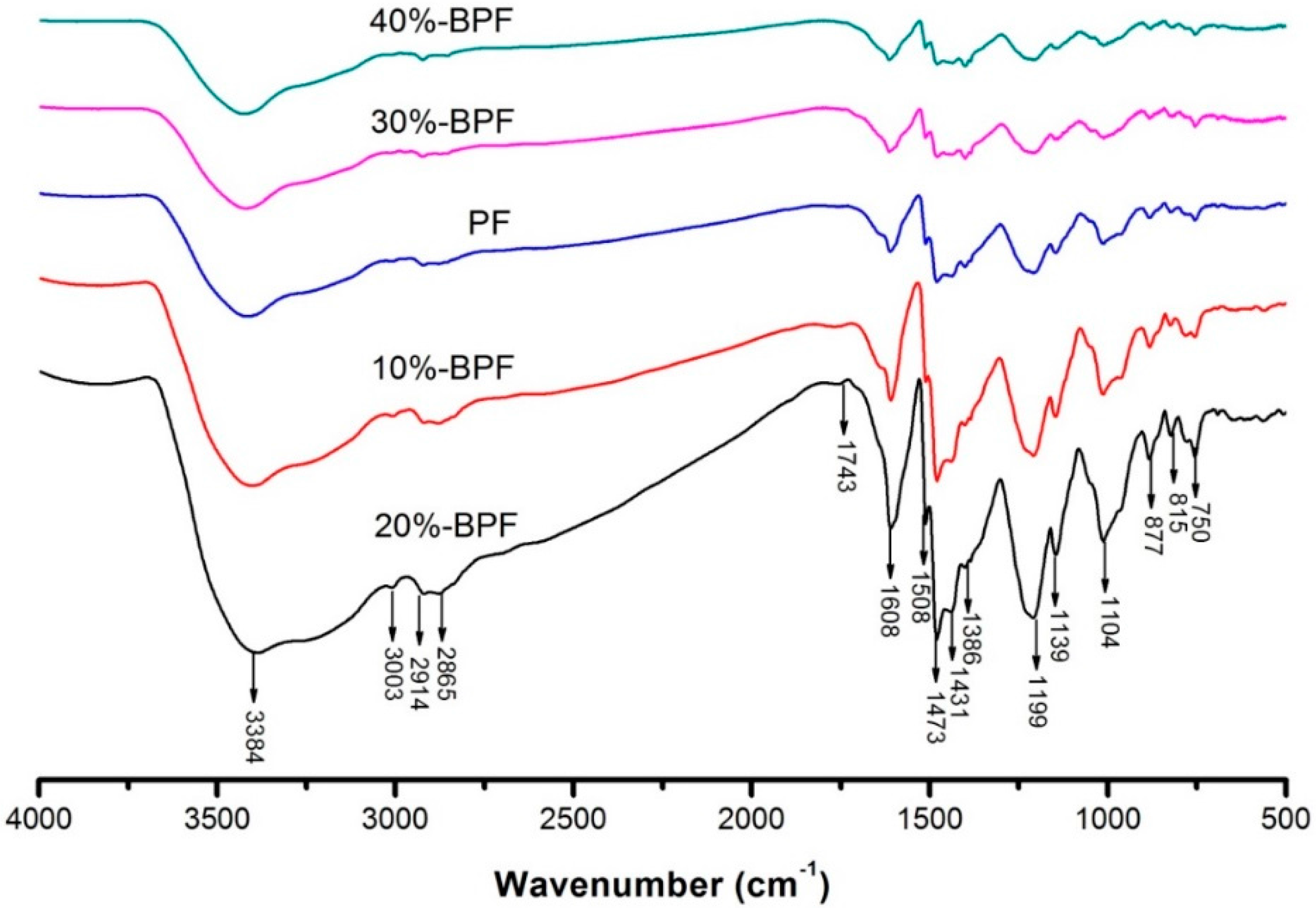
3.2. FTIR Spectrum Analysis
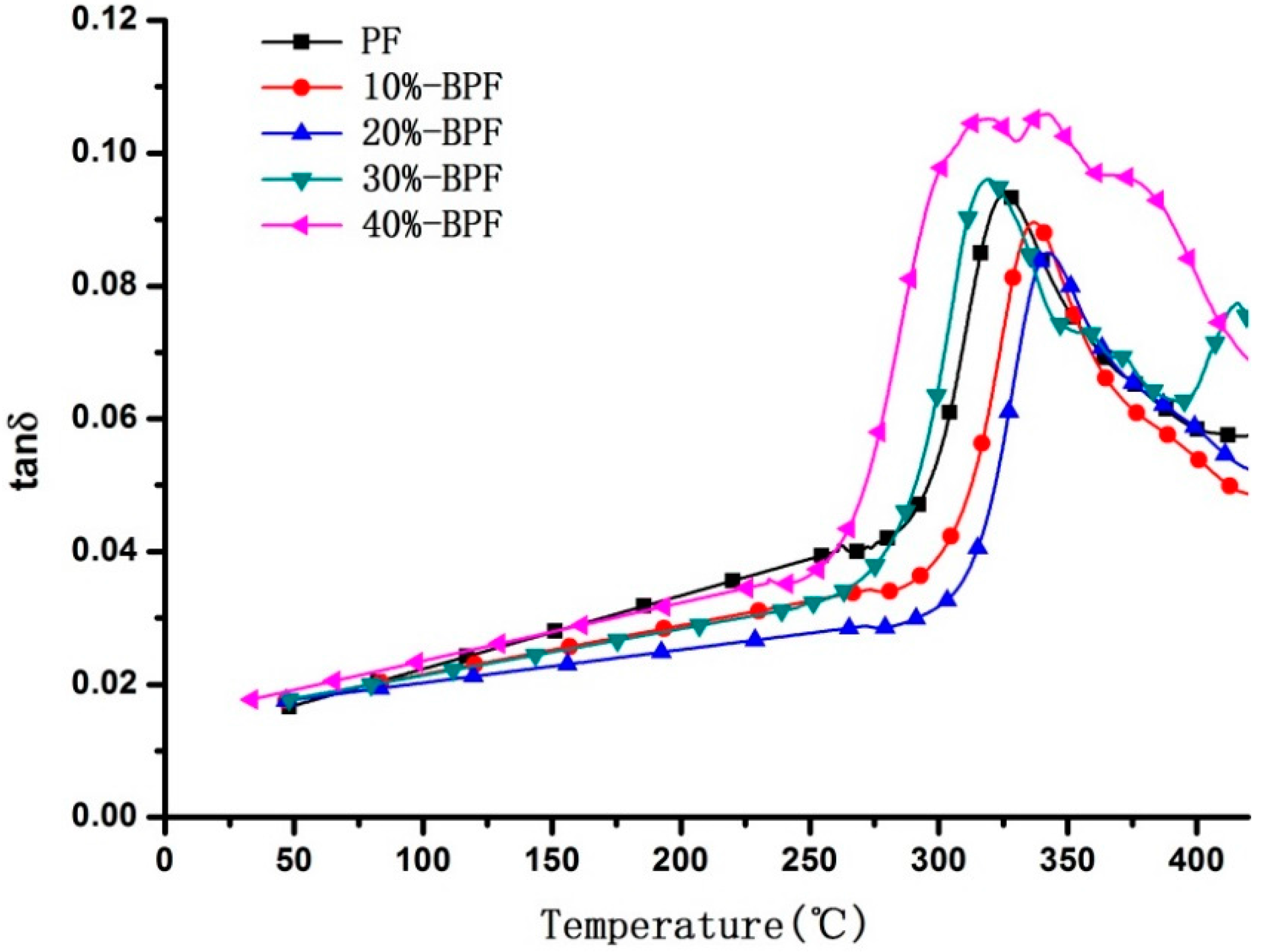
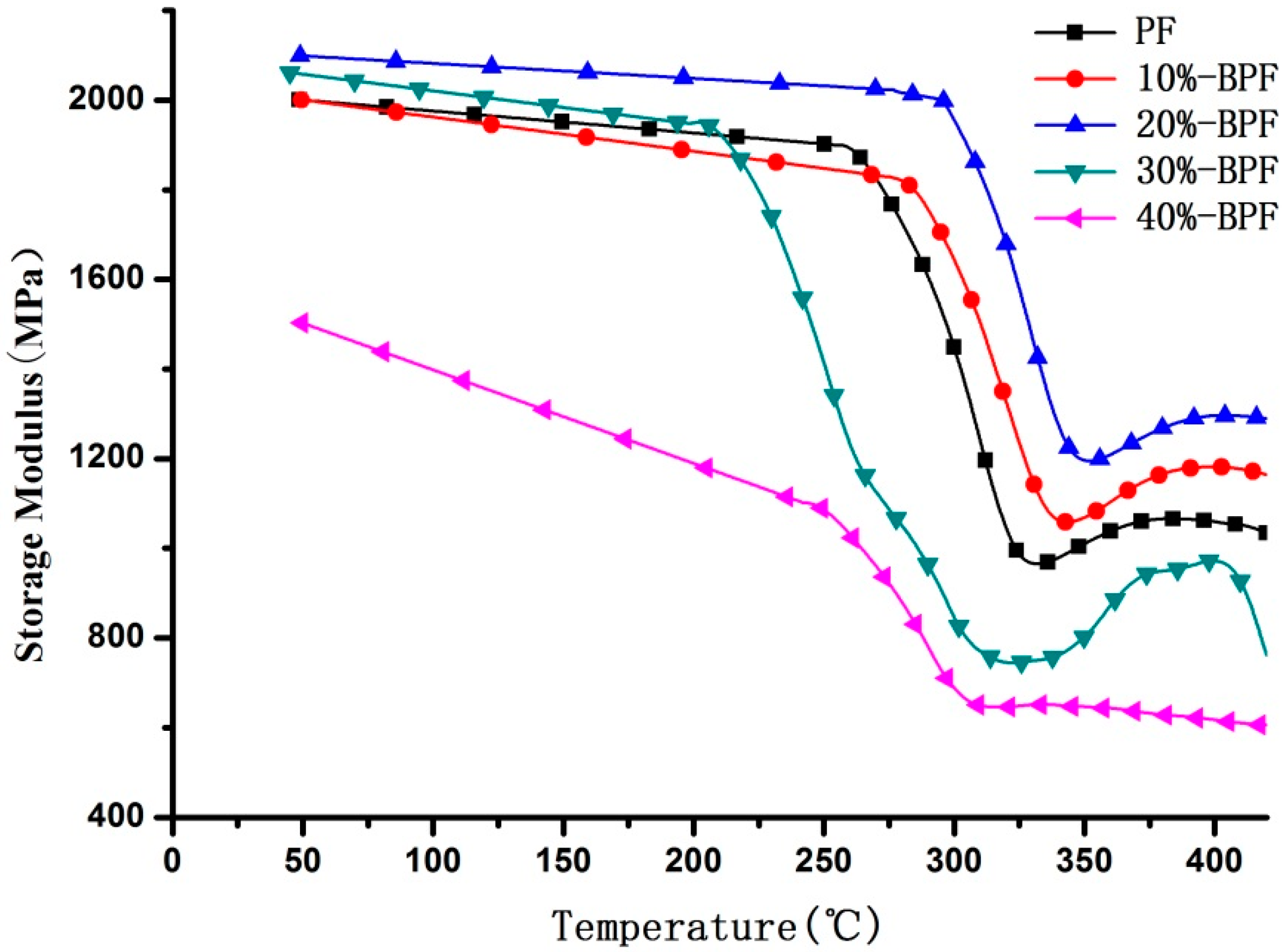
3.3. DMA Analysis
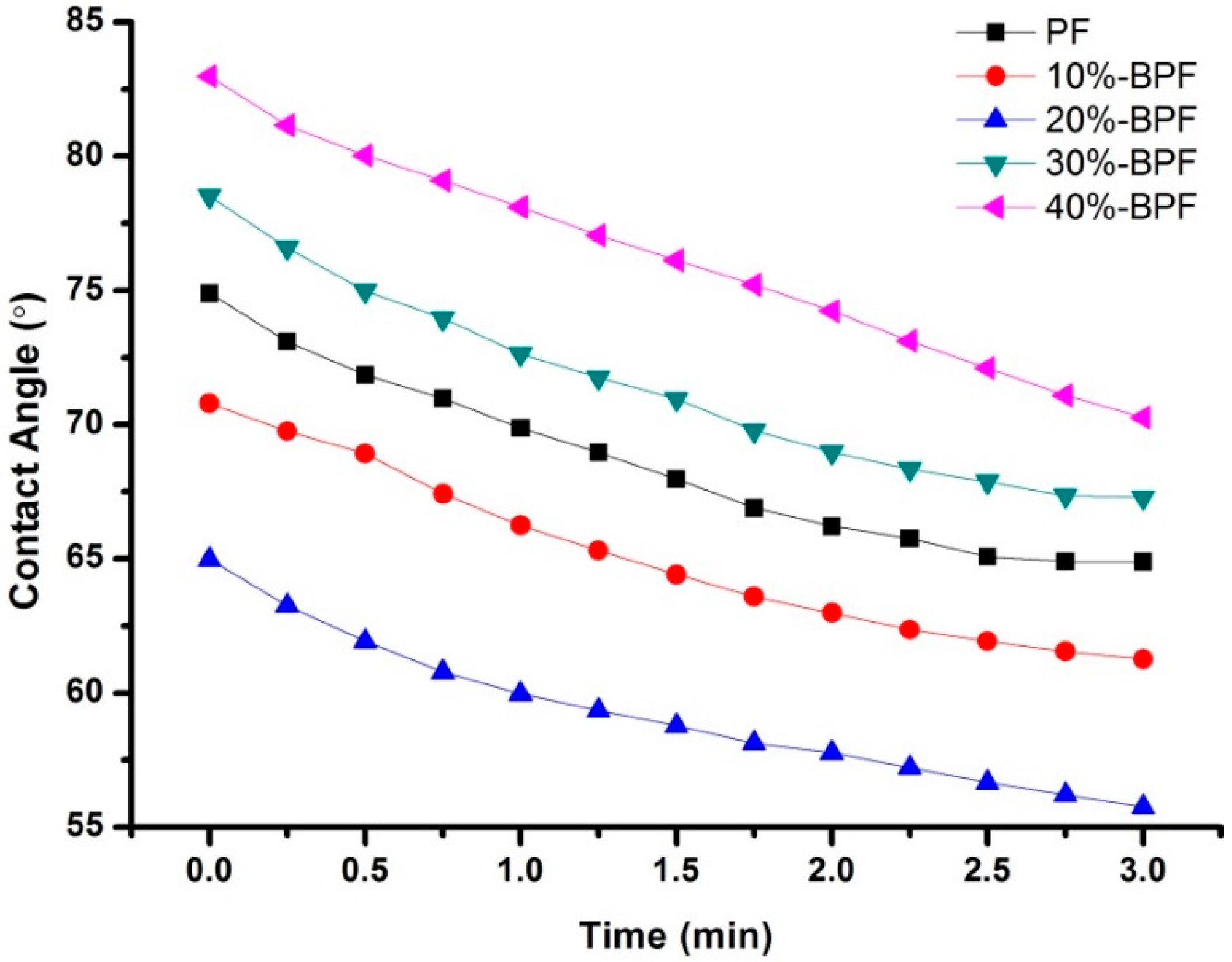
3.4. DW Analysis
3.5. SEM Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joseph, S.; Sreekala, M.S.; Oommen, Z.; Koshy, P.; Thomas, S. A comparison of the mechanical properties of phenol formaldehyde composites reinforced with banana fibres and glass fibres. Compos. Sci. Technol. 2002, 62, 1857–1868. [Google Scholar] [CrossRef]
- Wichmann, M.H.G.; Sumfleth, J.; Gojny, F.H.; Quaresimin, M.; Fiedler, B. Glass-fibre-reinforced composites with enhanced mechanical and electrical properties-Benefits and limitations of a nanoparticle modified matrix. Eng. Fract. Mech. 2006, 73, 2346–2359. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Hang, Z.S.; Wang, J.G.; Wu, S.Q. Thermal modifying methods and progress of phenol-formaldehyde resin. J. Mater. Eng. 2011, 1, 91–96. [Google Scholar]
- Mirski, R.; Dziurka, D.; Lecka, J. Properties of phenol-formaldehyde resin modified with organic acid esters. J. Appl. Polym. Sci. 2008, 107, 3358–3366. [Google Scholar] [CrossRef]
