High Throughput Screening of Valganciclovir in Acidic Microenvironments of Polyester Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Casting of PLGA/PCL Thin Films
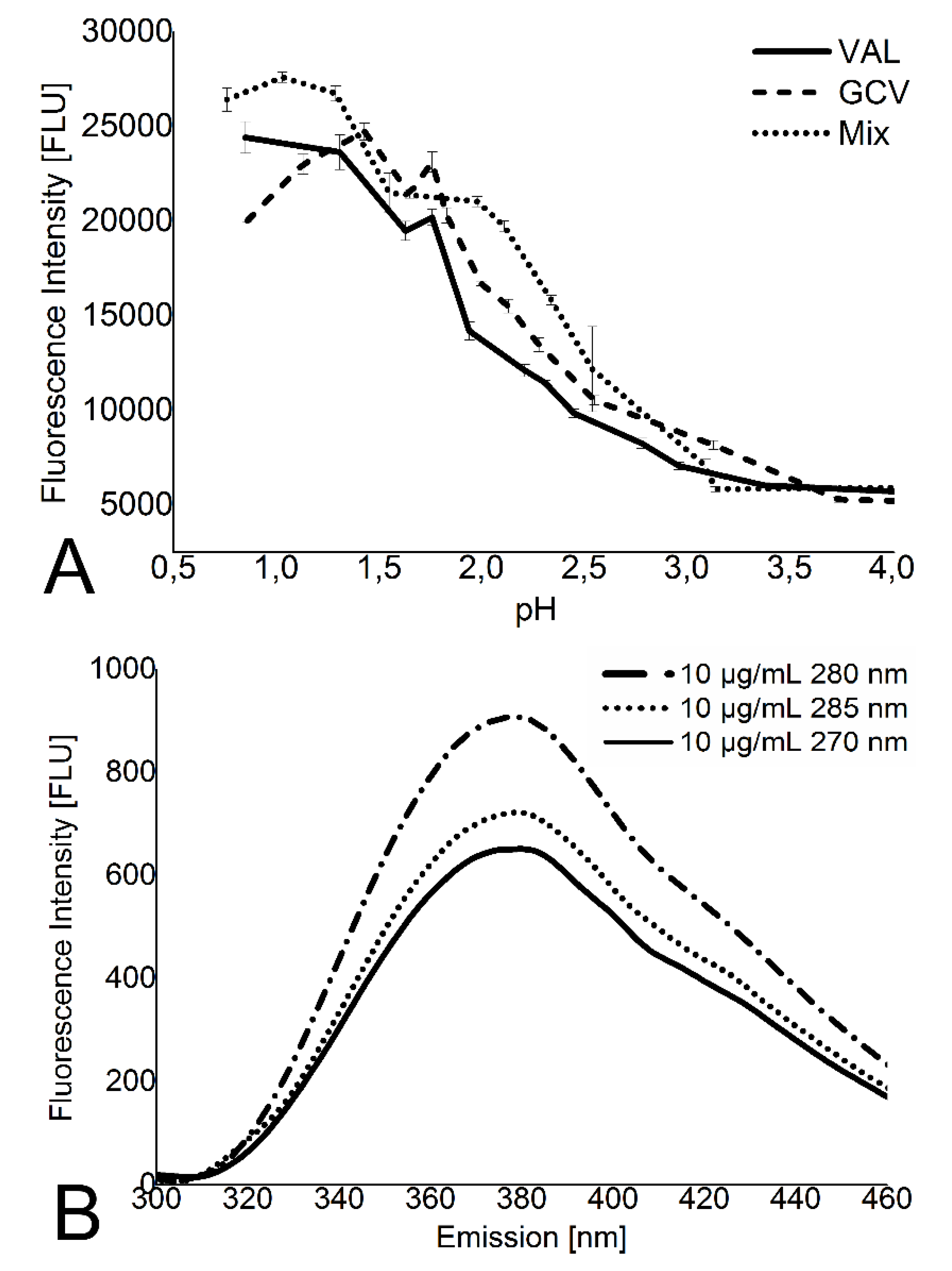
2.3. Spectrofluorometer Measurements of Valganciclovir
2.4. Polymer, Valganciclovir, and Thickness Quantification by 1H-NMR
2.5. Valganciclovir Conversion to Ganciclovir Inside of Thin Film by HPLC Quantification
2.6. Statistics Method Analysis
3. Results
3.1. High-Throughput Screening Leads to 50x Faster Development
| Thin Film | Valganciclovir (µg/cm2) | Valganciclovir added (%) | PLGA (µg/cm2) | PCL (µg/cm2) | PLGA:PCL | NMR estimated * thickness (µm) |
|---|---|---|---|---|---|---|
| PLGA | 106 ± 4 | 2.8 ± 0.1 | 3900 ± 130 | 0.0 ± 0.0 | N/A | 29.4 ± 1.0 |
| 1:4 PLGA: PCL | 805 ± 27 | 9.9 ± 0.3 | 1540 ± 50 | 7260 ± 250 | 1:4.71 | 79.1 ± 2.7 |
| 1:10 PLGA: PCL | 352 ± 12 | 7.1 ± 0.2 | 506 ± 17 | 4800 ± 160 | 1:9.54 | 47.8 ± 1.6 |
| PCL | 411 ± 14 | 12.6 ± 0.4 | 196 ± 7 | 3500 ± 120 | N/A | 34.2 ± 1.2 |
3.2. Valganciclovir Proves to be Stable within Polyester Films

3.3. Pure Films Allow Short and Long Term Valganciclovir Drug Delivery, but Polymer Phase Separation Prevents Tuning of Drug Release



4. Discussion
4.1. High-Throughput Screening Shows Significant Improvement Compared to HPLC
4.2. Acidic Microenvironment of Polyester Films Protects Valganciclovir from Physiological pH Degradation
4.3. Pure Films Allow Short and Long Term Valganciclovir Drug Delivery, but Polymer Phase Separation Prevents Tuning of Drug Release
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tseng, A.; Foisy, M. The role of ganciclovir for the management of cytomegalovirus retinitis in HIV patients: Pharmacological review and update on new developments. Can. J. Infect. Dis. 1996, 7, 183–194. [Google Scholar] [PubMed]
- Stefanidis, D.; Brandl, M. Reactivity of valganciclovir in aqueous solution. Drug Dev. Ind. Pharm. 2005, 31, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Alcami, A.; Koszinowski, U.H. Viral mechanisms of immune evasion. Immunol. Today 2000, 21, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Morlet, N.; Young, S.; Naidoo, D.; Graham, G.; Coroneo, M.T. High dose intravitreal ganciclovir injection provides a prolonged therapeutic intraocular concentration. Br. J. Ophthalmol. 1996, 80, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Jabs, D.A. Ocular manifestations of HIV infection. Trans. Am. Ophthalmol. Soc. 1995, 93, 623–683. [Google Scholar] [PubMed]
- Moss, P.; Rickinson, A. Cellular immunotherapy for viral infection after HSC transplantation. Nat. Rev. Immunol. 2005, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Loregian, A.; Gatti, R.; Palu, G.; de Palo, E.F. Separation methods for acyclovir and related antiviral compounds. J. Chromatogr. B 2001, 764, 289–311. [Google Scholar] [CrossRef]
