Growth of High-Density Zinc Oxide Nanorods on Porous Silicon by Thermal Evaporation
Abstract
:1. Introduction
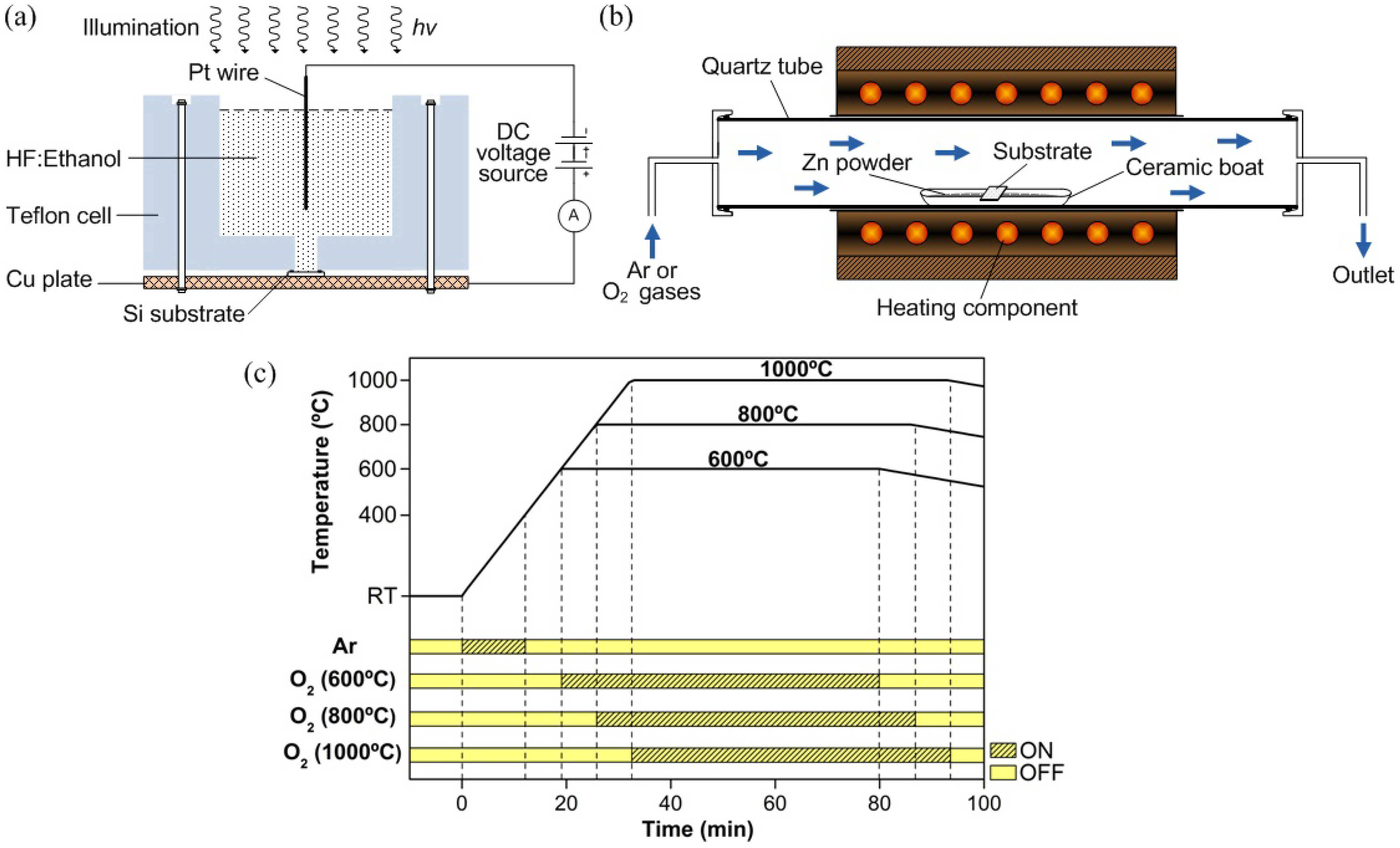
2. Experimental Section
2.1. PS Substrate Preparation
2.2. ZnO Nanorod Growth
3. Results and Discussion
3.1. Morphological and Compositional Properties
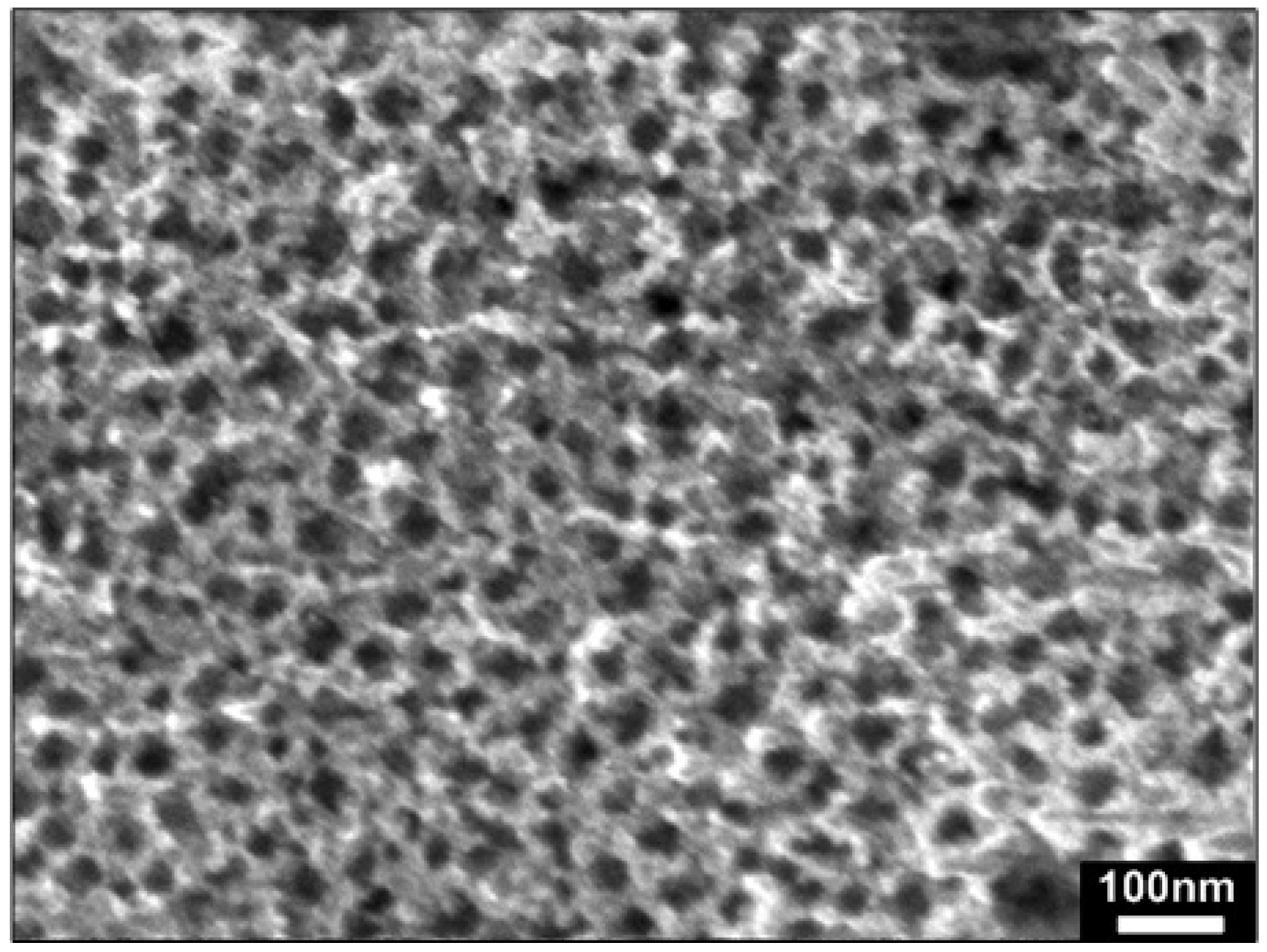
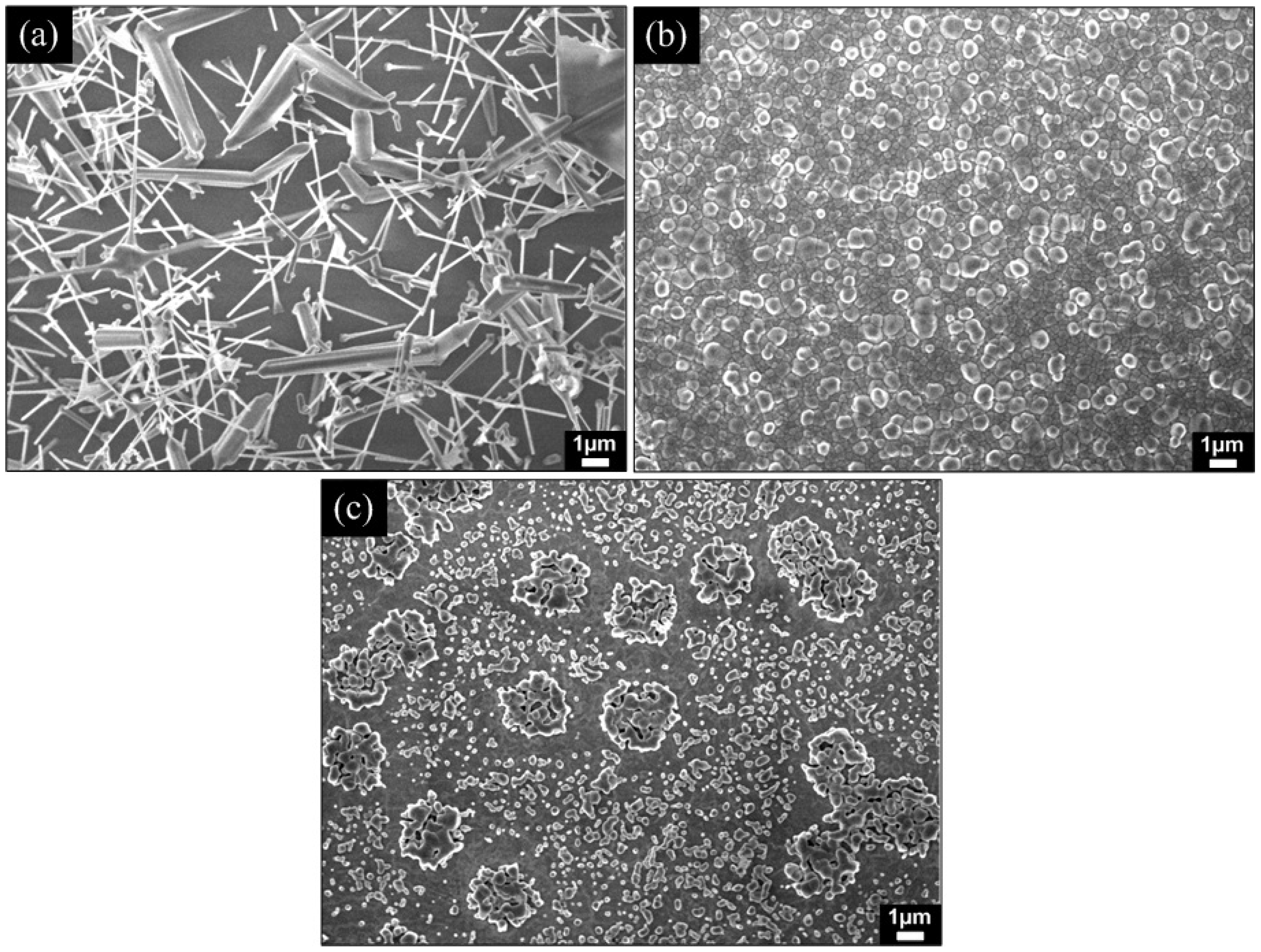
| O2 gas flow rate (sccm) | Temperature (°C) | Diameter of nanorods (nm) | Density (cm−2) | Size of ZnOx seed clusters/layer (nm) | |
|---|---|---|---|---|---|
| This work | 200 | 600 | 100–400 (base) 200–800 (top) | 271 × 106 | 100–220 (cluster size) |
| 800 | 200–500 (base) 600–950 (top) | 41 × 106 | 400–840 (cluster size) | ||
| 1000 | 200–500 | 120 × 106 | 900–1200 (layer thickness) | ||
| [11] | 500 | 700 | 350–400 (base) 150–200 (top) | 626 × 106 | Not reported |

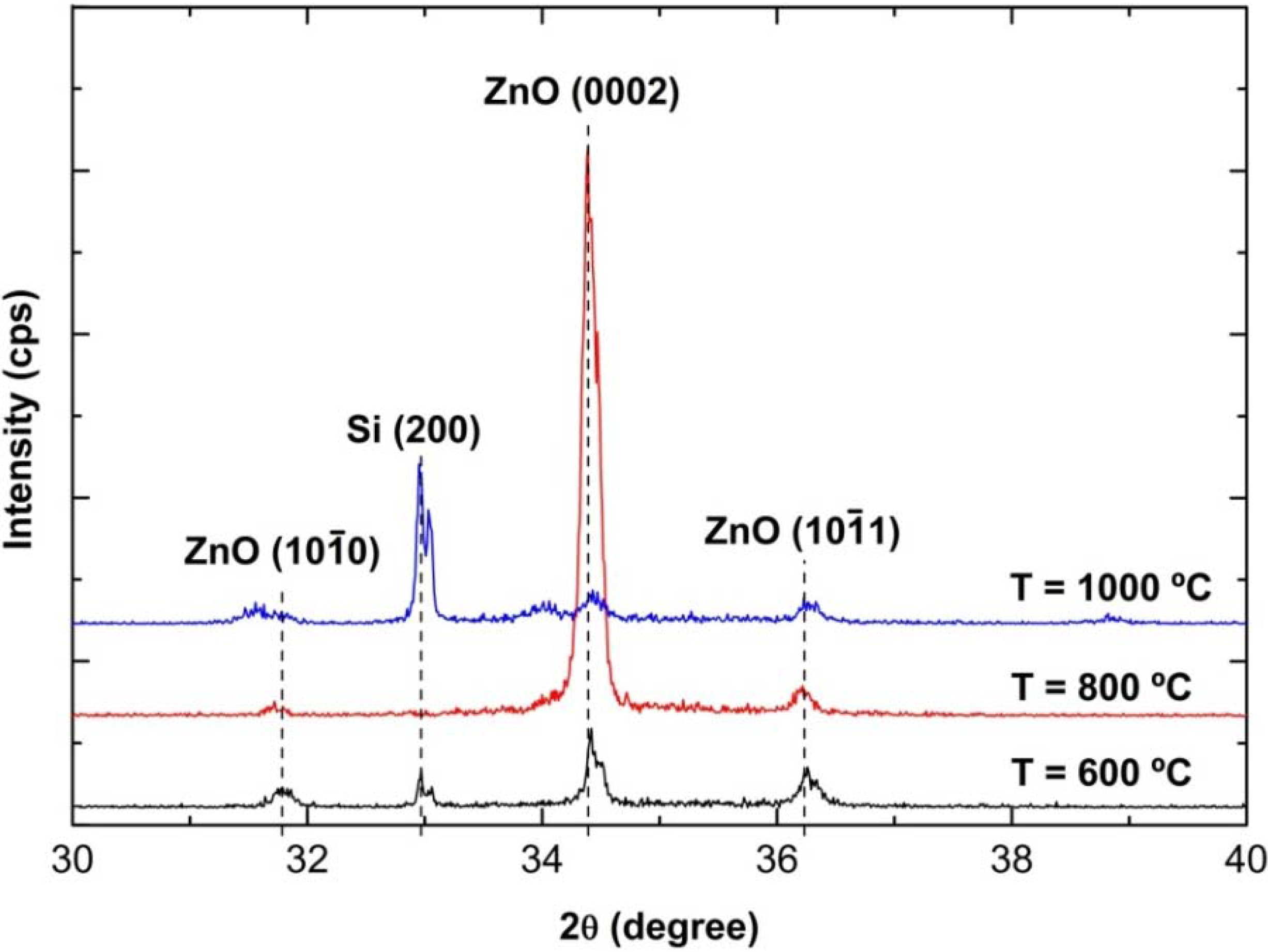
3.2. Crystallographic Properties
3.3. Structural Properties
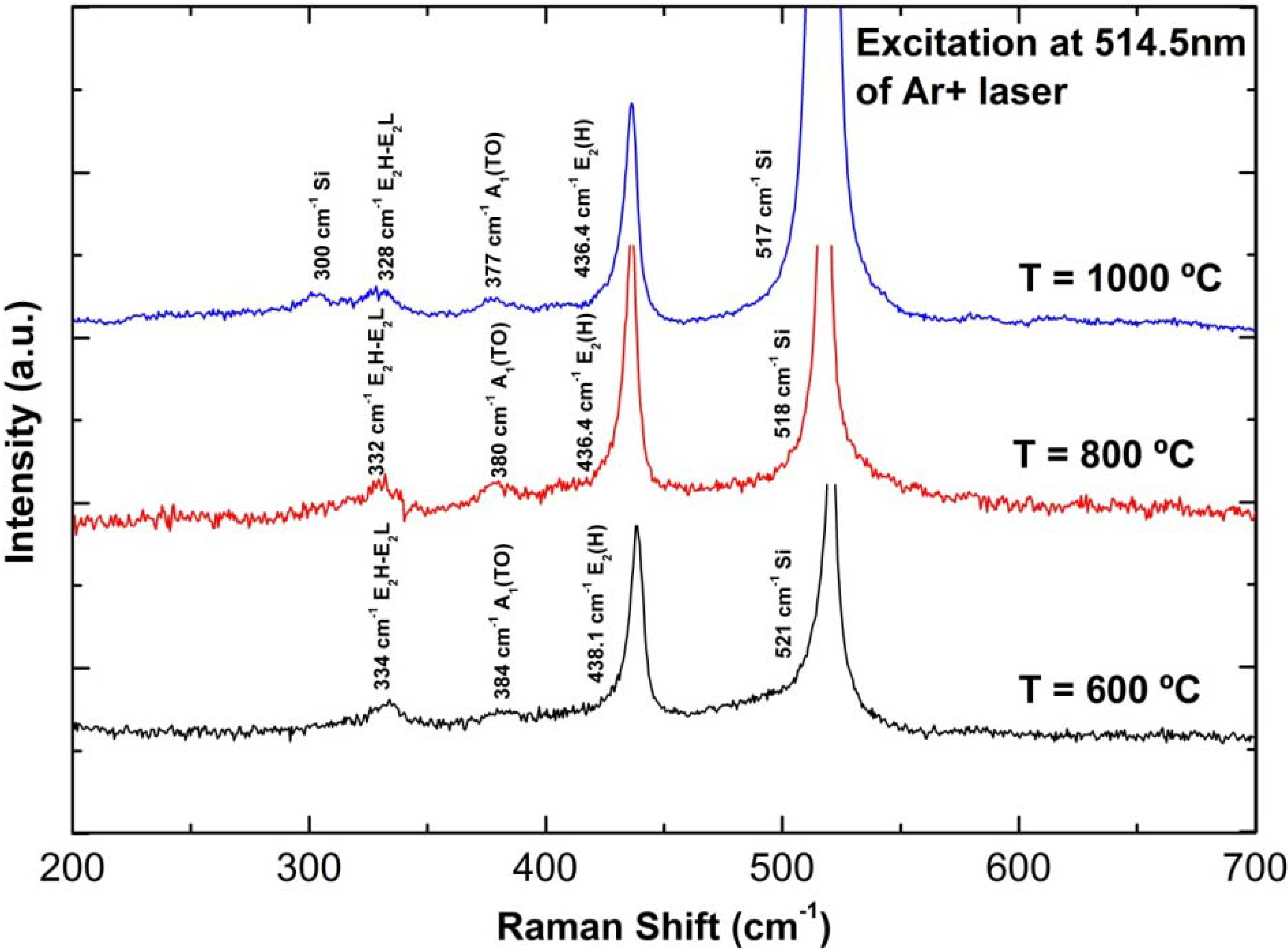
3.4. Photoluminescence (PL) Properties
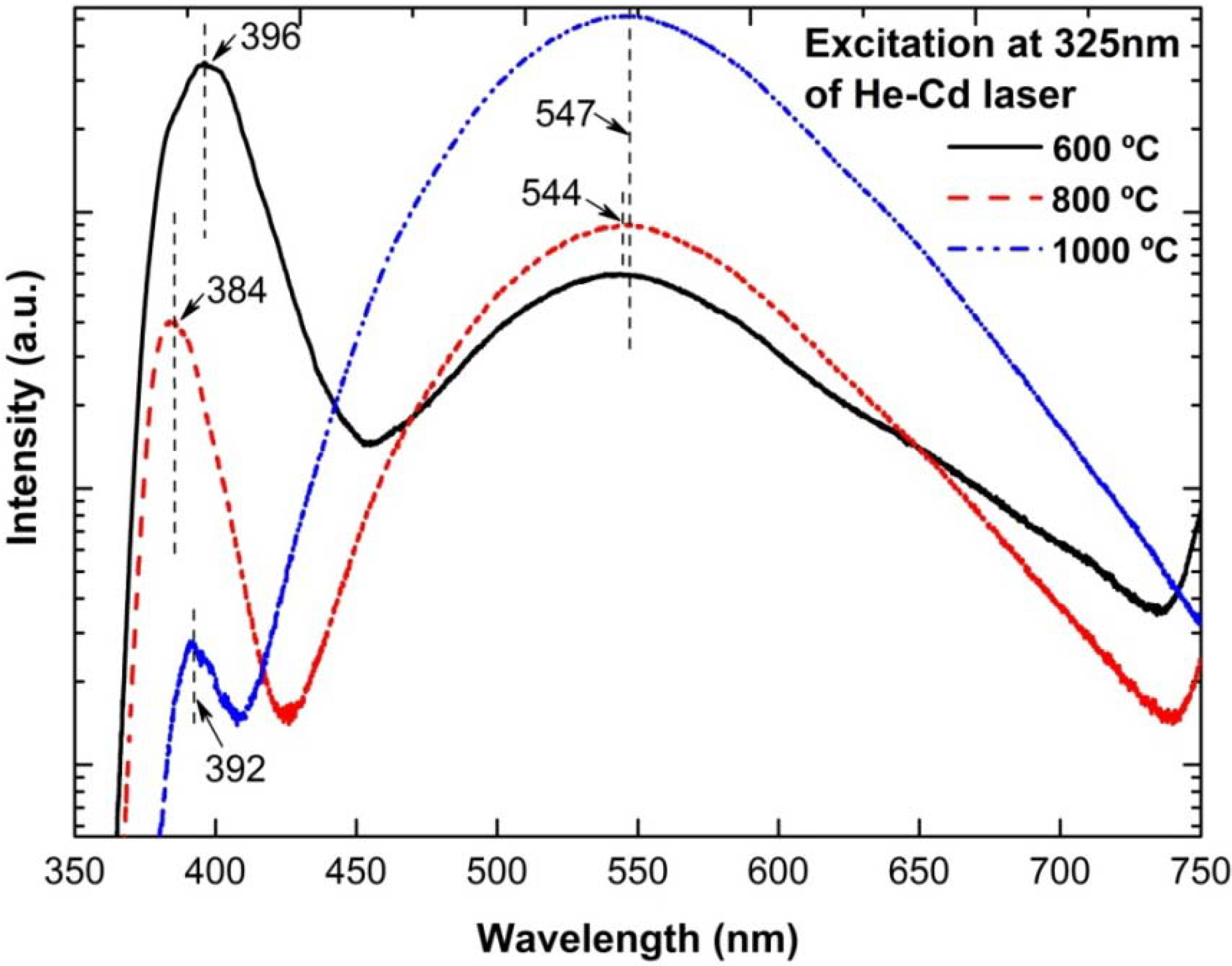
3.5. Possible Growth Mechanism of ZnO Nanorods

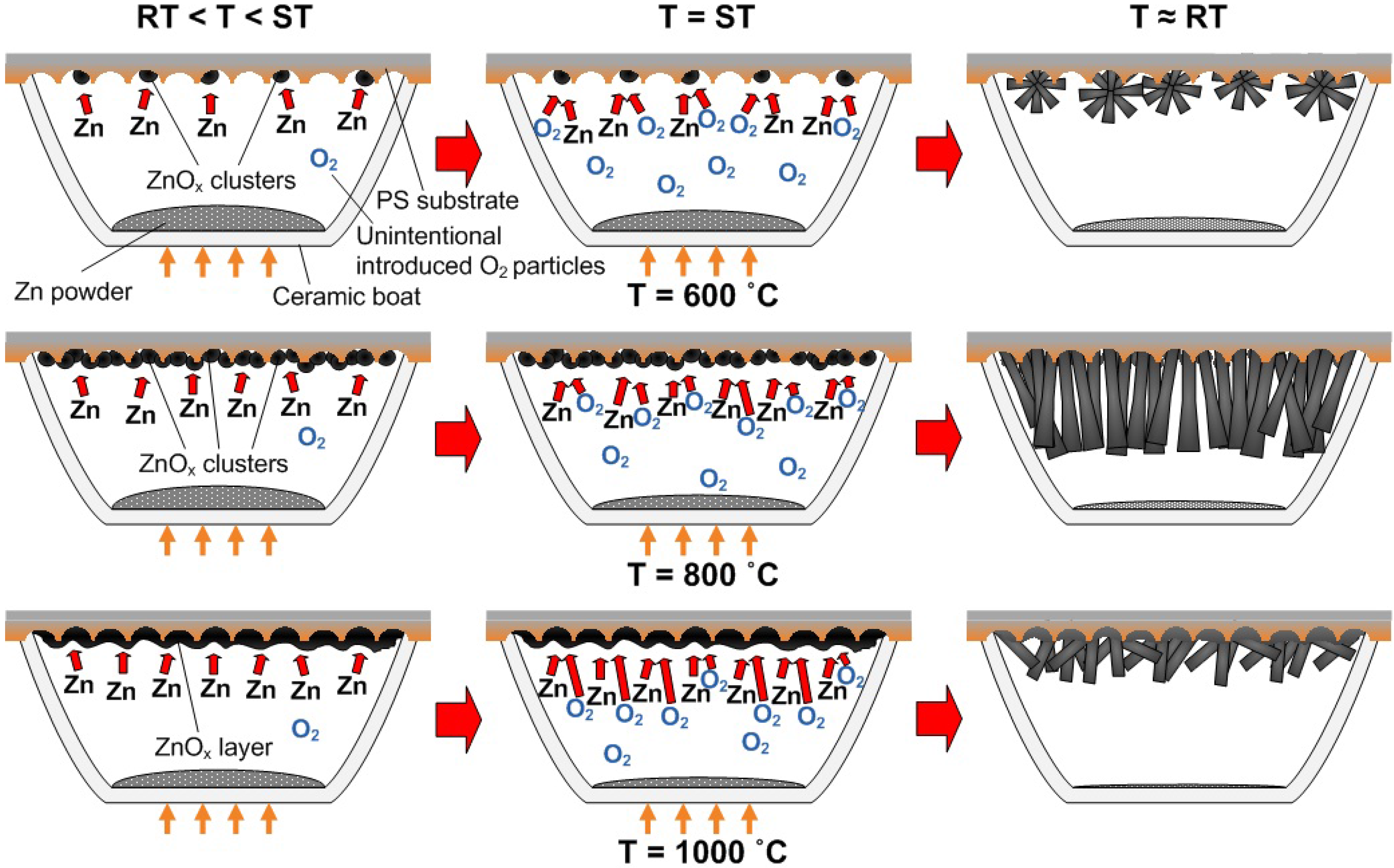
4. Conclusions
Acknowledgments
References
- Antohe, V.A.; Gence, L.; Srivastava, S.K.; Piraux, L. Template-free electrodeposition of highly oriented and aspect-ratio controlled ZnO hexagonal columnar arrays. Nanotechnology 2012, 23, 1–7. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Lockman, Z.; Aziz, A.A.; Razak, K.A. Properties of ZnO nanorods arrays growth via low temperature hydrothermal reaction. Adv. Mater. Res. 2012, 364, 422–426. [Google Scholar] [CrossRef]