- Su, D.; Chen, Z.F.; Yang, Y.; Chen, Z.; Wu, C. Effect of melamine/urea modified phenol formaldehyde resin binder on the mechanical properties of glass-fiber felt. Appl. Mech. Mater. 2014, 488, 18–21. [Google Scholar] [CrossRef]
- Tseng, R.L.; Wu, F.C.; Juang, R.S. Adsorption of CO2 at atmospheric pressure on activated carbons prepared from melamine-modified phenol-formaldehyde resins. Sep. Purif. Technol. 2015, 140, 53–60. [Google Scholar] [CrossRef]
- Kim, M.G.; Boyd, G.; Strickland, R. Adhesive properties of furfural-modified phenol formaldehyde resins as oriented strandboard binders. Holzforschung 1994, 48, 262–267. [Google Scholar] [CrossRef]
- Tjr, S.; Mcginnis, G.D.; Ruffin, T.M.; Janiga, E.R. Lignin-modified phenol-formaldehyde resin development for fiberboard. For. Prod. J. 2004, 54, 45–51. [Google Scholar]
- Hu, X.; Zeng, J.; Dai, W.; Shi, W.; Li, L. EPDM/vinyl triethoxysilane modified phenol formaldehyde resin composite. Polym. Bull. 2011, 66, 703–710. [Google Scholar] [CrossRef]
- Alaminov, H.; Andonova, N. Thermal decomposition of phenol formaldehyde resins modified with cyanuric acid. J. Appl. Polym. Sci. 2003, 14, 1083–1092. [Google Scholar] [CrossRef]
- Czarnecki, R.; Lecka, J. H2O2 as a modifier of phenol-formaldehyde resin used in the production of particleboards. J. Appl. Polym. Sci. 2003, 88, 3084–3092. