Initial Bacterial Adhesion on Different Yttria-Stabilized Tetragonal Zirconia Implant Surfaces in Vitro
Abstract
:1. Introduction
2. Results and Discussion
| Samples | Material Description | Coating |
|---|---|---|
| BES | Bovine enamel slabs | No |
| B1a | 3 mol% yttria-stabilized tetragonal zirconia polycrystal surface | No |
| B2a | 3 mol% yttria-stabilized tetragonal zirconia polycrystal surface | zirconium oxide (ZrO2) coating |
| B1b | 3 mol% yttria-stabilized tetragonal zirconia polycrystal surface | zirconia-based composite coating |
| B2b | 3 mol% yttria-stabilized tetragonal zirconia polycrystal surface | zirconia-based composite and zirconium oxide (ZrO2) coatings |
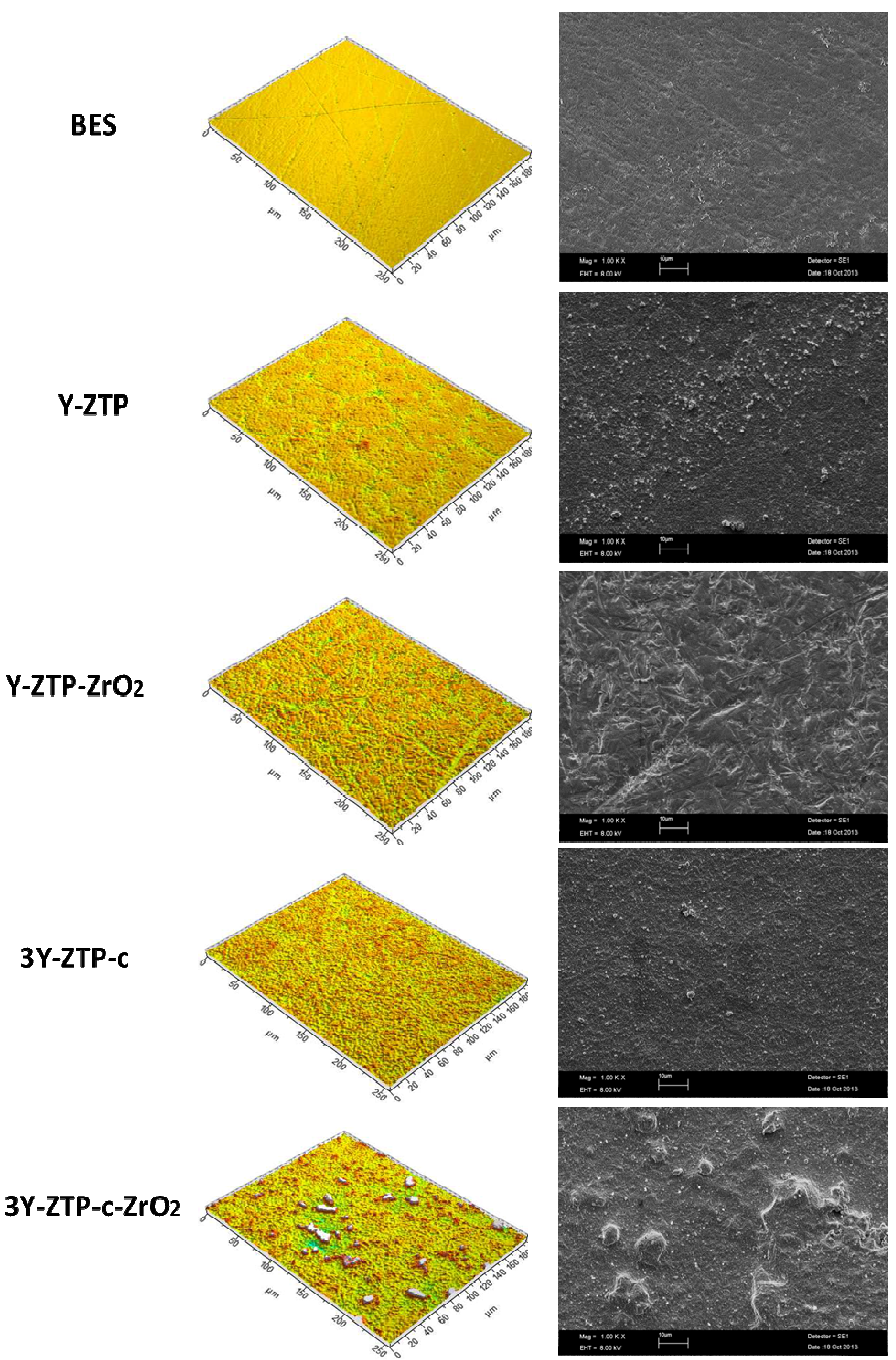
| Materials | Surface characteristics | ||||||
|---|---|---|---|---|---|---|---|
| Amplitude Parameters | Hybrid Parameters | Spatial Parameters | |||||
| Average surface roughness (Sa) | Root mean square surface roughness (Sq) | Ten-point average roughness (Sz) | Skewness (Ssk) | Summit density (Sds) | Developed area ratio (Sdr) | Texture aspect ratio (Str) | |
| BES (control) | 0.041 μm | 0.056 μm | 0.98 μm | −0.72 | 0.141/μm² | 0.48% | 0.220 |
| B1a | 0.119 μm | 0.162 μm | 2.44 μm | −0.64 | 0.108/μm² | 2.23% | 0.734 |
| B2a | 0.199 μm | 0.255 μm | 3.58 μm | −0.685 | 0.100/μm² | 5.98% | 0.841 |
| B1b | 0.252 μm | 0.384 μm | 4.54 μm | 1.43 | 0.110/μm² | 7.20% | 0.799 |
| B2b | 0.259 μm | 0.408 μm | 4.97 μm | 1.97 | 0.111/μm² | 7.44% | 0.353 |

3. Experimental Section
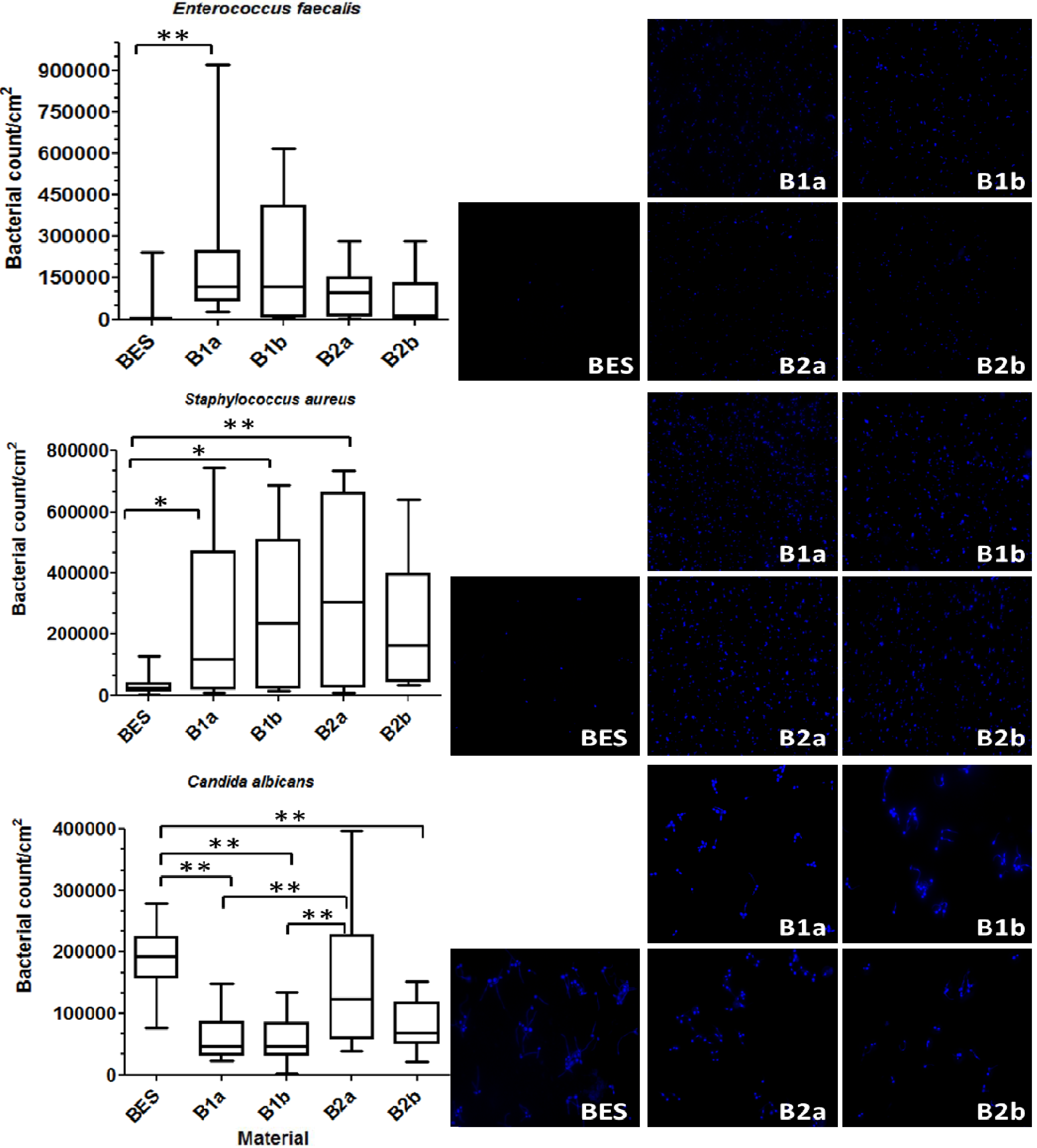
3.1. Total Bacterial Count (DAPI)
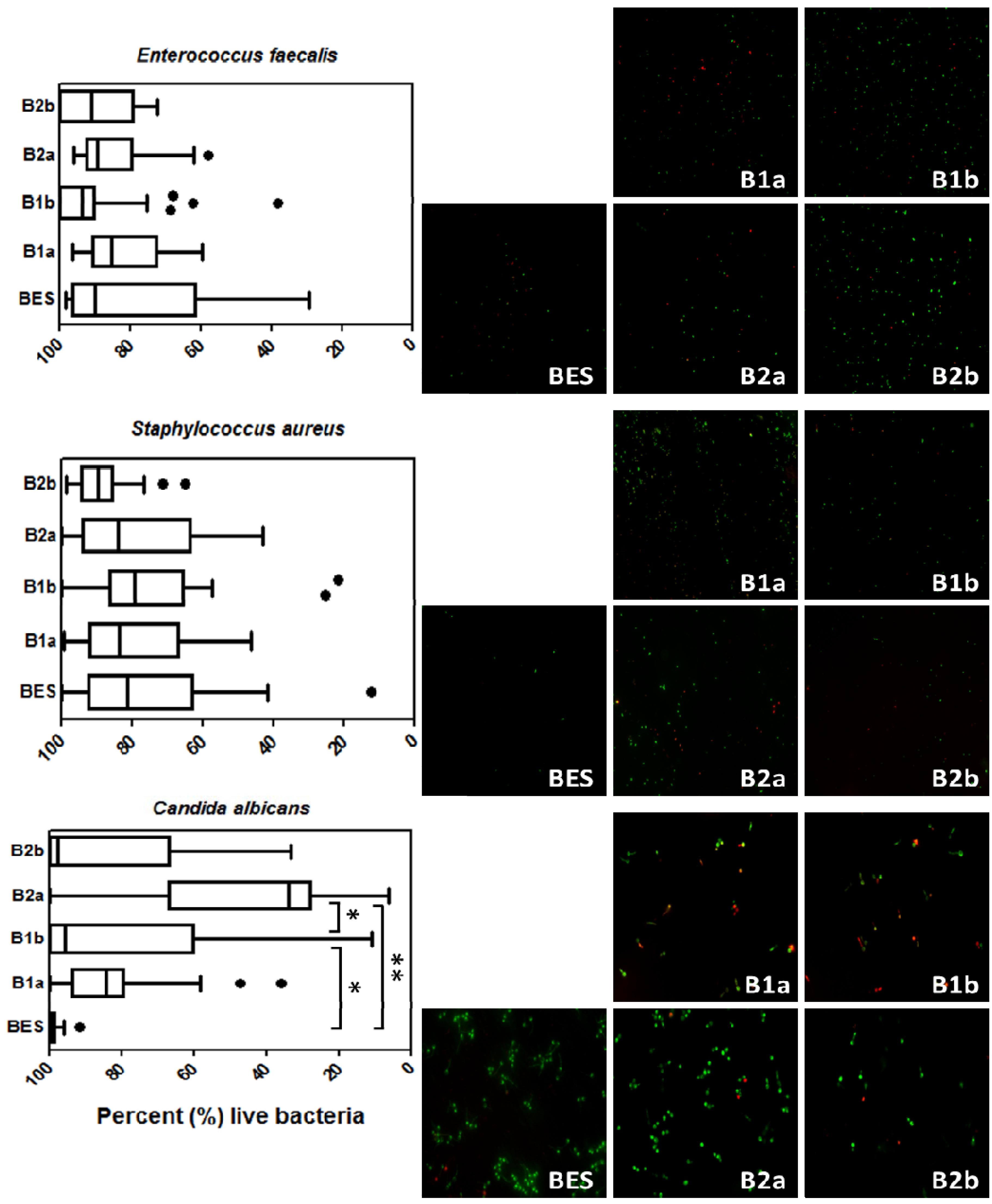
3.2. Live/Dead Staining