- Hassan, N.K.; Hashim, M.R.; Mahdi, M.A.; Allam, N.K. A catalyst-free growth of ZnO nanowires on Si (100) substrates: Effect of substrate position on morphological, structural and optical properties. ECS J. Solid State Sci. Technol. 2012, 1, P86–P89. [Google Scholar] [CrossRef]
- Yao, I.-C.; Tseng, T.-Y.; Lin, P. ZnO nanorods grown on polymer substrates as UV photodetectors. Sens. Actuators B 2012, 178, 26–31. [Google Scholar] [CrossRef]
- Sun, J.; Bian, J.; Wang, Y.; Wang, Y.; Gong, Y.; Li, Y.; Liu, K.; Zhang, S.; Liu, Y.; Liang, H.; Du, G.; Yu, N. Fabrication of a homojunction light emitting diode with ZnO-nanorods/ZnO:as-film structure. Electrochem. Solid-State Lett. 2012, 15, H164–H166. [Google Scholar] [CrossRef]
- Cai, F.; Wang, J.; Yuan, Z.; Duan, Y. Magnetic-field effect on dye-sensitized ZnO nanorods-based solar cells. J. Power Sources 2012, 216, 269–272. [Google Scholar] [CrossRef]
- Baxter, J.B.; Aydil, E.S. Nanowire-based dye-sensitized solar cells. Appl. Phys. Lett. 2005, 86, 1–4. [Google Scholar] [CrossRef]
- Tao, R.; Tomita, T.; Wong, R.A.; Waki, K. Electrochemical and structural analysis of Al-doped ZnO nanorod arrays in dye-sensitized solar cells. J. Power Sources 2012, 214, 159–165. [Google Scholar] [CrossRef]