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Pattiya, A. Bio-oil production via fast pyrolysis of biomass residues from cassava plants in a fluidized-bed reactor. Bioresour. Technol. 2011, 102, 1959–1967. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, R.; Yin, R.; Mei, Y. Upgrading of bio-oil from biomass fast pyrolysis in China: A review. Renew. Sustain. Energy Rev. 2013, 24, 66–72. [Google Scholar] [CrossRef]
- Chum, H.; Diebold, I.; Scahill, I.; Iohnson, D.; Black, S.; Schroeder, H.; Kreibich, R.E. Biomass pyrolysis oil feedstocks for phenolic adhesives. Am. Chem. Soc. 1989, 385, 135–151. [Google Scholar]
- Kelley, S.S.; Wang, X.M.; Myers, M.D.; Johnson, D.K.; Scahill, J.W. Use of biomass pyrolysis oil for preparation of modified phenol formaldehyde resins; Springer: Berlin, Germany, 1997; Volume 44, pp. 557–572. [Google Scholar]
- Chan, F.; Riedl, B.; Wang, X.M.; Lu, X.; Amen-Chen, C.; Roy, C. Performance of pyrolysis oil-based wood adhesives in OSB. For. Prod. J. 2002, 52, 31–38. [Google Scholar]
- Aslan, M.; Ozbay, G.; Ayrilmis, N. Performance of phenol formaldehyde modified with phenol-rich fraction of crude bio-oil. J. Adhes. Sci. Technol. 2015, 29, 2679–2691. [Google Scholar] [CrossRef]
