Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations
Abstract
:1. Introduction
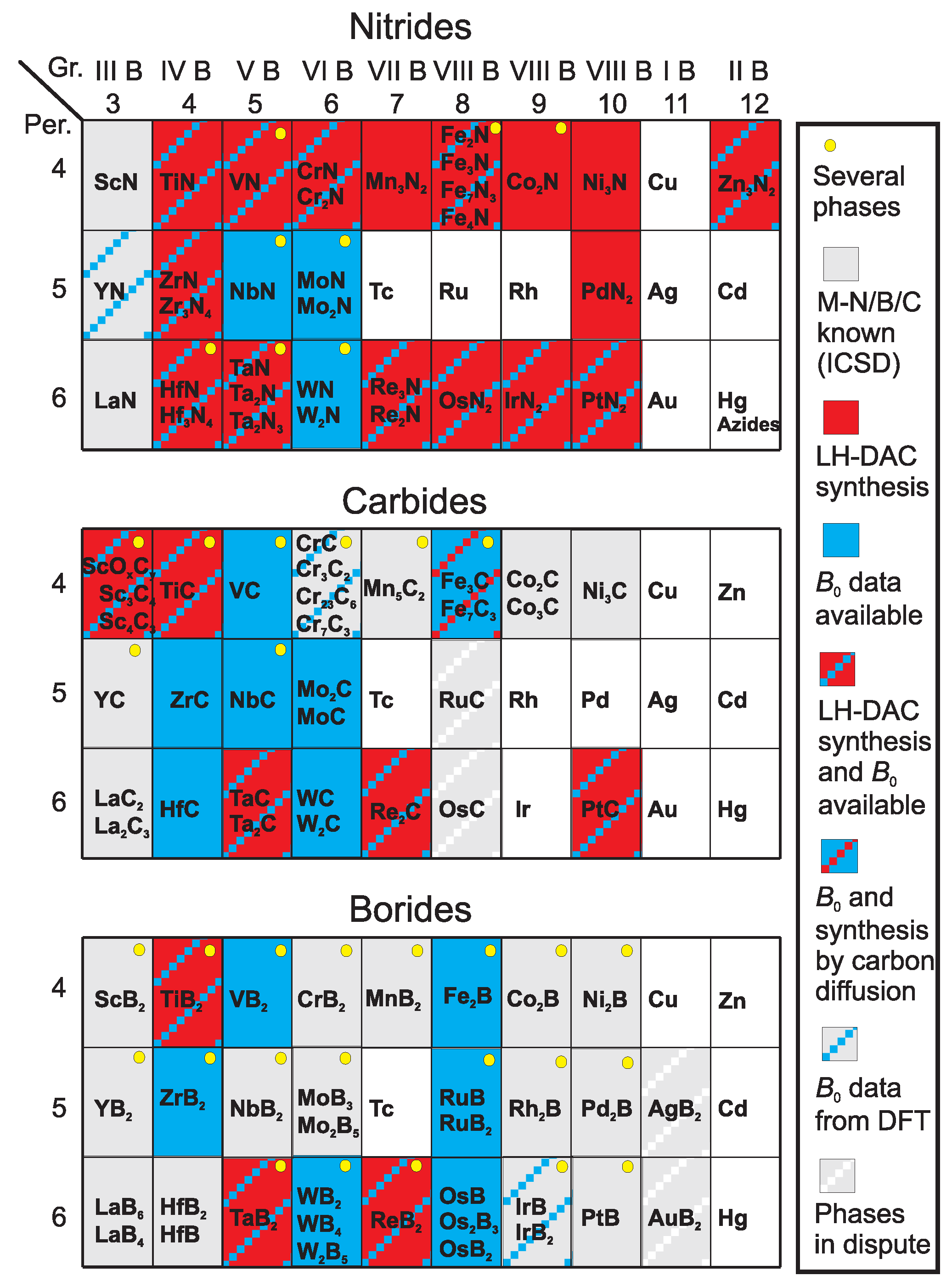
2. Experimental Approach
2.1. General Considerations