- Abdulgafour, H.I.; Yam, F.K.; Hassan, Z.; AL-Heuseen, K.; Jawad, M.J. ZnO nanocoral reef grown on porous silicon substrates without catalyst. J. Alloys Compd. 2011, 509, 5627–5630. [Google Scholar] [CrossRef]
- Thahab, S.M. Preparation and structural characterizations of ZnO nano-columns grown on porous silicon/silicon (PS/Si(111)) by thermal evaporation. Optoelectron. Adv. Mater. 2011, 5, 1107–1110. [Google Scholar]
- Srivatsa, K.M.K.; Chhikara, D.; Kumar, M.S. Synthesis of aligned ZnO nanorod array on silicon and sapphire substrates by thermal evaporation technique. J. Mater. Sci. Technol. 2011, 27, 701–706. [Google Scholar] [CrossRef]
- Cheng, Q.; Ostrikov, K.K. Temperature-dependent growth mechanisms of low-dimensional ZnO nanostructures. CrystEngComm 2011, 13, 3455–3461. [Google Scholar] [CrossRef]
- Calestani, D.; Zha, M.Z.; Zanotti, L.; Villani, M.; Zappettini, A. Low temperature thermal evaporation growth of aligned ZnO nanorods on ZnO film: A growth mechanism promoted by Zn nanoclusters on polar surfaces. CrystEngComm 2011, 13, 1707–1712. [Google Scholar] [CrossRef]
- Umar, A.; Kim, S.H.; Kim, J.H.; Al-Hajry, A.; Hahn, Y.B. Temperature-dependant non-catalytic growth of ultraviolet-emitting ZnO nanostructures on silicon substrate by thermal evaporation process. J. Alloys Compd. 2008, 463, 516–521. [Google Scholar] [CrossRef]