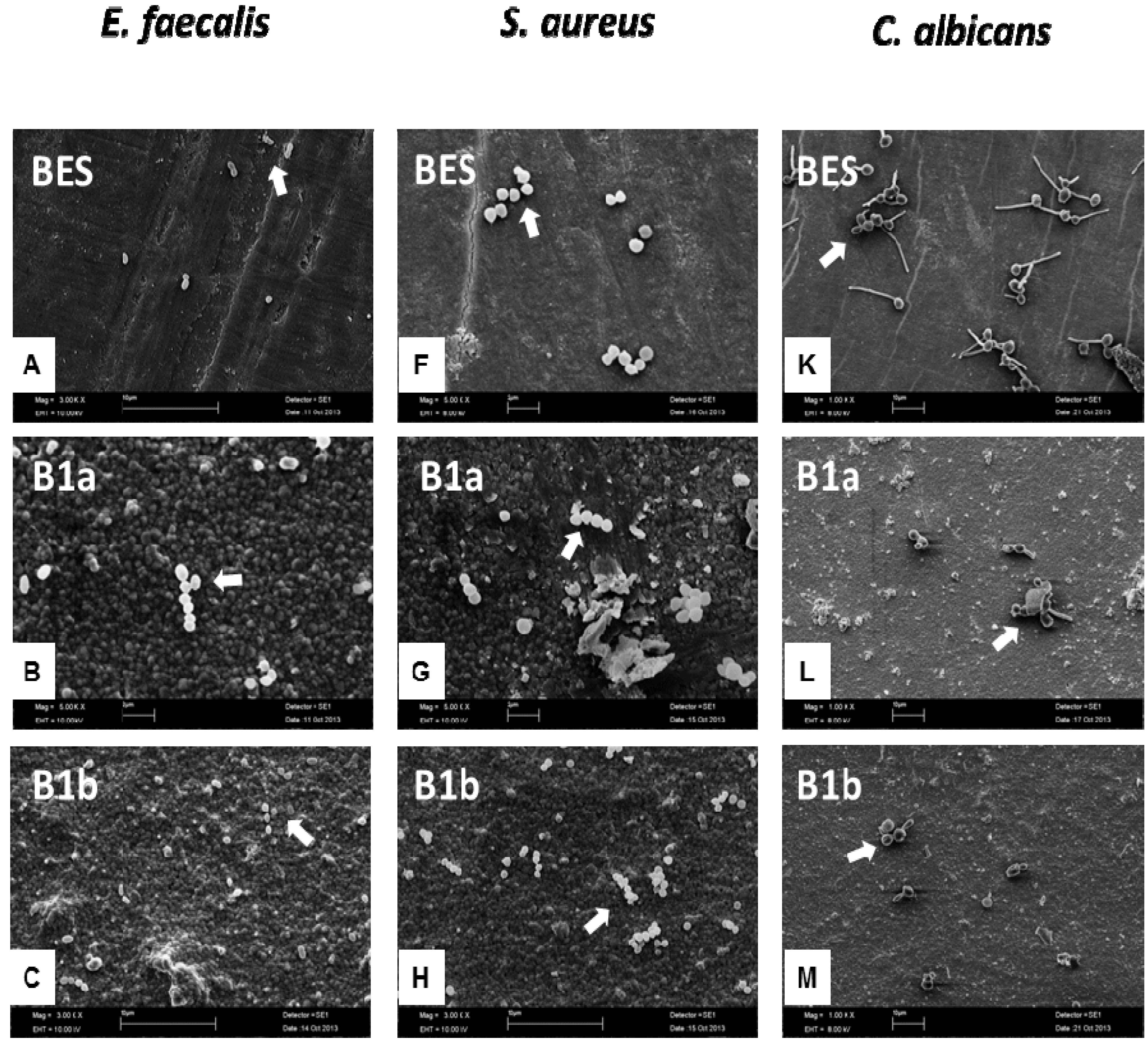
3.3. Scanning Electron Microscopy (SEM)
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wenz, H.J.; Bartsch, J.; Wolfart, S.; Kern, M. Osseointegration and clinical success of zirconia dental implants: A systematic review. Int. J. Prosthodont. 2008, 21, 27–36. [Google Scholar] [PubMed]
- Piconi, C.; Burger, W.; Richter, H.G.; Cittadini, A.; Maccauro, G.; Covacci, V.; Bruzzese, N.; Ricci, G.A.; Marmo, E. Y-TZP ceramics for artificial joint replacements. Biomaterials 1998, 19, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Andreiotelli, M.; Kohal, R.J. Fracture strength of zirconia implants after artificial aging. Clin. Implant Dent. Relat. Res. 2009, 11, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J. What future for zirconia as a biomaterial? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Deville, S.; Munch, E.; Jullian, R.; Lair, F. Critical effect of cubic phase on aging in 3 mol% yttria-stabilized zirconia ceramics for hip replacement prosthesis. Biomaterials 2004, 25, 5539–5545. [Google Scholar] [CrossRef] [PubMed]
- Sanon, C.; Chevalier, J.; Douillard, T.; Kohal, R.J.; Coelho, P.G.; Hjerppe, J.; Silva, N.R. Low temperature degradation and reliability of one-piece ceramic oral implants with a porous surface. Dent. Mater. 2013, 29, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Grandjean, S.; Kuntz, M.; Pezzotti, G. On the kinetics and impact of tetragonal to monoclinic transformation in an alumina/zirconia composite for arthroplasty applications. Biomaterials 2009, 30, 5279–5282. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, S7–S15. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [PubMed]
