Bone Regeneration and Remodeling within a Unidirectional Porous Hydroxyapatite Bone Substitute at a Cortical Bone Defect Site: Histological Analysis at One and Two Years after Implantation
Abstract
:1. Introduction
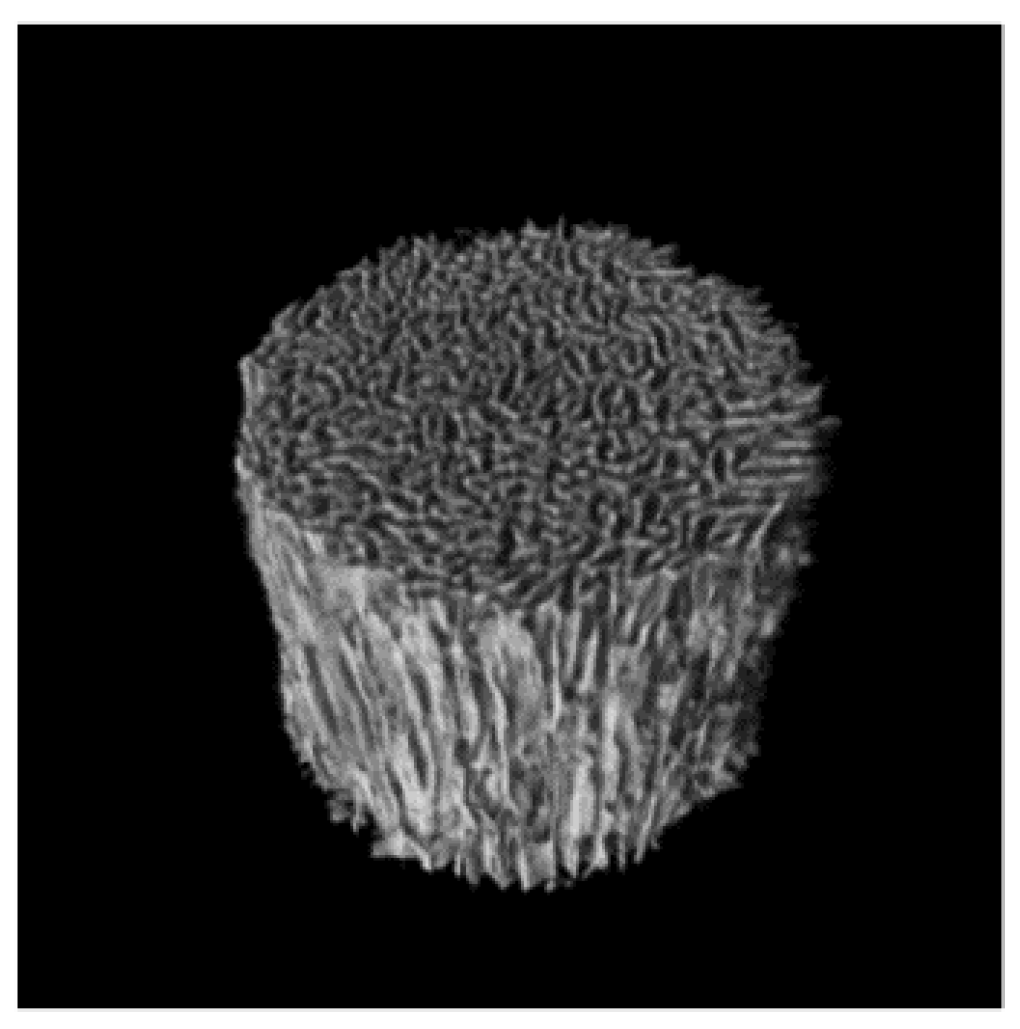
2. Results and Discussion
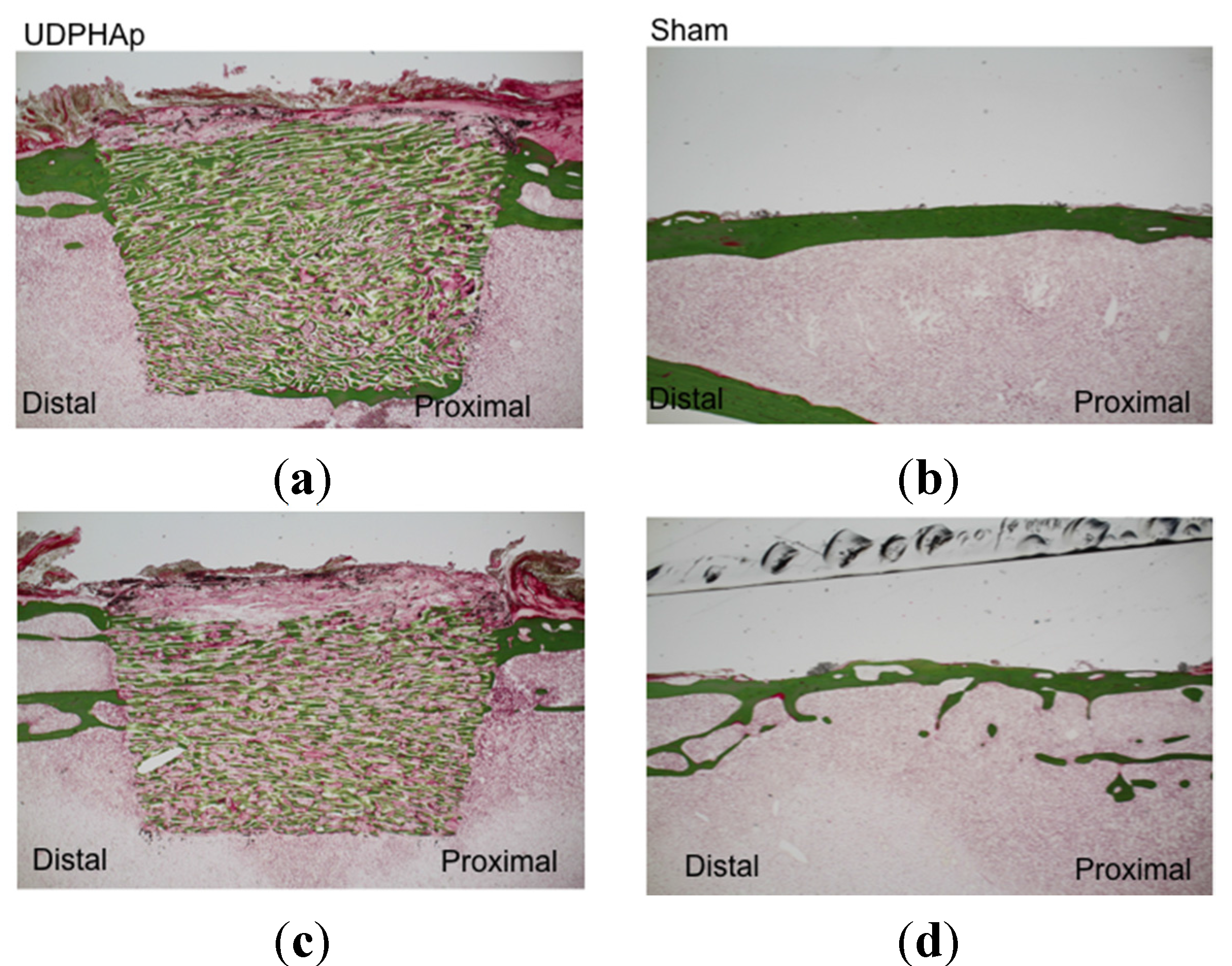
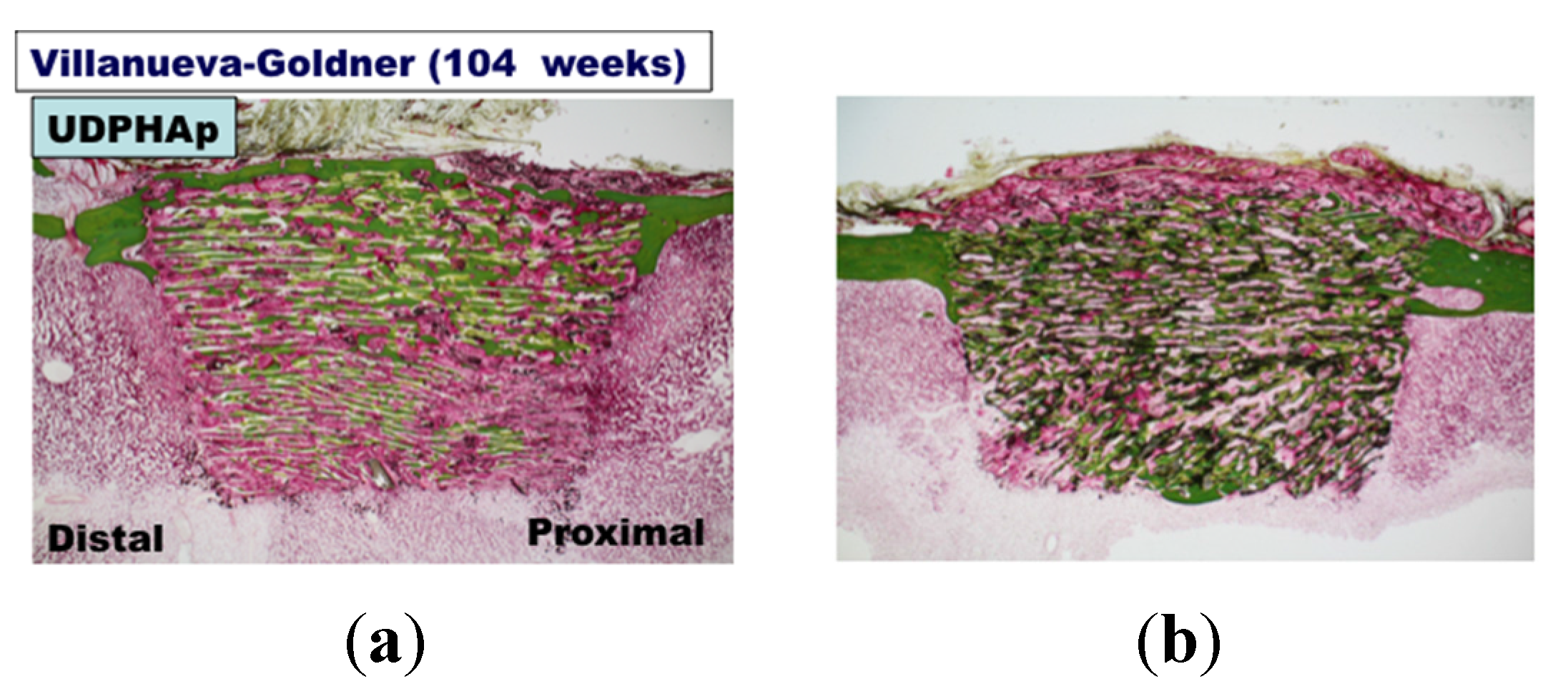
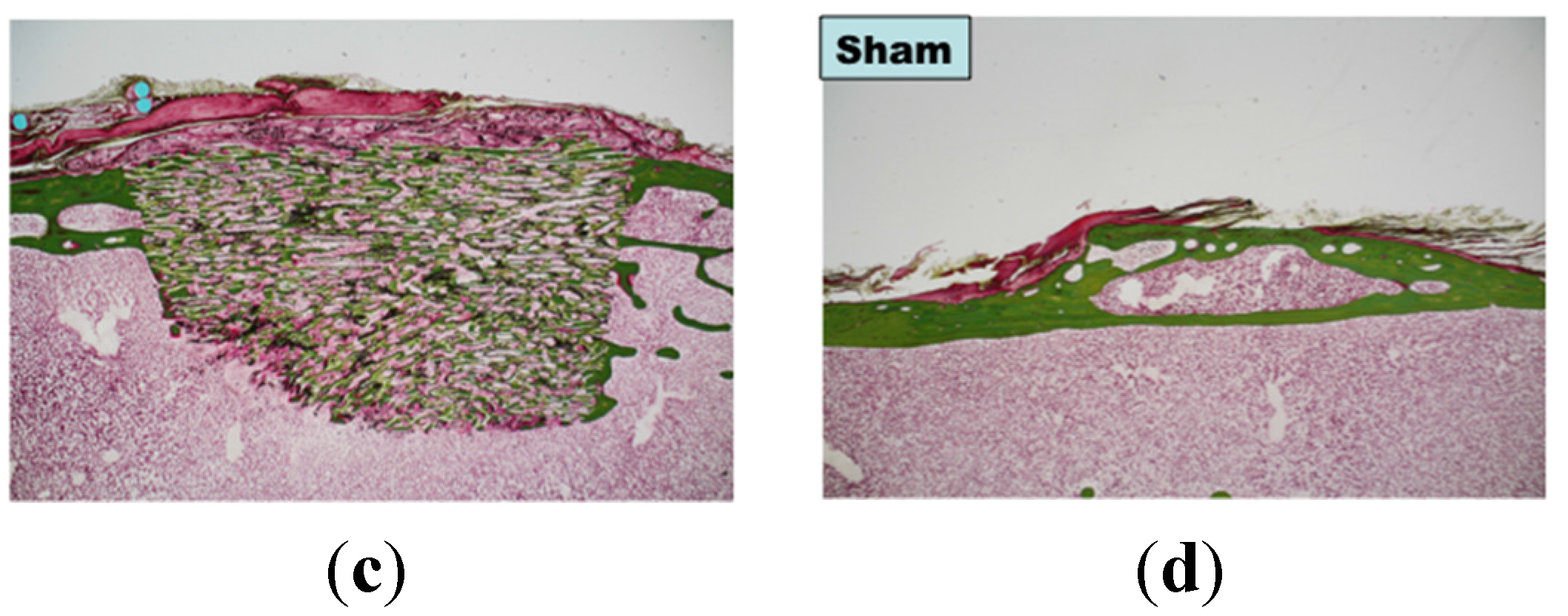
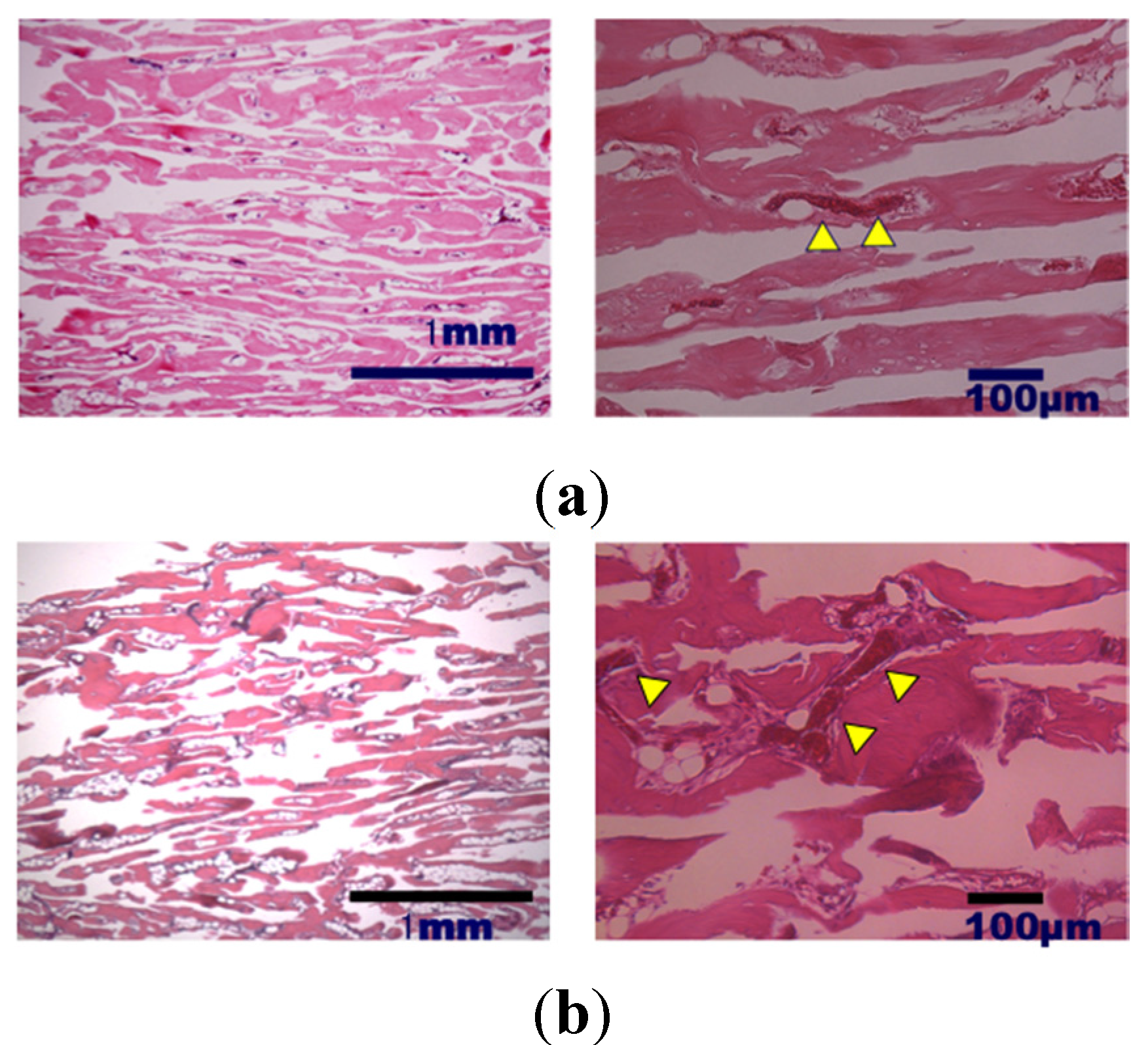

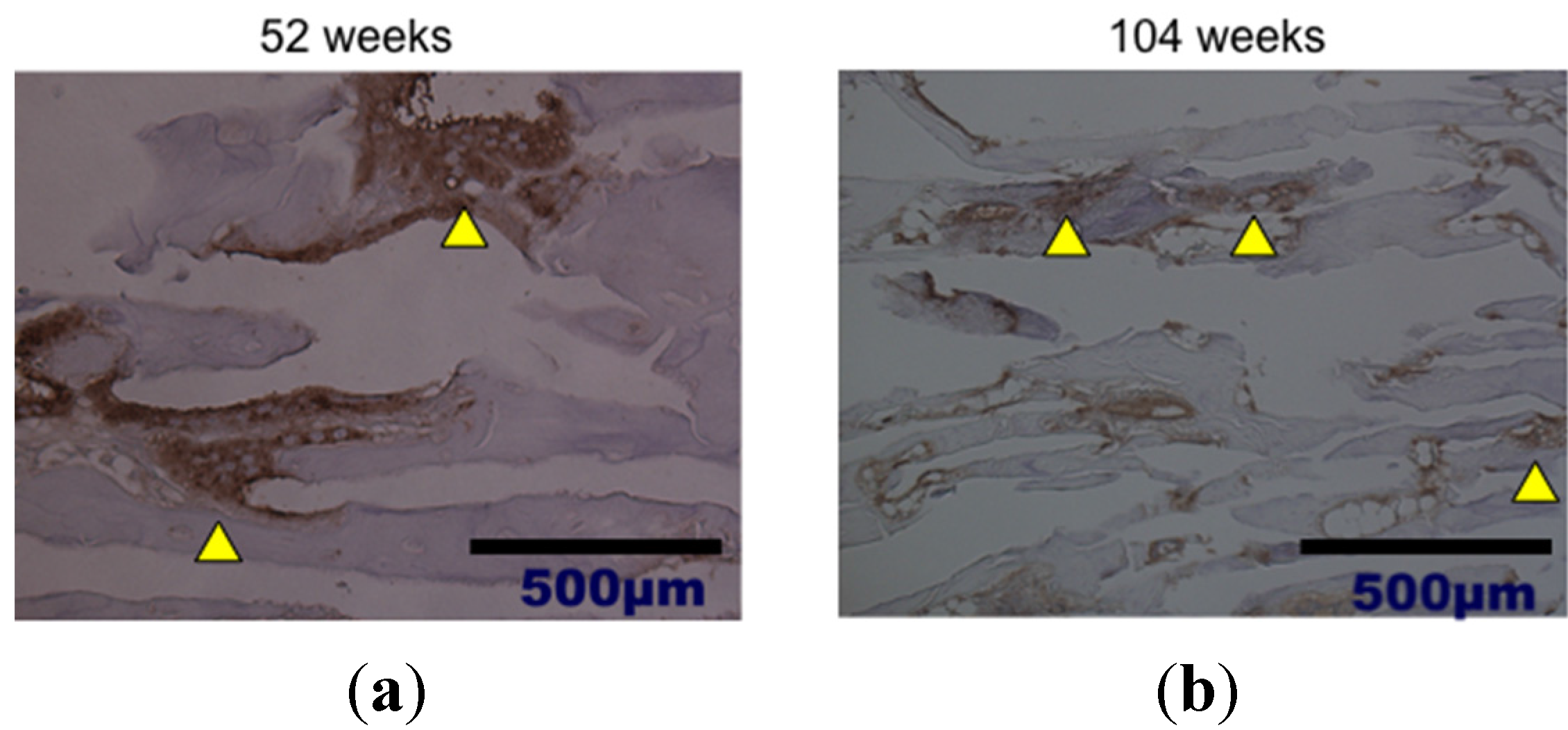
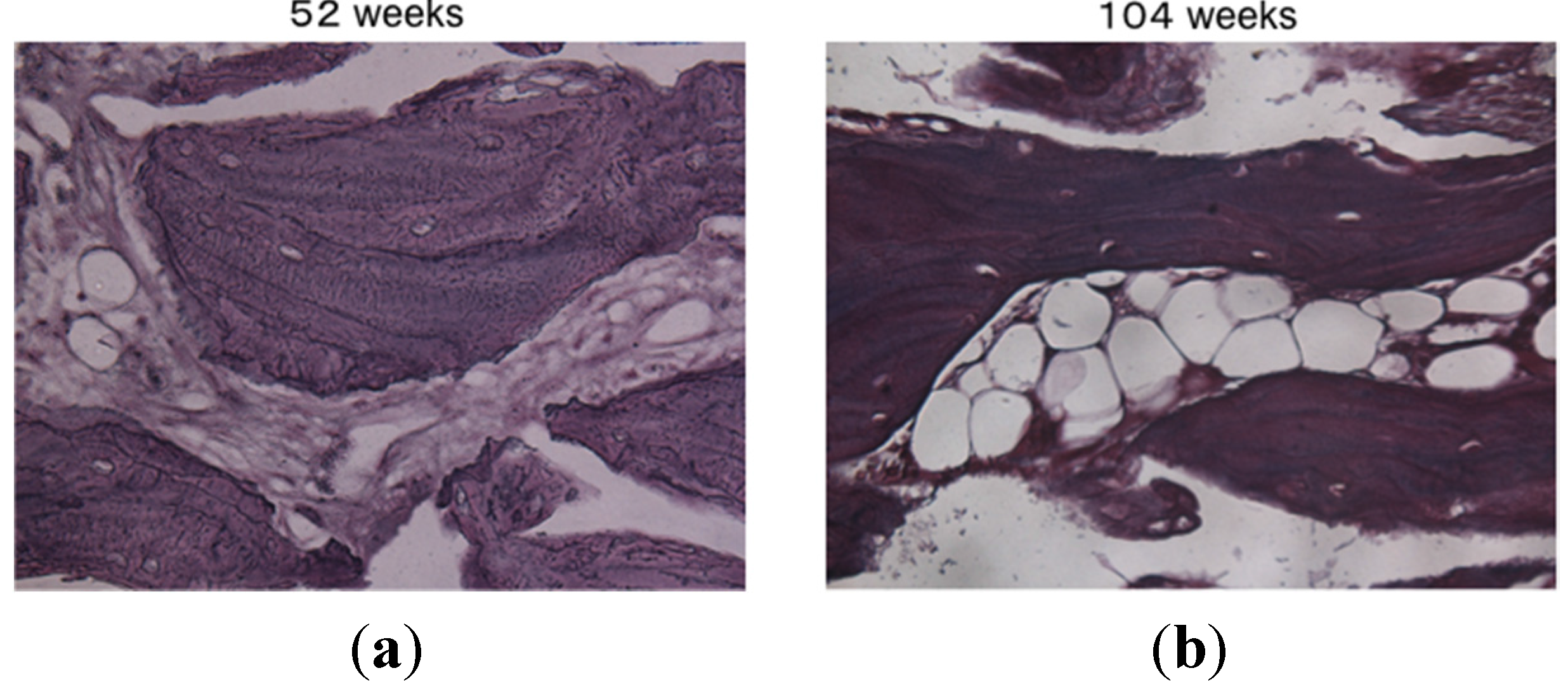
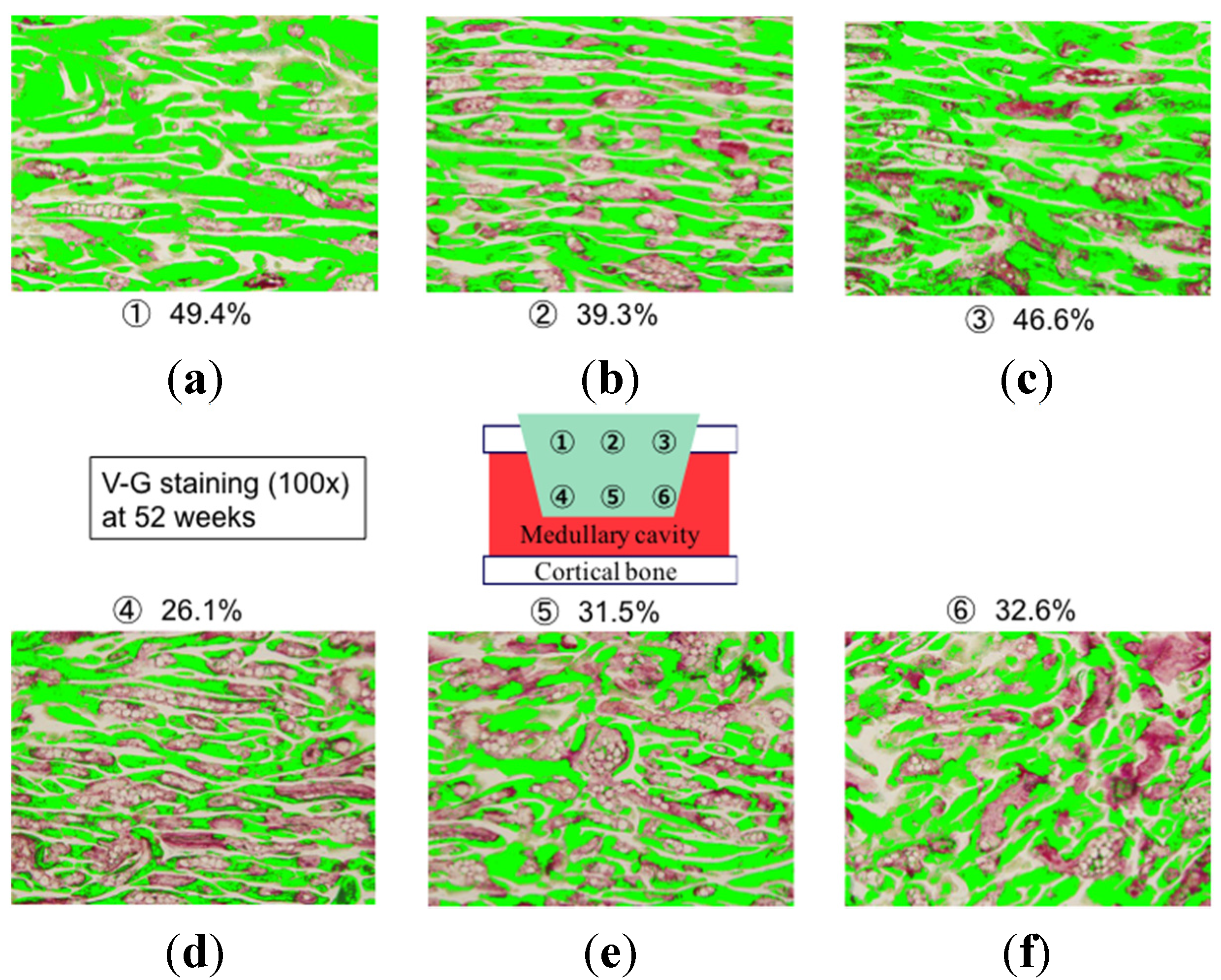
3. Experimental Section
3.1. Bone Defect Animal Model
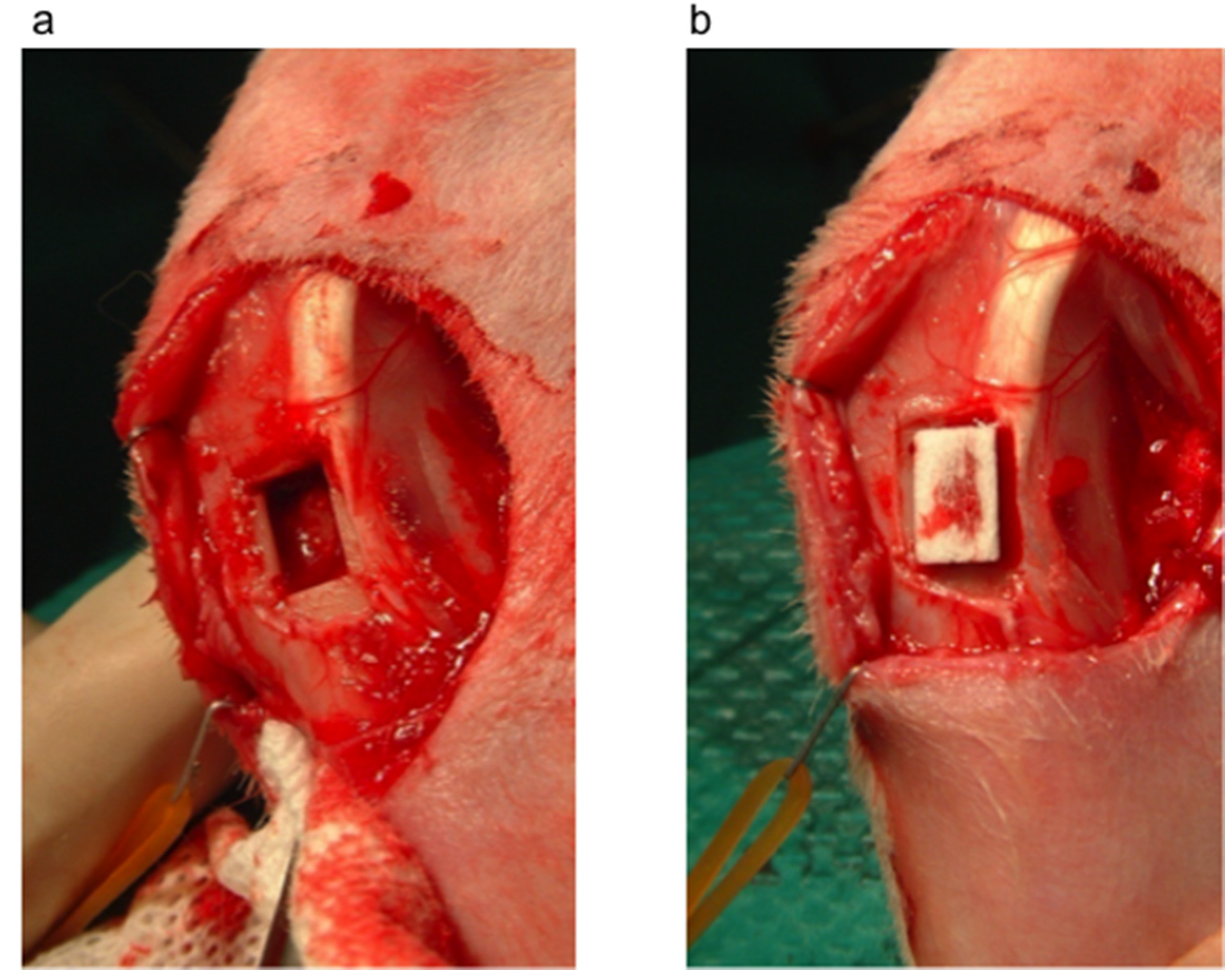
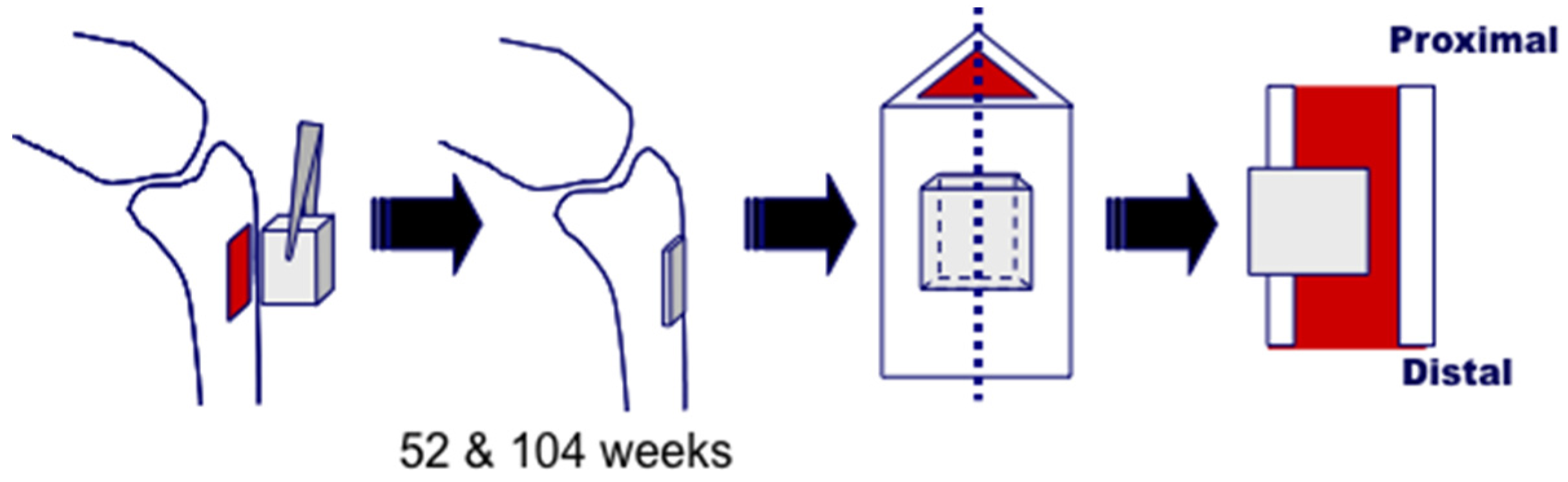
3.2. Histological Analysis
3.3. Bone Formation in Cortical and Medullary Bone Regions