- Kar, S.; Pal, B.N.; Chaudhuri, S.; Chakravorty, D. One-dimensional ZnO nanostructure arrays: Synthesis and characterization. J. Phys. Chem. B 2006, 110, 4605–4611. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Chang, C.-S. Growth of ZnO nanowires without catalyst on porous silicon. Jpn. J. Appl. Phys. 2004, 43, 8360–8364. [Google Scholar] [CrossRef]
- Pung, S.-Y.; Tee, C.-C.; Choy, K.-L.; Hou, X.H. Growth mechanism of Au-catalyzed ZnO nanowires: VLS or VS-VLS? Adv. Mater. Res. 2012, 364, 333–337. [Google Scholar] [CrossRef]
- Wu, J.-J.; Liu, S.-C. Low-temperature growth of well-aligned ZnO nanorods by chemical vapor deposition. Adv. Mater. 2002, 14, 215–218. [Google Scholar] [CrossRef]
- Yang, J.H.; Zheng, J.H.; Zhai, H.J.; Yang, L.L. Low temperature hydrothermal growth and optical properties of ZnO nanorods. Cryst. Res. Technol. 2009, 44, 87–91. [Google Scholar] [CrossRef]
- Rahman, I.B.A.; Ayob, M.T.M.; Mohamed, F.; Othman, N.K.; Lawi, R.L.M.; Radiman, S. Synthesis and characterization of ZnO, CuO and CuO/ZnO nanoparticles by a novel sol-gel route under ultrasonic conditions. Adv. Mater. Res. 2012, 545, 64–70. [Google Scholar] [CrossRef]
- Bechelany, M.; Amin, A.; Brioude, A.; Cornu, D.; Miele, P. ZnO nanotubes by template-assisted sol-gel route. J. Nanopart. Res. 2012, 14, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kashif, M.; Ali, S.M.U.; Ali, M.E.; Abdulgafour, H.I.; Hashim, U.; Willander, M.; Hassan, Z. Morphological, optical, and raman characteristics of ZnO nanoflakes prepared via a sol-gel method. Phys. Status Solidi A 2012, 209, 143–147. [Google Scholar] [CrossRef]