- Lee, W.J.; Tseng, I.M.; Kao, Y.P.; Lee, Y.Y.; Hu, M.S. Synthesis of alcohol-soluble phenol-formaldehyde resins from pyrolysis oil of Cunninghamia Lanceolata wood and properties of molding plates made of resin-impregnated materials. Holzforschung 2014, 68, 217–222. [Google Scholar] [CrossRef]
- Amen-Chen, C.; Riedl, B.; Wang, X.M.; Roy, C. Softwood bark pyrolysis oil-PF resols. Part1. Resin synthesis and OSB mechanical properties. Holzforschung 2002, 56, 167–175. [Google Scholar]
- Amen-Chen, C.; Riedl, B.; Roy, C. Softwood bark pyrolysis oil-PF resols. Part 2. Thermal analysis by DSC and TG. Holzforschung 2002, 56, 273–280. [Google Scholar] [CrossRef]
- Amen-Chen, C.; Riedl, B.; Wang, X.M.; Roy, C. Softwood bark pyrolysis oil-PF resols. Part 3. Use of propylene carbonate as resin cure accelerator. Holzforschung 2002, 56, 281–288. [Google Scholar] [CrossRef]
- Gagnon, M.; Roy, C.; Riedl, B. Adhesives made from isocyanates and pyrolysis oils for wood composites. Holzforschung 2004, 58, 400–407. [Google Scholar] [CrossRef]
- Mourant, D.; Yang, D.; Riedl, B. Mechanical properties of wood treated with PF-pyrolytic oil resins. Holz Roh Werkst 2008, 66, 163–171. [Google Scholar] [CrossRef]
- Cui, Y.; Chang, J.M.; Wang, W.L.; Li, B.; Ren, X.Y. Preparation of activated carbon using bio-oil phenol-formaldehyde resin. BioResources 2015, 10, 3865–3873. [Google Scholar] [CrossRef]