- Daikos, G.L.; Pulido, J.; Kathpalia, S.B.; Jackson, G.G. Intravenous and intraocular ganciclovir for CMV retinitis in patients with AIDS or chemotherapeutic immunosuppression. Br. J. Ophthalmol. 1988, 72, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.M.; Srivastava, S.K.; Fung, A.E.; Mahmoud, T.H.; Telander, D.G.; Hariprasad, S.M.; Ober, M.D.; Mruthyunjaya, P. Bacterial contamination of needles used for intravitreal injections: A prospective, multicenter study. Ocul. Immunol. Inflamm. 2011, 19, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Dorr, A. Single-dose pharmacokinetics of valganciclovir in HIV- and CMV-seropositive subjects. J. Clin. Pharmacol. 1999, 39, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, M.; Huang, W.; Fei, Y.J.; Leibach, F.H.; Ganapathy, V.; Ganapathy, M.E. Transport of valganciclovir, a ganciclovir prodrug, via peptide transporters PEPT1 and PEPT2. J. Pharm. Sci. 2000, 89, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Choonara, Y.E.; Pillay, V.; Danckwerts, M.P.; Carmichael, T.R.; du Toit, L.C. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J. Pharm. Sci. 2010, 99, 2219–2239. [Google Scholar] [CrossRef] [PubMed]
- Kadam, R.S.; Vooturi, S.K.; Kompella, U.B. Immunohistochemical and functional characterization of peptide, organic cation, neutral and basic amino acid, and monocarboxylate drug transporters in human ocular tissues. Drug Metabol. Dispos. 2013, 41, 466–474. [Google Scholar] [CrossRef]
- Huang, C.L.; Kumar, S.; Tan, J.J.Z.; Boey, F.Y.C.; Venkatraman, S.S.; Steele, T.W.J.; Loo, J.S.C. Modulating drug release from poly(lactic-co-glycolic acid) thin films through terminal end-groups and molecular weight. Polymer Degrad. Stab. 2013, 98, 619–626. [Google Scholar] [CrossRef]
- Steele, T.W.J.; Huang, C.L.; Kumar, S.; Iskandar, A.; Baoxin, A.; Boey, F.Y.C.; Loo, J.S.C.; Venkatraman, S.S. Tuning drug release in polyester thin films: Terminal end-groups determine specific rates of additive-free controlled drug release. NPG Asia Mater. 2013, 5, e46. [Google Scholar] [CrossRef]
- Steele, T.W.J.; Huang, C.L.; Kumar, S.; Widjaja, E.; Boey, F.Y.C.; Loo, J.S.; Venkatraman, S.S. High-throughput screening of PLGA thin films utilizing hydrophobic fluorescent dyes for hydrophobic drug compounds. J. Pharm. Sci. 2011, 100, 4317–4329. [Google Scholar] [CrossRef] [PubMed]
- Steele, T.W.J.; Huang, C.L.; Kumar, S.; Irvine, S.; Boey, F.Y.C.; Loo, J.S.; Venkatraman, S.S. Novel gradient casting method provides high-throughput assessment of blended polyester poly(lactic-co-glycolic acid) thin films for parameter optimization. Acta Biomater. 2012, 8, 2263–2270. [Google Scholar]
- Kai, M.; Ohkura, Y.; Yonekura, S.; Iwasaki, M. Selective determination of guanine and its nucleosides and nucleotides by reaction with phenylglyoxal as a fluorogenic reagent. Anal. Chim. Acta 1988, 207, 243–249. [Google Scholar] [CrossRef]
- Rao, M.; Prahhaka, T.; Sankar, G.; Jyothi, N. Development and validation of new stability indicating HPLC method for the determination of valganciclovir in tablet dosage forms. Int. J. Pharm. Sci. 2012, 2, 101–104. [Google Scholar]
- Li, W.; Anwar, F.; Jesurrun, J.; Erice, A. Cytomegalovirus UL97 and glycoprotein B (gB) sequences in tissues from immunocompromised patients with ganciclovir-resistant virus infection. Scand. J. Infect. Dis. 1999, 31, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Pack, D.W.; Klibanov, A.M.; Langer, R. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm. Res. 2000, 17, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Schwendeman, S.P. Mapping neutral microclimate pH in PLGA microspheres. J. Control. Release 2005, 101, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Mader, K.; Nitschke, S.; Stosser, R.; Borchert, H.H.; Domb, A. Non-destructive and localized assessment of acidic microenvironments inside biodegradable polyanhydrides by spectral spatial electron paramagnetic resonance imaging. Polymer 1997, 38, 4785–4794. [Google Scholar] [CrossRef]