- Hassan, N.K.; Hashim, M.R.; Al-Douri, Y.; Al-Heuseen, K. Current dependence growth of ZnO nanostructures by electrochemical deposition technique. Int. J. Electrochem. Sci. 2012, 7, 4625–4635. [Google Scholar]
- Cheng, C.; Liu, B.; Yang, H.; Zhou, W.; Sun, L.; Chen, R.; Yu, S.F.; Zhang, J.; Gong, H.; Sun, H.; Fan, H.J. Hierarchical assembly of ZnO nanostructures on SnO2 backbone nanowires: Low-temperature hydrothermal preparation and optical properties. J. Am. Chem. Soc. 2009, 3, 3069–3076. [Google Scholar]
- Hsu, H.-C.; Cheng, C.-S.; Chang, C.-C.; Yang, S.; Chang, C.-S.; Hsieh, W.-F. Orientation-enhanced growth and optical properties of ZnO nanowires grown on porous silicon substrates. Nanotechnology 2005, 16, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. Influence of pH, precursor concentration, growth time, and temperature on the morphology of ZnO nanostructures grown by the hydrothermal method. J. Nanomater. 2011. [Google Scholar] [CrossRef]
- Sun, Y.; Riley, D.J.; Ashfold, M.N.R. Mechanism of ZnO nanotube growth by hydrothermal methods on ZnO film-coated Si substrates. J. Phys. Chem. B 2006, 110, 15186–15192. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, M.; Dariani, R.S.; Zad, A.I. Growth of ZnO nanostructures on porous silicon and oxidized porous silicon substrates. Braz. J. Phys. 2011, 41, 113–117. [Google Scholar] [CrossRef]
