Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements
Abstract
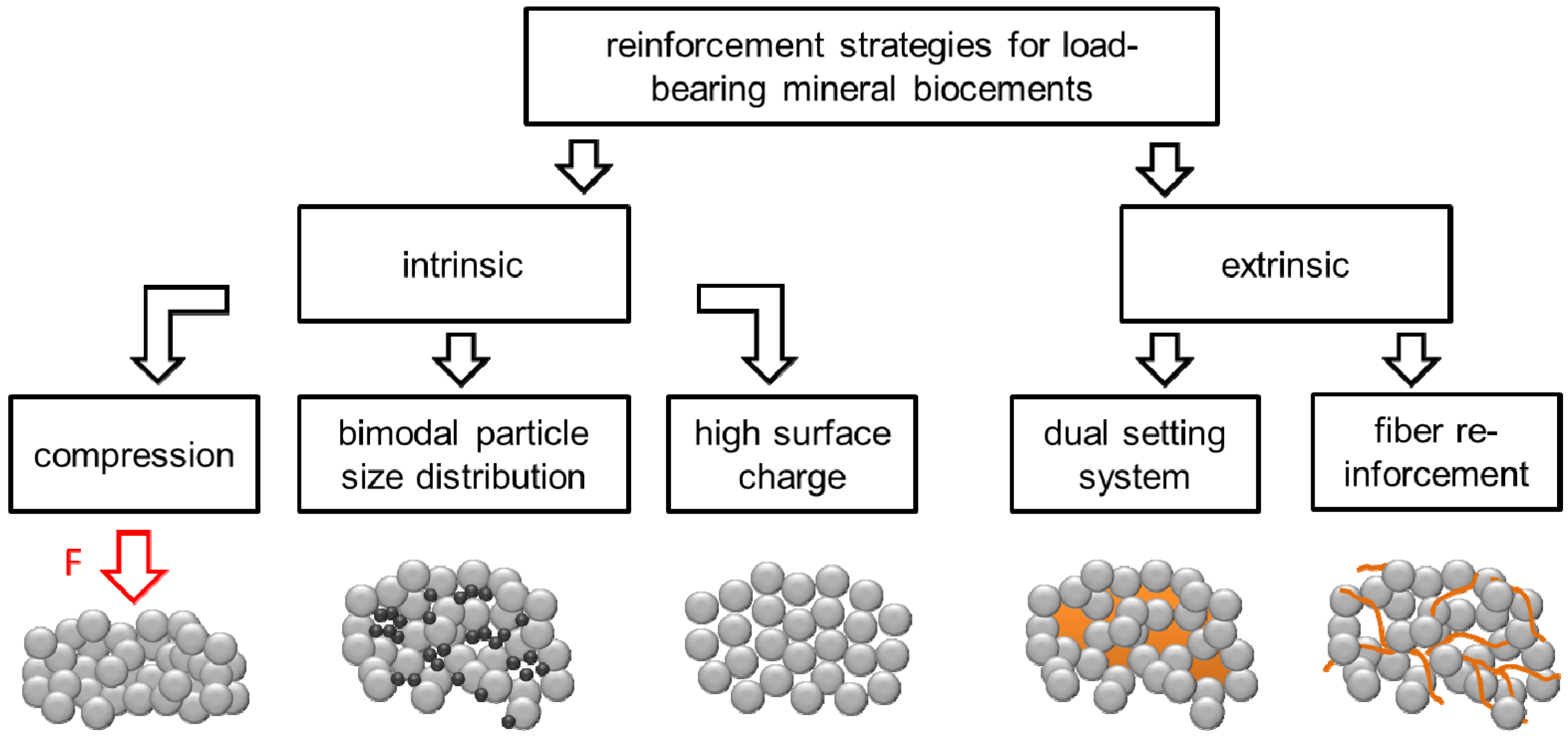
:1. Introduction
2. Porosity Reduction for Strength Improvement of CPC

3. Fiber Reinforcement of CPC

| Composition Fiber/Additive/Matrix | Fiber Volume Fraction | Strength [MPa] | Work of Fracture [kJ/m²] | Test Method | Ref. |
|---|---|---|---|---|---|
| DEGRADABLE FIBRES | |||||
| HA matrix (TTCP + DCPA (+ Na2HPO4 − solution)) | - | 10–15 | 0.032–0.05 | 3 p. b. | [76,77] |
| Polyglactin 910/-/HA (TTCP + DCPA) | 25 vol% | 17.5–25 | 2.6–3.6 | 3 p. b. | [76] |
| Polyglactin 910/-/HA (TTCP + DCPA) | Mesh multilayer | 8.5–24.5 | 0.75–3.1 | 3 p. b. | [78] |
| Polyglactin 910/chitosan lactate/HA (TTCP + DCPA) | 45 vol% | 41 | 11 | 3 p. b. | [74] |
| Polyglactin 910/chitosan lactate/HA (TTCP + DCPA) | Mesh multilayer | 43 | 9.8 | 3 p. b. | [79] |
| Polyglactin 910/(poly(caprolactone))/brushite (β-TCP + H3PO4) | 24 vol% random short 6–25 long fibers UD # | 7.5–20 | n.a. | 4 p. b. | [80] |
| NON-DEGRADABLE FIBRES | |||||
| Carbon/-/HA (TTCP + DCPA) | 2–10 vol% | 32–60 | 3.5–6.5 | 3 p. b. | [72] |
| CNT/-/HA (α-TCP + HA) | 0.2–1.0 wt% | 8.2–10.5 | n.a. | 3 p. b. | [81] |
| Aramid/-/macroporous HA (TTCP + DCPA + Na2HPO4) | 6 vol% | 7.5–13.5 | 0.8–6.5 | 3 p. b. | [75] |
| HAw/-/HA (TTCP + DCPA) | 10–40 vol% | 5.4–7.4 | 57–102 | 4 p. b. | [82] |
4. Dual Setting Cements

5. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bohner, M.; Gbureck, U.; Barralet, J.E. Technological issues for the development of more efficient calcium phosphate bone cements: A critical assessment. Biomaterials 2005, 26, 6423–6429. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Chow, L.C. Calcium phosphate cements. Monogr. Oral Sci. 2001, 18, 148–163. [Google Scholar] [PubMed]
- Nancollas, G.H.; Zawacki, S.J. Calcium phosphate mineralization. Connect. Tissue Res. 1989, 21, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Sheikh, Z.; Barralet, J. Dicalcium phosphate cements: Brushite and monetite. Acta Biomater. 2012, 8, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Sahin, E.; Ciftcioglu, M. Monetite promoting effect of NaCl on brushite cement setting kinetics. J. Mater. Chem. B 2013, 1, 2943–2950. [Google Scholar] [CrossRef]
- Bohner, M.; vanLanduyt, P.; Merkle, H.P.; Lemaitre, J. Composition effects on the pH of a hydraulic calcium phosphate cement. J. Mater. Sci. Mater. Med. 1997, 8, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M. Design of ceramic-based cements and putties for bone graft substitution. Eur. Cells Mater. 2010, 20, 1–12. [Google Scholar]
- Zhang, J.T.; Liu, W.Z.; Schnitzler, V.; Tancret, F.; Bouler, J.M. Calcium phosphate cements for bone substitution: Chemistry, handling and mechanical properties. Acta Biomater. 2014, 10, 1035–1049. [Google Scholar] [CrossRef] [PubMed]