3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tamai, N.; Myoui, A.; Kudawara, I.; Ueda, T.; Yoshikawa, H. Novel fully interconnected porous hydroxyapatite ceramic in surgical treatment of benign bone tumor. J. Orthop. Sci. 2010, 15, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, N.; Hirao, M.; Nanno, K.; Sugiyasu, K.; Tamai, N.; Hashimoto, N.; Yoshikawa, H.; Myoui, A. A comparative assessment of synthetic ceramic bone substitutes with different composition and microstructure in rabbit femoral condyle model. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Mota, C.; Gazzarri, M.; Dinucci, D.; Gloria, A.; Myrzabekova, M.; Ambrosio, L.; Chiellini, F. Additive manufacturing of wet-spun polymetric scaffolds for bone tissue engineering. Biomed. Microdevices 2012, 14, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; D’Antò, V.; Bollino, F.; Catauro, M.; Mollica, F.; Rengo, S.; Ambrosio, L. Advanced composites for hard-tissue engineering based on PCL/organic-inorganic hybrid fillers: From the design of 2D substrates to 3D rapid prototyped scaffolds. Polym. Compos. 2013, 34, 1413–1417. [Google Scholar] [CrossRef]
- Suetsugu, Y.; Hotta, Y.; Iwasashi, M.; Sakane, M.; Kikuchi, M.; Ikoma, T.; Higaki, T.; Ochiai, N.; Tanaka, J. Structural and tissue reaction properties of novel hydroxyapatite ceramics with unidirectional pores. Key Eng. Mater. 2007, 330–332, 1003–1006. [Google Scholar] [CrossRef]