- Madianos, P.N.; Bobetsis, Y.A.; Kinane, D.F. Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. J. Clin. Periodontol. 2005, 32, S57–S71. [Google Scholar] [CrossRef]
- Roos-Jansaker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine- to fourteen-year follow-up of implant treatment. Part II: Presence of peri-implant lesions. J. Clin. Periodontol. 2006, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Schaudinn, C.; Gorur, A.; Keller, D.; Sedghizadeh, P.P.; Costerton, J.W. Periodontitis: An archetypical biofilm disease. J. Am. Dent. Assoc. 2009, 140, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Bumgardner, J.D.; Adatrow, P.; Haggard, W.O.; Norowski, P.A. Emerging antibacterial biomaterial strategies for the prevention of peri-implant inflammatory diseases. Int. J. Oral Maxillofac. Implants 2011, 26, 553–560. [Google Scholar] [PubMed]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol. 2000 2002, 28, 12–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Follo, M.; Selzer, A.C.; Hellwig, E.; Hannig, M.; Hannig, C. Bacterial colonization of enamel in situ investigated using fluorescence in situ hybridization. J. Med. Microbiol. 2009, 58, 1359–1366. [Google Scholar] [PubMed]
- Yeo, I.S.; Kim, H.Y.; Lim, K.S.; Han, J.S. Implant surface factors and bacterial adhesion: A review of the literature. Int. J. Artif. Organs 2012, 35, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Muller, P.; Attin, T.; Wieland, M.; Hofer, D.; Guggenheim, B. Polyspecies biofilm formation on implant surfaces with different surface characteristics. J. Appl. Oral Sci. 2013, 21, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Fackler, A.; Follo, M.; Hellwig, E.; Bachle, M.; Hannig, C.; Han, J.S.; Wolkewitz, M.; Kohal, R. In vivo study of the initial bacterial adhesion on different implant materials. Arch. Oral Biol. 2013, 58, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Missirlis, Y.F. Concise review of mechanisms of bacterial adhesion to biomaterials and of techniques used in estimating bacteria-material interactions. Eur. Cell Mater. 2004, 8, 37–57. [Google Scholar] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Rosan, B.; Lamont, R.J. Dental plaque formation. Microbes Infect. 2000, 2, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- Katsikogianni, M.; Spiliopoulou, I.; Dowling, D.P.; Missirlis, Y.F. Adhesion of slime producing Staphylococcus epidermidis strains to PVC and diamond-like carbon/silver/fluorinated coatings. J. Mater. Sci. Mater. Med. 2006, 17, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implants Res. 2006, 17, S68–S81. [Google Scholar] [CrossRef]
- Truong, V.K.; Lapovok, R.; Estrin, Y.S.; Rundell, S.; Wang, J.Y.; Fluke, C.J.; Crawford, R.J.; Ivanova, E.P. The influence of nano-scale surface roughness on bacterial adhesion to ultrafine-grained titanium. Biomaterials 2010, 31, 3674–3683. [Google Scholar] [CrossRef] [PubMed]
- Crawford, R.J.; Webb, H.K.; Truong, V.K.; Hasan, J.; Ivanova, E.P. Surface topographical factors influencing bacterial attachment. Adv. Colloid Interface Sci. 2012, 179–182, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Shmueli, S.; Shur, I.; Benayahu, D.; Aronov, D.; Rosenman, G. The effect of surface treatment on the surface texture and contact angle of electrochemically deposited hydroxyapatite coating and on its interaction with bone-forming cells. Acta Biomater. 