- Constantz, B.R.; Barr, B.M.; Ison, I.C.; Fulmer, M.T.; Baker, J.; McKinney, L.A.; Goodman, S.B.; Gunasekaren, S.; Delaney, D.C.; Ross, J.; Poser, R.D. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J. Biomed. Mater. Res. 1998, 43, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Apelt, D.; Theiss, F.; El-Warrak, A.O.; Zlinszky, K.; Bettschart-Wolfisberger, R.; Bohner, M.; Matter, S.; Auer, J.A.; von Rechenberg, B. In vivo behavior of three different injectable hydraulic calcium phosphate cements. Biomaterials 2004, 25, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Tas, A.C. The use of physiological solutions or media in calcium phosphate synthesis and processing. Acta Biomater. 2014, 10, 1771–1792. [Google Scholar] [CrossRef] [PubMed]
- Grossardt, C.; Ewald, A.; Grover, L.M.; Barralet, J.E.; Gbureck, U. Passive and active in vitro resorption of calcium and magnesium phosphate cements by osteoclastic cells. Tissue Eng. A 2010, 16, 3687–3695. [Google Scholar] [CrossRef]
- Detsch, R.; Mayr, H.; Ziegler, G. Formation of osteoclast-like cells on HA and TCP ceramics. Acta Biomater. 2008, 4, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Wenisch, S.; Stahl, J.P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Gbureck, U.; Wright, A.J.; Tremayne, M.; Barralet, J.E. Biologically mediated resorption of brushite cement in vitro. Biomaterials 2006, 27, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Noth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Theiss, F.; Apelt, D.; Brand, B.A.; Kutter, A.; Zlinszky, K.; Bohner, M.; Matter, S.; Frei, C.; Auer, J.A.; von Rechenberg, B. Biocompatibility and resorption of a brushite calcium phosphate cement. Biomaterials 2005, 26, 4383–4394. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Torres, J.; Lopez-Cabarcos, E.; Bassett, D.C.; Habibovic, P.; Luceron, E.; Barralet, J.E. Minimally invasive maxillofacial vertical bone augmentation using brushite based cements. Biomaterials 2009, 30, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Penel, G.; Leroy, N.; van Landuyt, P.; Flautre, B.; Hardouin, P.; Lemaitre, J.; Leroy, G. Raman microspectrometry studies of brushite cement: In vivo evolution in a sheep model. Bone 1999, 25, 81S–84S. [Google Scholar] [CrossRef] [PubMed]
- Kuemmerle, J.M.; Oberle, A.; Oechslin, C.; Bohner, M.; Frei, C.; Boecken, I.; von Rechenberg, B. Assessment of the suitability of a new brushite calcium phosphate cement for cranioplasty—An experimental study in sheep. J. Cranio Maxillofac. Surg. 2005, 33, 37–44. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Leng, Y. Theoretical analysis of calcium phosphate precipitation in simulated body fluid. Biomaterials 2005, 26, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Klammert, U.; Ignatius, A.; Wolfram, U.; Reuther, T.; Gbureck, U. In vivo degradation of low temperature calcium and magnesium phosphate ceramics in a heterotopic model. Acta Biomater. 2011, 7, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Kanter, B.; Geffers, M.; Ignatius, A.; Gbureck, U. Control of in vivo mineral bone cement degradation. Acta Biomater. 2014, 10, 3279–3287. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Theiss, F.; Apelt, D.; Hirsiger, W.; Houriet, R.; Rizzoli, G.; Gnos, E.; Frei, C.; Auer, J.A.; von Rechenberg, B. Compositional changes of a dicalcium phosphate dihydrate cement after implantation in sheep. Biomaterials 2003, 24, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Gbureck, U.; Spatz, K.; Thull, R.; Barralet, J.E. Rheological enhancement of mechanically activated alpha-tricalcium phosphate cements. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Charriere, E.; Terrazzoni, S.; Pittet, C.; Mordasini, P.; Dutoit, M.; Lemaitre, J.; Zysset, P. Mechanical characterization of brushite and hydroxyapatite cements. Biomaterials 2001, 22, 2937–2945. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate-based biocomposites and hybrid biomaterials. J. Mater. Sci. 2009, 44, 2343–2387. [Google Scholar] [CrossRef]
- Von Gonten, A.S.; Kelly, J.R.; Antonucci, J.M. Load-bearing behavior of a simulated craniofacial structure fabricated from a hydroxyapatite cement and bioresorbable fiber-mesh. J. Mater. Sci. Mater. Med. 2000, 11, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Blattert, T.R.; Jestaedt, L.; Weckbach, A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation a controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine 2009, 34, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Maestretti, G.; Cremer, C.; Otten, P.; Jakob, R.P. Prospective study of standalone balloon kyphoplasty with calcium phosphate cement augmentation in traumatic fractures. Eur. Spine J. 2007, 16, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Tarsuslugil, S.M.; O’Hara, R.M.; Dunne, N.J.; Buchanan, F.J.; Orr, J.F.; Barton, D.C.; Wilcox, R.K. Development of calcium phosphate cement for the augmentation of traumatically fractured porcine specimens using vertebroplasty. J. Biomech. 2013, 46, 711–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heini, P.F. Vertebroplastie: Ein Update. Orthopäde 2010, 39, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Grafe, I.A.; Baier, M.; Noldge, G.; Weiss, C.; da Fonseca, K.; Hillmeier, J.; Libicher, M.; Rudofsky, G.; Metzner, C.; Nawroth, P.; et al. Calcium-phosphate and polymethylmethacrylate cement in long-term outcome after kyphoplasty of painful osteoporotic vertebral fractures. Spine 2008, 33, 1284–1290. [Google Scholar] [CrossRef] [PubMed]
- Kiyasu, K.; Takemasa, R.; Ikeuchi, M.; Tani, T. Differential blood contamination levels and powder–liquid ratios can affect the compressive strength of calcium phosphate cement (CPC): A study using a transpedicular vertebroplasty model. Eur. Spine J. 2013, 22, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Weir, M.D.; Xu, H.H.K. Self-setting collagen-calcium phosphate bone cement: Mechanical and cellular properties. J. Biomed. Mater. Res. A 2009, 91, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, W.; Reinstorf, A.; Biewener, A.; Serra, A.; Grass, R.; Kinscher, M.; Heineck, J.; Rehberg, S.; Zwipp, H.; Rammelt, S. In Vivo Effects of modification of hydroxyapatite/collagen composites with and without chondroitin sulphate on bone remodeling in the sheep tibia. J. Orthop. Res. 2009, 27, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tamimi, F.; Kumarasami, B.; Doillon, C.; Gbureck, U.; le Nihouannen, D.; Cabarcos, E.L.; Barralet, J.E. Brushite-collagen composites for bone regeneration. Acta Biomater. 2008, 4, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, R.M.; Orr, J.F.; Buchanan, F.J.; Wilcox, R.K.; Barton, D.C.; Dunne, N.J. Development of a bovine collagen-apatitic calcium phosphate cement for potential fracture treatment through vertebroplasty. Acta Biomater. 2012, 8, 4043–4052. [Google Scholar] [CrossRef] [PubMed]