2.2. Laser-Heated Diamond Anvil Cell Experiments
2.3. Characterization of the Synthesis Products
3. Computational Approaches
4. Transition Metal Nitrides
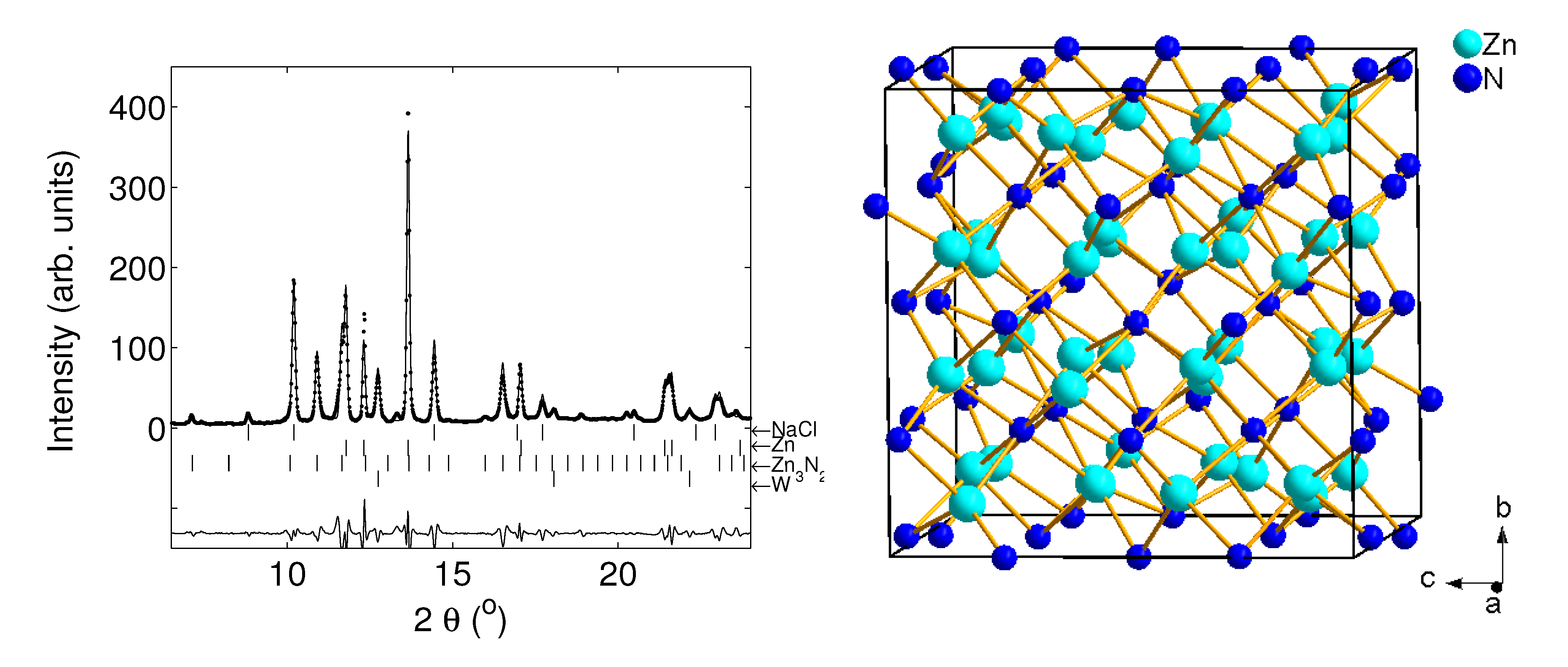
4.1. Period 4 (3d) Transition Metal Nitrides