- Rajabi, M.; Dariani, R.S.; Zad, A.I. Comparative study of ZnO nanostructures grown on silicon (1 0 0) and oxidized porous silicon substrates with and without Au catalyst by chemical vapor transport and condensation. J. Alloys Compd. 2011, 509, 4295–4299. [Google Scholar] [CrossRef]
- Polishchuk, V.; Souteyrand, E.; Martin, J.R.; Strikha, V.I.; Skryshevsky, V.A. A study of hydrogen detection with palladium modified porous silicon. Anal. Chim. Acta 1998, 375, 205–210. [Google Scholar] [CrossRef]
- Bomchil, G.; Herino, R.; Barla, K.; Pfister, J.C. Pore size distribution in porous silicon studied by adsorption isotherms. J. Electrochem. Soc. 1983, 130, 1611–1614. [Google Scholar] [CrossRef]
- Galun, E.; Reuben, C.; Matlis, S.; Tenne, R.; LCvy-ClCment, C. Morphology of n-type macroporous silicon: Doping density dependence. J. Phys. Chem. 1995, 99, 4132–4140. [Google Scholar] [CrossRef]
- Lehmann, V. Porous silicon formation and other photoelectrochemical effects at silicon electrodes anodized in hydrofluoric acid. Appl. Surf. Sci. 1996, 106, 402–405. [Google Scholar] [CrossRef]
- Ouyang, H.; Christophersen, M.; Fauchet, P.M. Enhanced control of porous silicon morphology from macropore to mesopore formation. Phys. Status Solidi A 2005, 202, 1396–1401. [Google Scholar] [CrossRef]
- Wang, X.N.; Wang, Y.; Mei, Z.X.; Dong, J.; Zeng, Z.Q.; Yuan, H.T.; Zhang, T.C.; Dua, X.L.; Jia, J.F.; Xue, Q.K.; Zhang, X.N.; Zhang, Z.; Li, Z.F.; Lu, W. Low-temperature interface engineering for high-quality ZnO epitaxy on Si(111) substrate. Appl. Phys. Lett. 2007, 90, 1–3. [Google Scholar]