- Canal, C.; Ginebra, M.P. Fibre-reinforced calcium phosphate cements: A review. J. Mechan. Behav. Biomed. Mater. 2011, 4, 1658–1671. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; de Oliveira, L.C.; Rigo, E.C.D.; Carrodeguas, R.G.; Boschi, A.O.; de Arruda, A.C.F. Fiber reinforced calcium phosphate cement. Artif. Org. 2000, 24, 212–216. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Carrodeguas, R.G.; Boschi, A.O.; de Arruda, A.C.F. Fiber-enriched double-setting calcium phosphate bone cement. J. Biomed. Mater. Res. A 2003, 65, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.A.; Carrodeguas, R.G.; Boschi, A.O.; de Arruda, A.C.F. Dual-setting calcium phosphate cement modified with ammonium polyacrylate. Artif. Org. 2003, 27, 412–418. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.S.; Liu, Y.F.; Zhang, S. Double-network interpenetrating bone cement via in situ hybridization protocol. Adv. Funct. Mater. 2010, 20, 3997–4011. [Google Scholar] [CrossRef]
- Barralet, J.E.; Gaunt, T.; Wright, A.J.; Gibson, I.R.; Knowles, J.C. Effect of porosity reduction by compaction on compressive strength and microstructure of calcium phosphate cement. J. Biomed. Mater. Res. 2002, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Espanol, M.; Perez, R.A.; Montufar, E.B.; Marichal, C.; Sacco, A.; Ginebra, M.P. Intrinsic porosity of calcium phosphate cements and its significance for drug delivery and tissue engineering applications. Acta Biomater. 2009, 5, 2752–2762. [Google Scholar] [CrossRef] [PubMed]
- Geffers, M.; Barralet, J.E.; Groll, J.; Gbureck, U. Dual-setting brushite-silica gel cements. Acta Biomater. 2015, 11, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Engstrand, J.; Persson, C.; Engqvist, H. The effect of composition on mechanical properties of brushite cements. J. Mech. Behav. Biomed. Mater. 2014, 29, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, M.P.; Mohammed, A.R.; Perrie, Y.; Gbureck, U.; Barralet, J.E. High-strength resorbable brushite bone cement with controlled drug-releasing capabilities. Acta Biomater. 2009, 5, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Knowles, J.C.; Fleming, G.J.P.; Barralet, J.E. In vitro ageing of brushite calcium phosphate cement. Biomaterials 2003, 24, 4133–4141. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Delgado, J.A.; Harr, I.; Almirall, A.; del Valle, S.; Planell, J.A. Factors affecting the structure and properties of an injectable self-setting calcium phosphate foam. J. Biomed. Mater. Res. A 2007, 80, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Burguera, E.F.; Guitian, F.; Chow, L.C. A water setting tetracalcium phosphate-dicalcium phosphate dihydrate cement. J. Biomed. Mater. Res. A 2004, 71, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Unosson, J.E.; Persson, C.; Engqvist, H. An evaluation of methods to determine the porosity of calcium phosphate cements. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 103, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Bohner, M.; Baroud, G. Injectability of calcium phosphate pastes. Biomaterials 2005, 26, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.E.; Tremayne, M.; Lilley, K.J.; Gbureck, U. Modification of calcium phosphate cement with alpha-hydroxy acids and their salts. Chem. Mater. 2005, 17, 1313–1319. [Google Scholar] [CrossRef]