- Iwasashi, M.; Sakane, M.; Suetsugu, Y.; Ochiai, N. Bone regeneration at cortical bone defect with unidirectional porous hydroxyapatite in vivo. Key Eng. Mater. 2009, 396–398, 11–14. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.S.; Hong, K.S.; Youn, H.J.; Ryu, H.S.; Chung, S.S.; Park, K.W. Osteoconduction at porous hydroxyapatite with various pore configurations. Biomaterials 2000, 21, 1291–1298. [Google Scholar] [CrossRef]
- Gotza, W.; Reicherta, C.; Canullob, L.; Jagera, A.; Heinemannc, F. Coupling of osteogenesis and angiogenesis in bone substitute healing—A brief overview. Ann. Anat. 2012, 194, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Watanabe, A.; Funayama, T.; Tsukanishi, T.; Wadano, Y.; Sakane, M. A novel unidirectional porous hydroxyapatite cylinder implanted in the dorsal muscles of dogs promotes fibrous tissue vascularization and invasion. Key Eng. Mater. 2013, 529–530, 275–278. [Google Scholar] [CrossRef]
- Noguchi, H.; Sakane, M.; Watanabe, A.; Tsukanishi, T.; Wadano, Y.; Yamazaki, M. A novel unidirectional porous hydroxyapatite in canines. Bioinspir. Biomim. Nanobiomat. 2014, 3, 228–234. [Google Scholar] [CrossRef]
- Matsumine, A.; Myoui, A.; Kusuzaki, K.; Araki, N.; Seto, M.; Yoshikawa, H.; Uchida, A. Calcium hydroxyapatite ceramic implants in bone tumor surgery. J. Bone Joint Surg. Br. 2004, 86, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Rampel, E.; Wolf, E.; Kauschke, E.; Biengraber, V.; Bayerlein, T.; Gerange, T.; Proff, P. The biodegradation of hydroxyapatite bone graft substitute in vivo. Folia Morphol. 2006, 65, 43–68. [Google Scholar]