- Rieger, M.M.; Kohl, P.A. Mechanism of (111) silicon etching in HF-acetonitrile. J. Electrochem. Soc. 1995, 142, 1490–1495. [Google Scholar] [CrossRef]
- Petersen, K.E. Silicon as a mechanical material. Proc. IEEE 1982, 70, 420–457. [Google Scholar] [CrossRef]
- Li, Y.J.; Duan, R.; Shi, P.B.; Qin, G.G. Synthesis of ZnO nanoparticles on Si substrates using a ZnS source. J. Cryst. Growth 2004, 260, 309–315. [Google Scholar] [CrossRef]
- Fan, D.; Zhang, R.; Wang, X. Synthesis and optical property of ZnO nanonail arrays with controllable morphology. Physica E 2010, 42, 2081–2085. [Google Scholar] [CrossRef]
- Kim, M.S.; Yim, K.G.; Kim, D.Y.; Kim, S.; Nam, G.; Lee, D.-Y.; Kim, S.-O.; Kim, J.S.; Kim, J.S.; Son, J.-S.; Leem, J.-Y. Growth and characterization of seed layer-free ZnO thin films deposited on porous silicon by hydrothermal method. Electron. Mater. Lett. 2012, 8, 75–80. [Google Scholar] [CrossRef]
- Ghosh, M.; Raychaudhuri, A.K. Shape transition in ZnO nanostructures and its effect on blue-green photoluminescence. Nanotechnology 2008, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.Q.; Zhao, D.X.; Zhang, J.Y.; Shen, D.Z.; Lu, Y.M.; Liu, Y.C.; Fan, X.W. Growth temperature controlled shape variety of ZnO nanowires. Chem. Phys. Lett. 2005, 407, 91–94. [Google Scholar] [CrossRef]