- Gu, T.; Shi, H.; Ye, J. Reinforcement of calcium phosphate cement by incorporating with high-strength ß-tricalcium phosphate aggregates. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Del Real, R.P.; Wolke, J.G.C.; Vallet-Regi, M.; Jansen, J.A. A new method to produce macropores in calcium phosphate cements. Biomaterials 2002, 23, 3673–3680. [Google Scholar] [CrossRef] [PubMed]
- Vorndran, E.; Geffers, M.; Ewald, A.; Lemm, M.; Nies, B.; Gbureck, U. Ready-to-use injectable calcium phosphate bone cement paste as drug carrier. Acta Biomater. 2013, 9, 9558–9567. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Sariibrahimoglu, K.; Yang, W.; Bohner, M.; Yamashita, D.; Kunstar, A.; van Apeldoorn, A.A.; Bronkhorst, E.M.; Lanao, R.P.F.; Leeuwenburgh, S.C.G.; et al. Influence of the pore generator on the evolution of the mechanical properties and the porosity and interconnectivity of a calcium phosphate cement. Acta Biomater. 2012, 8, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Barralet, J.E.; Hofmann, M.; Grover, L.M.; Gbureck, U. High-strength apatitic cement by modification with alpha-hydroxy acid salts. Adv. Mater. 2003, 15, 2091–2094. [Google Scholar] [CrossRef]
- Cama, G.; Barberis, F.; Botter, R.; Cirillo, P.; Capurro, M.; Quarto, R.; Scaglione, S.; Finocchio, E.; Mussi, V.; Valbusa, U. Preparation and properties of macroporous brushite bone cements. Acta Biomater. 2009, 5, 2161–2168. [Google Scholar] [CrossRef] [PubMed]
- Unosson, J.E.; Montufar, E.B.; Engqvist, H.; Ginebra, M.P.; Persson, C. Brushite foams—The effect of Tween 80 and Pluronic F-127 on foam porosity and mechanical properties. J. Biomed. Res. B Appl. Biomater. 2015. [Google Scholar] [CrossRef] [Green Version]
- Barralet, J.E.; Grover, L.M.; Gbureck, U. Ionic modification of calcium phosphate cement viscosity. Part II: hypodermic injection and strength improvement of brushite cement. Biomaterials 2004, 25, 2197–2203. [Google Scholar] [CrossRef] [PubMed]
- Gravius, S.; Wirtz, D.C.; Marx, R.; Maus, U.; Andereya, S.; Mueller-Rath, R.; Mumme, T. Mechanical in vitro testing of fifteen commercial bone cements based on polymethylmethacrylate. Z. Orthop. Unfall. 2007, 145, 579–585. [Google Scholar] [PubMed]
- Chow, L.C.; Hirayama, S.; Takagi, S.; Parry, E. Diametral tensile strength and compressive strength of a calcium phosphate cement: Effect of applied pressure. J. Biomed. Mater. Res. 2000, 53, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Asaoka, K. Estimation of ideal mechanical strength and critical porosity of calcium-phosphate cement. J. Biomed. Mater. Res. 1995, 29, 1537–1543. [Google Scholar] [CrossRef] [PubMed]
- Dewith, G.; Corbijn, A.J. Metal fiber reinforced hydroxy-apatite ceramics. J. Mater. Sci. 1989, 24, 3411–3415. [Google Scholar] [CrossRef]