- Mascher, H.; Kikuta, C.; Metz, R.; Vergin, H. New, high-sensitivity high-performance liquid-chromatographic method for the determination of acyclovir in human plasma, using fluorometric detection. J. Chromatogr. Biomed. Appl. 1992, 583, 122–127. [Google Scholar] [CrossRef]
- Dogan-Topal, B.; Uslu, B.; Ozkan, S.A. Development and validation of an RP-HPLC method for determination of valganciclovir in human serum and tablets. Chroma 2007, 66, 97–101. [Google Scholar]
- Yasukawa, T.; Tabata, Y.; Kimura, H.; Ogura, Y. Recent advances in intraocular drug delivery systems. Recent Pat. Drug Deliv. Formul. 2011, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Asai, T.; Oku, N.; Araki, Y.; Tanaka, M.; Ebihara, N. Liposomes and nanotechnology in drug development: Focus on ocular targets. Int. J. Nanomed. 2013, 8, 495–503. [Google Scholar] [CrossRef]
- Diban, N.; Haimi, S.; Bolhuis-Versteeg, L.; Teixeira, S.; Miettinen, S.; Poot, A.; Grijpma, D.; Stamatialis, D. Hollow fibers of poly(lactide-co-glycolide) and poly(epsilon-caprolactone) blends for vascular tissue engineering applications. Acta Biomater. 2013, 9, 6450–6458. [Google Scholar] [CrossRef] [PubMed]
- Lao, L.L.; Venkatraman, S.S.; Peppas, N.A. Modeling of drug release from biodegradable polymer blends. Eur. J. Pharm. Biopharm. 2008, 70, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Barichello, J.M.; Morishita, M.; Takayama, K.; Nagai, T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev. Ind. Pharm. 1999, 25, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Williams, D.; Dash, A.; Leslie-Pelecky, D.; Labhasetwar, V. Solid-state solubility influences encapsulation and release of hydrophobic drugs from PLGA/PLA nanoparticles. J. Pharm. Sci. 2004, 93, 1804–1814. [Google Scholar] [CrossRef] [PubMed]
- Hans, M.L.; Lowman, A.M. Biodegradable nanoparticles for drug delivery and targeting. Curr. Opin. Solid State Mater. Sci. 2002, 6, 319–327. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaller, T.; Wenner, T.; Agrawal, R.; Teoh, S.; Phua, L.T.; Loo, J.S.C.; Steele, T.W.J. High Throughput Screening of Valganciclovir in Acidic Microenvironments of Polyester Thin Films. Materials 2015, 8, 1714-1728. https://doi.org/10.3390/ma8041714
Schaller T, Wenner T, Agrawal R, Teoh S, Phua LT, Loo JSC, Steele TWJ. High Throughput Screening of Valganciclovir in Acidic Microenvironments of Polyester Thin Films. Materials. 2015; 8(4):1714-1728. https://doi.org/10.3390/ma8041714
Chicago/Turabian StyleSchaller, Teilo, Tobias Wenner, Rupesh Agrawal, Stephen Teoh, Li Ting Phua, Joachim S. C. Loo, and Terry W. J. Steele. 2015. "High Throughput Screening of Valganciclovir in Acidic Microenvironments of Polyester Thin Films" Materials 8, no. 4: 1714-1728. https://doi.org/10.3390/ma8041714