- Huang, M.H.; Wu, Y.; Feick, H.; Tran, N.; Weber, E.; Yang, P. Catalytic growth of zinc oxide nanowires by vapor transport. Adv. Mater. 2001, 13, 113–116. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, Y.; Li, Q.K.; Nan, C.W. Synthesis and optical properties of tetrapod-like zinc oxide nanorods. Chem. Phys. Lett. 2002, 358, 83–86. [Google Scholar] [CrossRef]
- Quang, L.H.; Chua, S.J.; Loh, K.P.; Fitzgerald, E. The effect of post-annealing treatment on photoluminescence of ZnO nanorods prepared by hydrothermal synthesis. J. Cryst. Growth 2006, 287, 157–161. [Google Scholar] [CrossRef]
- Mamat, M.H.; Khusaimi, Z.; Zahidi, M.M.; Mahmood, M.R. Performance of an ultraviolet photoconductive sensor using well-aligned aluminium-doped zinc-oxide nanorod arrays annealed in an air and oxygen environment. Jpn. J. Appl. Phys. 2011, 50, 06GF05:1–06GF05:4. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rusli, N.I.; Tanikawa, M.; Mahmood, M.R.; Yasui, K.; Hashim, A.M. Growth of High-Density Zinc Oxide Nanorods on Porous Silicon by Thermal Evaporation. Materials 2012, 5, 2817-2832. https://doi.org/10.3390/ma5122817
Rusli NI, Tanikawa M, Mahmood MR, Yasui K, Hashim AM. Growth of High-Density Zinc Oxide Nanorods on Porous Silicon by Thermal Evaporation. Materials. 2012; 5(12):2817-2832. https://doi.org/10.3390/ma5122817
Chicago/Turabian StyleRusli, Nurul Izni, Masahiro Tanikawa, Mohamad Rusop Mahmood, Kanji Yasui, and Abdul Manaf Hashim. 2012. "Growth of High-Density Zinc Oxide Nanorods on Porous Silicon by Thermal Evaporation" Materials 5, no. 12: 2817-2832. https://doi.org/10.3390/ma5122817