- Krueger, R.; Groll, J. Fiber reinforced calcium phosphate cements—On the way to degradable load bearing bone substitutes? Biomaterials 2012, 33, 5887–5900. [Google Scholar] [CrossRef] [PubMed]
- Rösler, J.; Harders, H.; Bäker, M. Mechanisches Verhalten der Werkstoffe, 2nd ed.; Teubner Verlag: Wiesbaden, Germany, 2008. [Google Scholar]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Brandt, A.M. Cement-Based Composites—Materials, Mechanical Properties and Performance, 2nd ed.; Taylor & Francis: Abingdon, UK, 2009. [Google Scholar]
- Xu, H.H.K.; Eichmiller, F.C.; Barndt, P.R. Effects of fiber length and volume fraction on the reinforcement of calcium phosphate cement. J. Mater. Sci. Mater. Med. 2001, 12, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Eichmiller, F.C.; Giuseppetti, A.A. Reinforcement of a self-setting calcium phosphate cement with different fibers. J. Biomed. Mater. Res. 2000, 52, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, H.H.K. Effects of synergistic reinforcement and absorbable fiber strength on hydroxyapatite bone cement. J. Biomed. Mater. Res. A 2005, 75, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Quinn, J.B.; Takagi, S.; Chow, L.C.; Eichmiller, F.C. Strong and macroporous calcium phosphate cement: Effects of porosity and fiber reinforcement on mechanical properties. J. Biomed. Mater. Res. 2001, 57, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Quinn, J.B. Calcium phosphate cement containing resorbable fibers for short-term reinforcement and macroporosity. Biomaterials 2002, 23, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Simon, C.G. Self-hardening calcium phosphate cement-mesh composite: Reinforcement, macropores, and cell response. J. Biomed. Mater. Res. A 2004, 69, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Quinn, J.B.; Takagi, S.; Chow, L.C. Synergistic reinforcement of in situ hardening calcium phosphate composite scaffold for bone tissue engineering. Biomaterials 2004, 25, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Gorst, N.J.S.; Perrie, Y.; Gbureck, U.; Hutton, A.L.; Hofmann, M.P.; Grover, L.M.; Barralet, J.E. Effects of fibre reinforcement on the mechanical properties of brushite cement. Acta Biomater. 2006, 2, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Sun, K.; Zhao, T.; Ren, X. Effect of CNTs on property of calcium phosphate cement. Key Eng. Mater. 2007, 336–338, 1606–1608. [Google Scholar]
- Muller, F.A.; Gbureck, U.; Kasuga, T.; Mizutani, Y.; Barralet, J.E.; Lohbauer, U. Whisker-reinforced calcium phosphate cements. J. Am. Ceram. Soc. 2007, 90, 3694–3697. [Google Scholar] [CrossRef]
- Maenz, S.; Kunisch, E.; Muehlstaedt, M.; Boehm, A.; Kopsch, V.; Bossert, J.; Kinne, R.W.; Jandt, K.D. Enhanced mechanical properties of a novel, injectable, fiber-reinforced brushite cement. J. Mech. Behav. Biomed. Mater. 2014, 39, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.D.; Xu, H.H.K.; Simon, C.G. Strong calcium phosphate cement-chitosan-mesh construct containing cell-encapsulating hydrogel beads for bone tissue engineering. J. Biomed. Mater. Res. A 2006, 77, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.; Seitz, J.-M.; Ewald, A.; Bach, F.