2009, 5, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Okada, A.; Nikaido, T.; Ikeda, M.; Okada, K.; Yamauchi, J.; Foxton, R.M.; Sawada, H.; Tagami, J.; Matin, K. Inhibition of biofilm formation using newly developed coating materials with self-cleaning properties. Dent. Mater. J. 2008, 27, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahmad, A.; Wiedmann-Al-Ahmad, M.; Faust, J.; Bachle, M.; Follo, M.; Wolkewitz, M.; Hannig, C.; Hellwig, E.; Carvalho, C.; Kohal, R. Biofilm formation and composition on different implant materials in vivo. J. Biomed. Mater. Res. Appl. Biomater. 2010, 95, 101–109. [Google Scholar] [CrossRef]
- Rimondini, L.; Cerroni, L.; Carrassi, A.; Torricelli, P. Bacterial colonization of zirconia ceramic surfaces: An in vitro and in vivo study. Int. J. Oral Maxillofac. Implants 2002, 17, 793–798. [Google Scholar] [PubMed]
- Marchi, J.; Amorim, E.M.; Lazar, D.R.; Ussui, V.; Bressiani, A.H.; Cesar, P.F. Physico-chemical characterization of zirconia-titania composites coated with an apatite layer for dental implants. Dent. Mater. 2013, 29, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Pardun, K.; Treccani, L.; Volkmann, E.; Li Destri, G.; Marletta, G.; Streckbein, P.; Heiss, C.; Rezwan, K. Characterization of wet powder-sprayed zirconia/calcium phosphate coating for dental implants. Clin. Implant Dent. Relat. Res. 2013. [Google Scholar] [CrossRef]
- Piconi, C.; Maccauro, G.; Muratori, F.; Brach Del Prever, E. Alumina and zirconia ceramics in joint replacements. J. Appl. Biomater. Biomech. 2003, 1, 19–32. [Google Scholar] [PubMed]