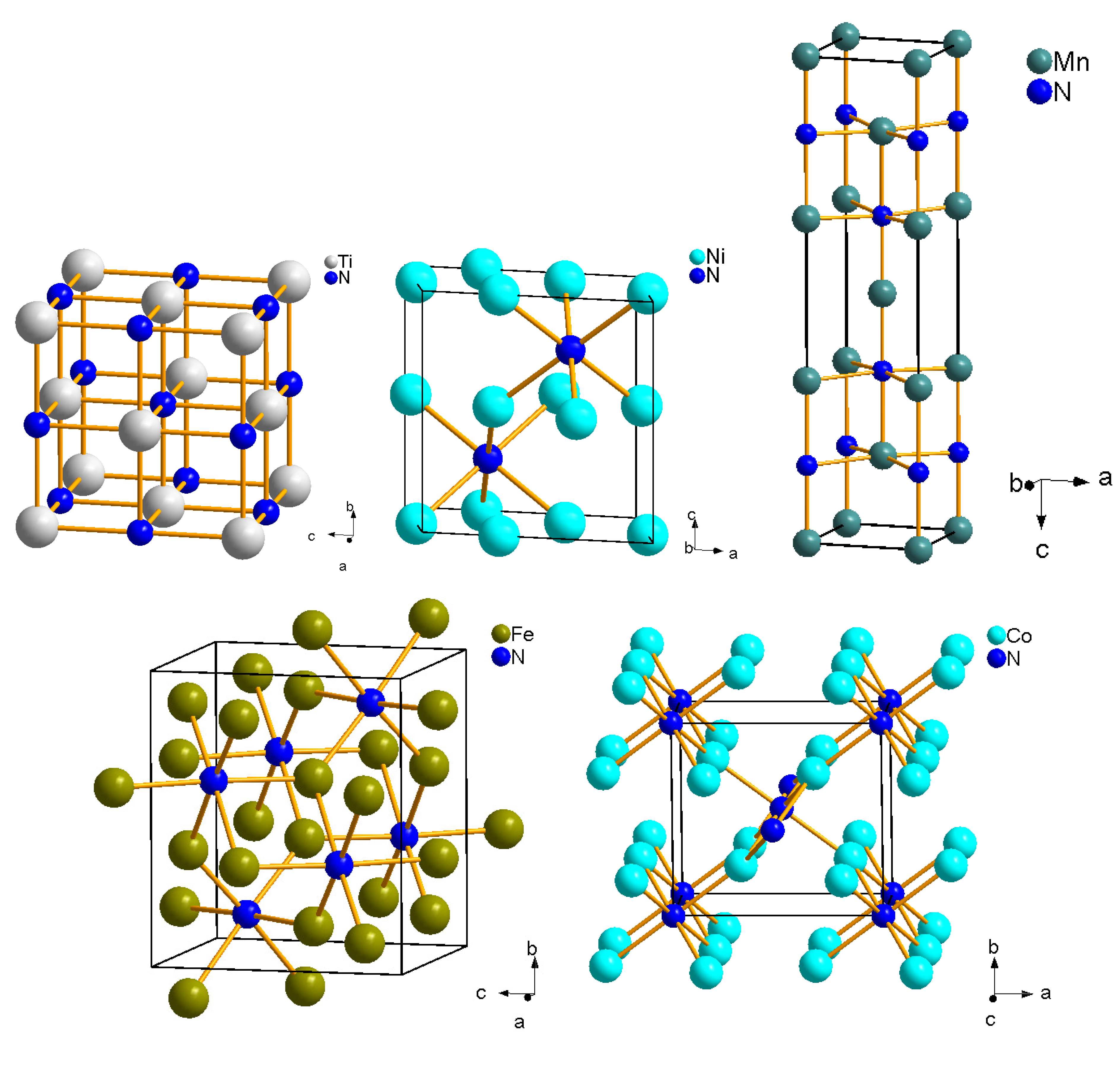
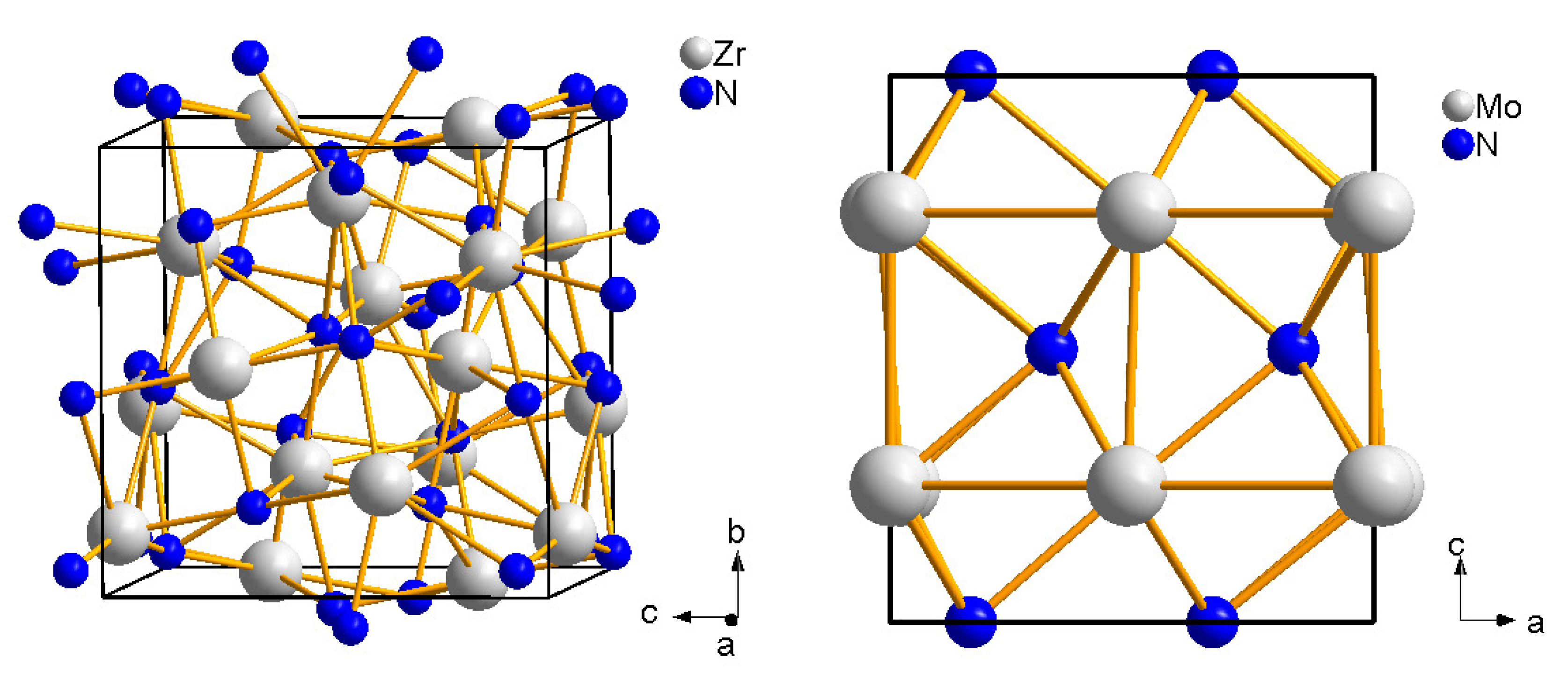
4.2. Zirconium and Hafnium Nitrides
4.3. Molybdenum Nitrides
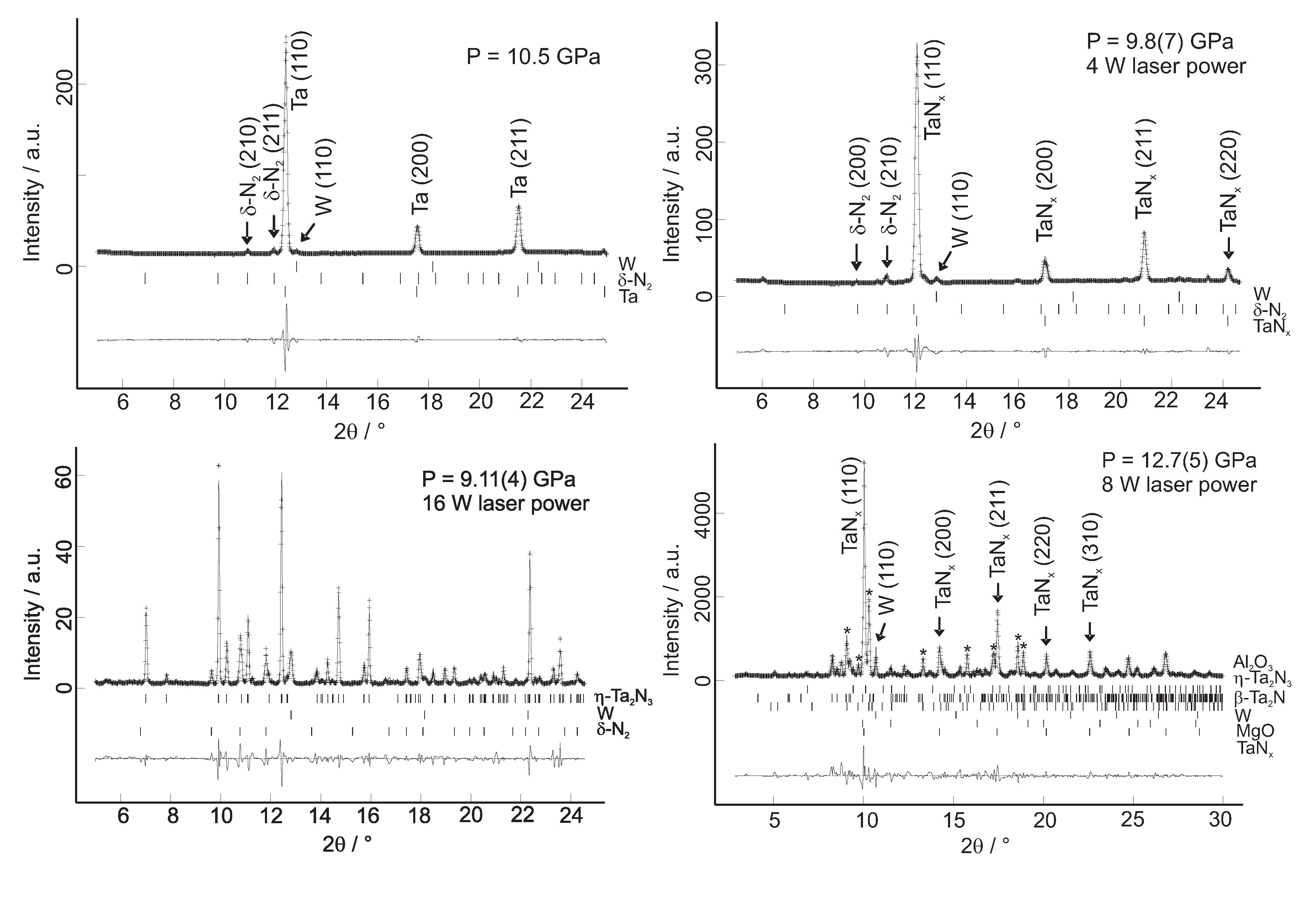