-W.; Groll, J. Strong and tough magnesium wire reinforced phosphate cement composites for load-bearing bone replacement. J. Mech. Behav. Biomed. Mater. 2013, 20, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, I.; Driessens, F.C.M.; Boltong, M.G.; Planell, J.A.; Wenz, R. Addition of cohesion promoters to calcium phosphate cements. Biomaterials 1999, 20, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Alkhraisat, M.H.; Rueda, C.; Marino, F.T.; Torres, J.; Jerez, L.B.; Gbureck, U.; Cabarcos, E.L. The effect of hyaluronic acid on brushite cement cohesion. Acta Biomater. 2009, 5, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Ju, C.-P.; Wang, J.-C.; Hung, C.-C.; Lin, J.-H.C. Brittle and ductile adjustable cement derived from calcium phosphate cement/polyacrylic acid composites. Dent. Mater. 2008, 24, 1616–1622. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.E.; Tenhuisen, K.S.; Brown, P.W. The formation of hydroxyapatite-calcium polyacrylate composites. J. Mater. Sci. Mater. Med. 1999, 10, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Khashaba, R.M.; Moussa, M.; Koch, C.; Jurgensen, A.R.; Missimer, D.M.; Rutherford, R.L.; Chutkan, N.B.; Borke, J.L. Preparation, physical-chemical characterization, and cytocompatibility of polymeric calcium phosphate cements. Int. J. Biomater. 2011, 2011, 467641. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, A.O.; Deb, S.; Nicholson, J.W. Effect of molecular weight and concentration of poly(acrylic acid) on the formation of a polymeric calcium phosphate cement. J. Mater. Sci. Mater. Med. 2003, 14, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Majekodunmi, A.O.; Deb, S. Poly(acrylic acid) modified calcium phosphate cements: The effect of the composition of the cement powder and of the molecular weight and concentration of the polymeric acid. J. Mater. Sci. Mater. Med. 2007, 18, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Matsuya, Y.; Antonucci, J.M.; Matsuya, S.; Takagi, S.; Chow, L.C. Polymeric calcium phosphate cements derived from poly(methyl vinyl ether-maleic acid). Dent. Mater. 1996, 12, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Greish, Y.E.; Brown, P.W.; Bender, J.D.; Allcock, H.R.; Lakshmi, S.; Laurencin, C.T. Hydroxyapatite-polyphosphazane composites prepared at low temperatures. J. Am. Ceram. Soc. 2007, 90, 2728–2734. [Google Scholar] [CrossRef]
- Greish, Y.E.; Brown, P.W. Chemically formed HAp-Ca poly(vinyl phosphonate) composites. Biomaterials 2001, 22, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Antonucci, J.M.; Takagi, S.; Chow, L.C.; Ohashi, M. Formation of hydroxyapatite in hydrogels from tetracalcium phosphate/dicalcium phosphate mixtures. J. Nihon Univ. Sch. Dent. 1989, 31, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Rigo, E.C.S.; dos Santos, L.A.; Vercik, L.C.O.; Carrodeguas, R.G.; Boschi, A.O. alpha-tricalcium phosphate- and tetracalcium phosphate/dicalcium phosphate-based dual setting cements. Lat. Am. Appl. Res. 2007, 37, 267–274. [Google Scholar]