- Treccani, L.; Klein, T.Y.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef] [PubMed]
- Nganga, S.; Travan, A.; Marsich, E.; Donati, I.; Soderling, E.; Moritz, N.; Paoletti, S.; Vallittu, P.K. In vitro antimicrobial properties of silver-polysaccharide coatings on porous fiber-reinforced composites for bone implants. J. Mater. Sci. Mater. Med. 2013, 24, 2775–2785. [Google Scholar] [CrossRef] [PubMed]
- Van Merode, A.E.; Duval, J.F.; van der Mei, H.C.; Busscher, H.J.; Krom, B.P. Increased adhesion of Enterococcus faecalis strains with bimodal electrophoretic mobility distributions. Colloids Surf. B Biointerfaces 2008, 64, 302–306. [Google Scholar]
- Busscher, H.J.; van der Mei, H.C. How do bacteria know they are on a surface and regulate their response to an adhering state? PLoS Pathog. 2012, 8. [Google Scholar] [CrossRef]
- Tiller, J.C.; Liao, C.J.; Lewis, K.; Klibanov, A.M. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 2001, 98, 5981–5985. [Google Scholar] [CrossRef] [PubMed]
- Kugler, R.; Bouloussa, O.; Rondelez, F. Evidence of a charge-density threshold for optimum efficiency of biocidal cationic surfaces. Microbiology 2005, 151, 1341–1348. [Google Scholar] [PubMed]
- Karygianni, L.; Follo, M.; Hellwig, E.; Burghardt, D.; Wolkewitz, M.; Anderson, A.; Al-Ahmad, A. Microscope-based imaging platform for large-scale analysis of oral biofilms. Appl. Environ. Microbiol. 2012, 78, 8703–8711. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.D.; Pita, M.S.; Fernandes, F.H.