4.4. Tantalum Nitrides
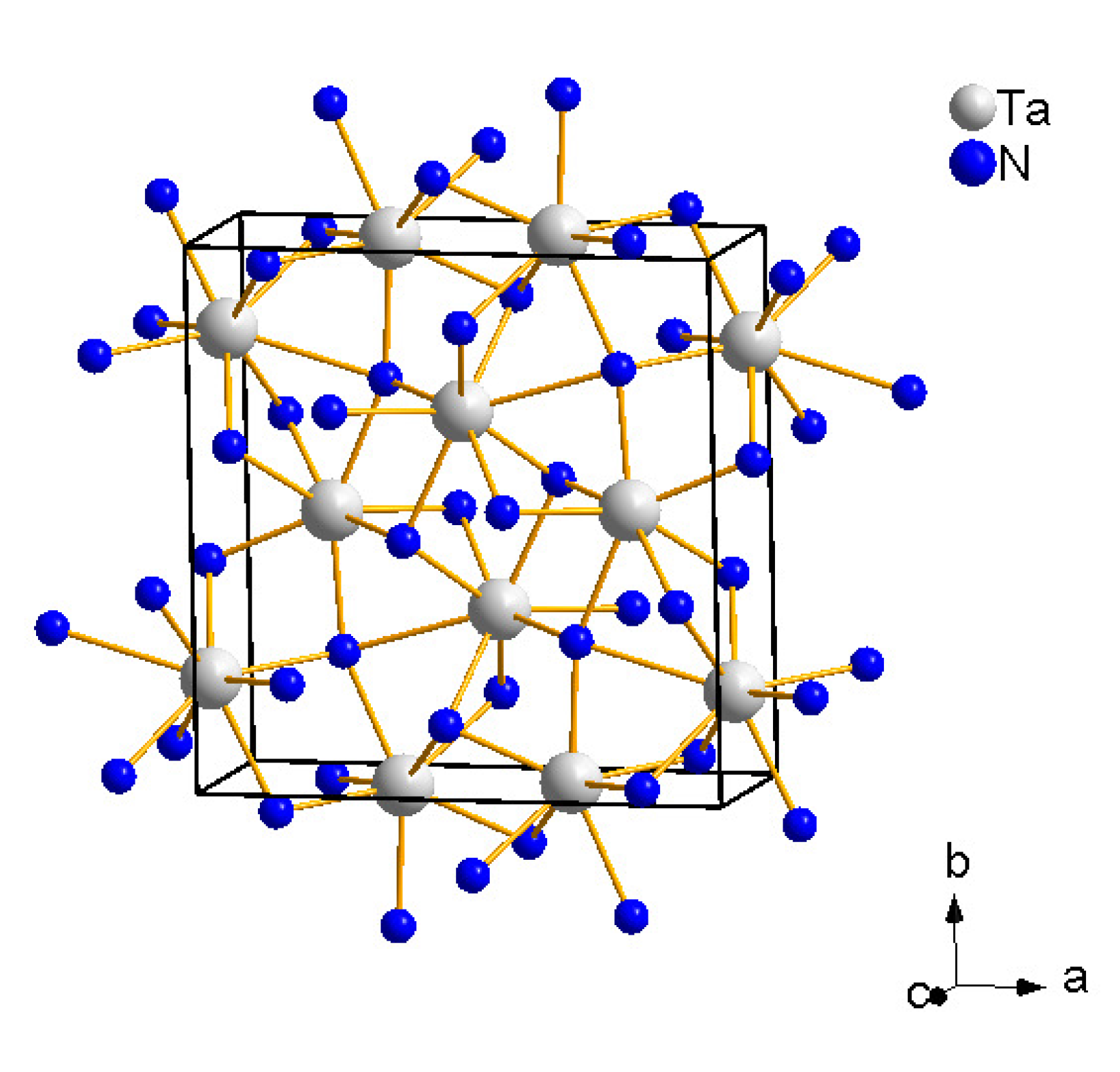
4.5. Rhenium Nitrides
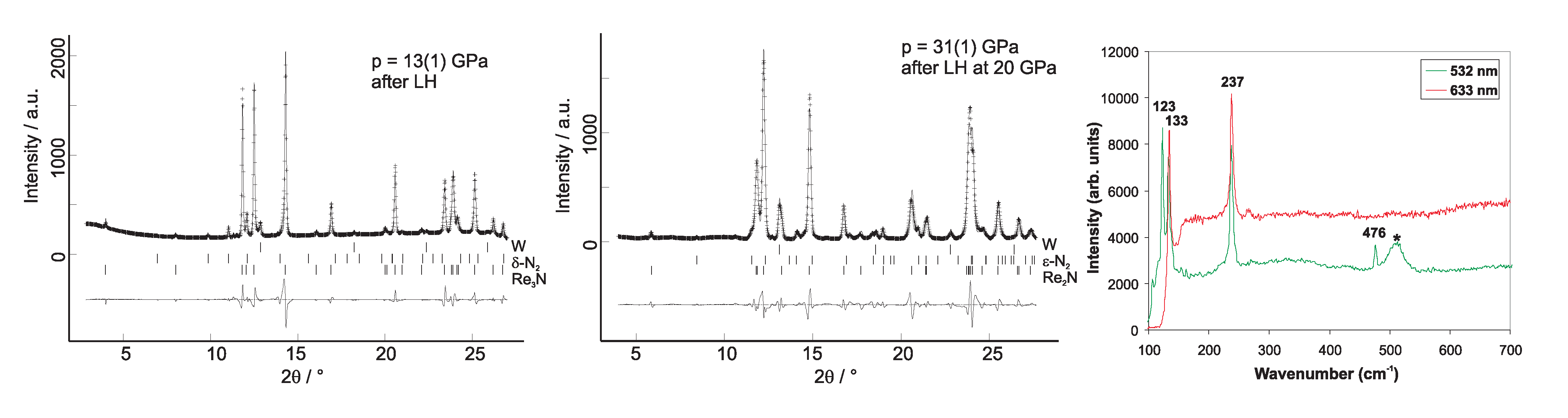
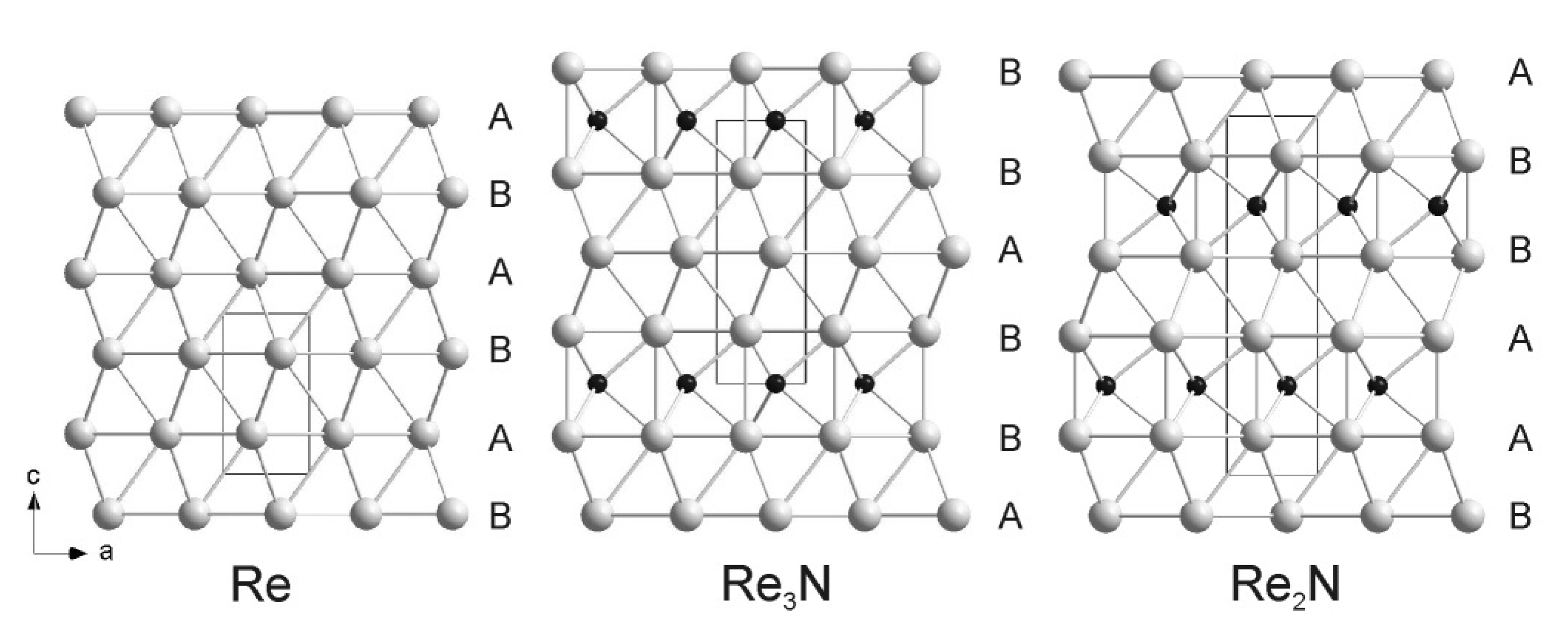
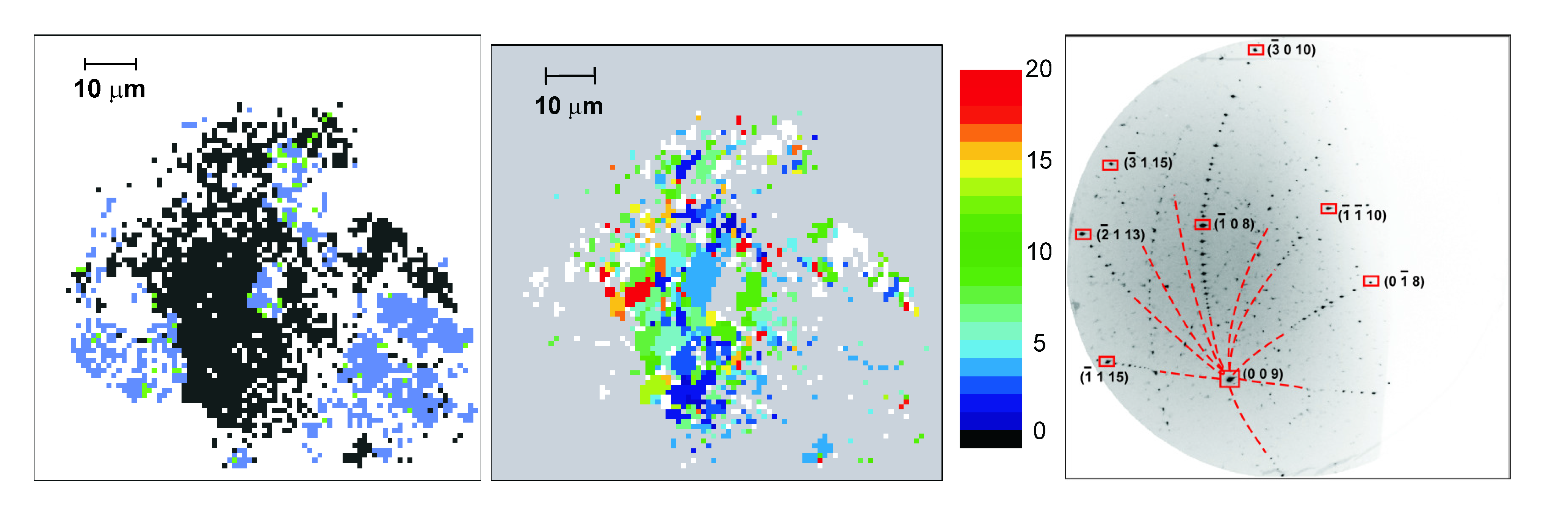