- Akazawa, T.; Murata, M.; Tazaki, J.; Nakamura, K.; Hino, J.; Ito, K.; Yamamoto, M.; Tabata, Y.; Takahata, M.; Ito, M. Biomimetic microstructure and biocompatibility of functionally graded hydroxyapatite derived from animal bone by a supersonic dissolution-precipitation method. Bioceramics 2009, 22, 155–158. [Google Scholar]
- Akazawa, T.; Murata, M.; Hino, J.; Tazaki, J.; Ito, K.; Nakamura, K.; Takahata, M.; Abe, Y.; Xianjun, D.; Itoh, M.; et al. Microstructure and bio-absorption characteristics of hydroxyapatite modified by a partially supersonic dissolution-precipitation technique. Arch. BioCeram. Res. 2009, 9, 123–126. [Google Scholar]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Redini, F.; Heymann, D.; et al. Induction of osteogenesis in mesenchymal stem cells by activated monocytes/macrophages depends on oncostatin M signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolaidou, V.; Wong, M.W.; Redpath, A.N.; Ersek, A.; Baban, D.F.; Williams, L.M.; Cope, A.P.; Horwood, N.J. Monocytes induce STAT3 activation in human mesenchymal stem cells to promote osteoblast formation. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwasashi, M.; Funayama, T.; Watanabe, A.; Noguchi, H.; Tsukanishi, T.; Suetsugu, Y.; Makihara, T.; Ochiai, N.; Yamazaki, M.; Sakane, M. Bone Regeneration and Remodeling within a Unidirectional Porous Hydroxyapatite Bone Substitute at a Cortical Bone Defect Site: Histological Analysis at One and Two Years after Implantation. Materials 2015, 8, 4884-4894. https://doi.org/10.3390/ma8084884
Iwasashi M, Funayama T, Watanabe A, Noguchi H, Tsukanishi T, Suetsugu Y, Makihara T, Ochiai N, Yamazaki M, Sakane M. Bone Regeneration and Remodeling within a Unidirectional Porous Hydroxyapatite Bone Substitute at a Cortical Bone Defect Site: Histological Analysis at One and Two Years after Implantation. Materials. 2015; 8(8):4884-4894. https://doi.org/10.3390/ma8084884
Chicago/Turabian StyleIwasashi, Masashi, Toru Funayama, Arata Watanabe, Hiroshi Noguchi, Toshinori Tsukanishi, Yasushi Suetsugu, Takeshi Makihara, Naoyuki Ochiai, Masashi Yamazaki, and Masataka Sakane. 2015. "Bone Regeneration and Remodeling within a Unidirectional Porous Hydroxyapatite Bone Substitute at a Cortical Bone Defect Site: Histological Analysis at One and Two Years after Implantation" Materials 8, no. 8: 4884-4894. https://doi.org/10.3390/ma8084884