- Christel, T.; Kuhlmann, M.; Vorndran, E.; Groll, J.; Gbureck, U. Dual setting alpha-tricalcium phosphate cements. J. Mater. Sci. Mater. Med. 2013, 24, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Luchini, T.J.F.; Agarwal, A.K.; Goel, V.K.; Bhaduri, S.B. Development of monetite-nanosilica bone cement: A preliminary study. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.; Lee, J.Y.; Seol, D.-W.; Pyo, S.G.; Lee, D. The effect of calcium phosphate cement-silica composite materials on proliferation and differentiation of pre-osteoblast cells. Mater. Lett. 2013, 109, 302–305. [Google Scholar] [CrossRef]
- Van den Vreken, N.M.F.; de Canck, E.; Ide, M.; Lamote, K.; van der Voort, P.; Verbeeck, R.M.H. Calcium phosphate cements modified with pore expanded SBA-15 materials. J. Mater. Chem. 2012, 22, 14502–14509. [Google Scholar] [CrossRef]
- Hesaraki, S.; Alizadeh, M.; Borhan, S.; Pourbaghi-Masouleh, M. Polymerizable nanoparticulate silica-reinforced calcium phosphate bone cement. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Hamdan Alkhraisat, M.; Rueda, C.; Blanco Jerez, L.; Marino, F.T.; Torres, J.; Gbureck, U.; Lopez Cabarcos, E. Effect of silica gel on the cohesion, properties and biological performance of brushite cement. Acta Biomater. 2010, 6, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Andersson, J.; Areva, S.; Spliethoff, B.; Linden, M. Sol-gel synthesis of a multifunctional, hierarchically porous silica/apatite composite. Biomaterials 2005, 26, 6827–6835. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, S.; Heinemann, C.; Bernhardt, R.; Reinstorf, A.; Nies, B.; Meyer, M.; Worch, H.; Hanke, T. Bioactive silica-collagen composite xerogels modified by calcium phosphate phases with adjustable mechanical properties for bone replacement. Acta Biomater. 2009, 5, 1979–1990. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.; Souza, K.C.; Sousa, E.M.B. Mesoporous silica/apatite nanocomposite: Special synthesis route to control local drug delivery. Acta Biomater. 2008, 4, 671–679. [Google Scholar] [CrossRef] [PubMed]
- The International Organization for Standardization (ISO). International Standard ISO 5833 Implants for Surgery—Acrylic Resin Cements; ISO: Geneva, Switzerland, 2002. [Google Scholar]
- Wilke, H.-J.; Mehnert, U.; Claes, L.E.; Bierschneider, M.M.; Jaksche, H.; Boszczyk, B.M. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethyl methacrylate or calcium phosphate cement under cyclic loading. Spine 2006, 31, 2934–2941. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.; Schwardt, J.D.; Slater, T.A.; Janna, S. Evaluation of a synthetic vertebral body augmentation model for rapid and reliable cyclic compression life testing of materials for balloon kyphoplasty. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 179–188. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geffers, M.; Groll, J.; Gbureck, U. Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements. Materials 2015, 8, 2700-2717. https://doi.org/10.3390/ma8052700
Geffers M, Groll J, Gbureck U. Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements. Materials. 2015; 8(5):2700-2717. https://doi.org/10.3390/ma8052700
Chicago/Turabian StyleGeffers, Martha, Jürgen Groll, and Uwe Gbureck. 2015. "Reinforcement Strategies for Load-Bearing Calcium Phosphate Biocements" Materials 8, no. 5: 2700-2717. https://doi.org/10.3390/ma8052700