- Yi, J.P.; Zhang, J.Z.; Yao, S.X.; Chang, J.M.; Li, B. Preparation of bio-oil-phenol-formaldehyde resins from biomass pyrolysis oil. Appl. Mech. Mater. 2012, 174, 1429–1432. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, H.Y.; Lv, M.L.; Kong, Y.; Yu, Y.W.; Xu, X.Y. Component analysis of extracellular polymeric substances (EPS) during aerobic sludge granulation using FTIR and 3D-EEM technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, N.; Feng, M.W. Thermal degradation characteristics of phenol-formaldehyde resins derived from beetle infested pine barks. Thermochim. Acta 2013, 555, 46–52. [Google Scholar] [CrossRef]
- Huang, Y.X.; Ma, E.N.; Zhao, G.J. Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers. Ind. Crops Prod. 2015, 69, 447–455. [Google Scholar] [CrossRef]
- Dominguez, J.D.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodriguez, F. Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to commercial resol resin. Ind. Crops Prod. 2013, 42, 308–314. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137. [Google Scholar] [CrossRef] [PubMed]

| Resin | Resin Properties | Composites Performances | ||||||
|---|---|---|---|---|---|---|---|---|
| Viscosiy (mPa·s) | Solid Content (%) | Gel Time (min) | Free Formaldehyde (%) | Free Phenol (%) | Oxygen Index (%) | Bending Strength (MPa) | MOE 1 (MPa) | |