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral. Implants Res. 2013. [Google Scholar] [CrossRef]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral. Implants Res. 2007, 18, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.; Hoffmann, S.; Obst, U. Formation of natural biofilms during chlorine dioxide and u.v. disinfection in a public drinking water distribution system. J. Appl. Microbiol. 2003, 95, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Tawakoli, P.N.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Comparison of different live/dead stainings for detection and quantification of adherent microorganisms in the initial oral biofilm. Clin. Oral Investig. 2013, 17, 841–850. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karygianni, L.; Jähnig, A.; Schienle, S.; Bernsmann, F.; Adolfsson, E.; Kohal, R.J.; Chevalier, J.; Hellwig, E.; Al-Ahmad, A. Initial Bacterial Adhesion on Different Yttria-Stabilized Tetragonal Zirconia Implant Surfaces in Vitro. Materials 2013, 6, 5659-5674. https://doi.org/10.3390/ma6125659
Karygianni L, Jähnig A, Schienle S, Bernsmann F, Adolfsson E, Kohal RJ, Chevalier J, Hellwig E, Al-Ahmad A. Initial Bacterial Adhesion on Different Yttria-Stabilized Tetragonal Zirconia Implant Surfaces in Vitro. Materials. 2013; 6(12):5659-5674. https://doi.org/10.3390/ma6125659
Chicago/Turabian StyleKarygianni, Lamprini, Andrea Jähnig, Stefanie Schienle, Falk Bernsmann, Erik Adolfsson, Ralf J. Kohal, Jérôme Chevalier, Elmar Hellwig, and Ali Al-Ahmad. 2013. "Initial Bacterial Adhesion on Different Yttria-Stabilized Tetragonal Zirconia Implant Surfaces in Vitro" Materials 6, no. 12: 5659-5674. https://doi.org/10.3390/ma6125659