| PF | 920 | 79.1 | 16 | 1.8 | 2.8 | 68.2 | 100.2 | 3367.8 |
| 10%-BPF | 850 | 77.8 | 22 | 1.3 | 3.1 | 85.6 | 121.4 | 4080.4 |
| 20%-BPF | 730 | 76.5 | 25 | 1.1 | 3.5 | 90.2 | 135.5 | 4554.3 |
| 30%-BPF | 590 | 74.2 | 29 | 0.9 | 3.8 | 73.5 | 92.5 | 3109.2 |
| 40%-BPF | 450 | 72.1 | 32 | 0.85 | 4.6 | 60.4 | 81.6 | 2742.7 |
| Wavenumber (cm−1) | Peak Assignment |
|---|---|
| 3650–3200 | OH stretch |
| 3100–3000 | Aromatic CH stretch |
| 3000–2850 | Aliphatic CH stretch |
| 1960–1680 | C=O stretch |
| 1650–1430 | Benzene ring stretch |
| 1450 ± 10, 1375 ± 5 | Aliphatic CH3 bend |
| 1465 ± 20 | Aliphatic CH2 bend |
| 1300–1000 | C–O stretch |
| 910–650 | Aromatic CH bend |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Chang, J.; Wang, W. Fabrication of Glass Fiber Reinforced Composites Based on Bio-Oil Phenol Formaldehyde Resin. Materials 2016, 9, 886. https://doi.org/10.3390/ma9110886
Cui Y, Chang J, Wang W. Fabrication of Glass Fiber Reinforced Composites Based on Bio-Oil Phenol Formaldehyde Resin. Materials. 2016; 9(11):886. https://doi.org/10.3390/ma9110886
Chicago/Turabian StyleCui, Yong, Jianmin Chang, and Wenliang Wang. 2016. "Fabrication of Glass Fiber Reinforced Composites Based on Bio-Oil Phenol Formaldehyde Resin" Materials 9, no. 11: 886. https://doi.org/10.3390/ma9110886