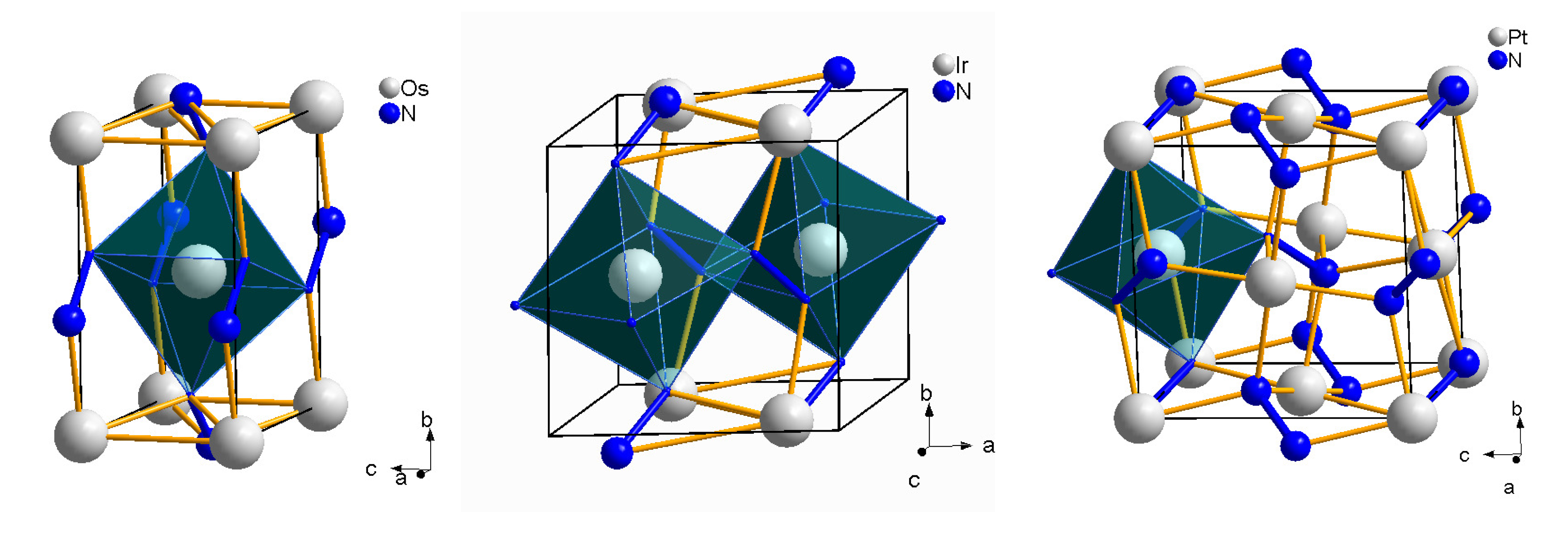
4.6. Osmium, Iridium, Platinum and Palladium Nitrides
4.7. Mechanical Properties of the Transition Metal Nitrides
| Compound | Space group | Structure | (GPa) | Ref. | Hardness (GPa) | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|
| Group III | ScN | NaCl | |||||||
| YN | NaCl | DFT: 157 | 3.5 | [75] | |||||
| LaN | NaCl | ||||||||
| Group IV | TiN | NaCl | 277–289 | [76,77,78] | 18–21 | [1,76,79] | |||
| δ-ZrN | NaCl | 248 | [80] | 15.8–17.4 | [1,80] | ||||
| c-ZrN | c-ThP | 217–223 | 4–4.4 | [81,82] | 18 | [81] | |||
| δ-HfN | NaCl | 260 | [80] | 16.3–19.5 | [1,80] | ||||
| c-HfN | c-ThP | 227–241 | 4–5.3 | [83] | DFT: 21.3/18.7 | [84] | |||
| Group V | VN | NaCl | 265(5) | [85] | 6–15 | [1,85,86] | |||
| δ-NbN | NaCl | 348 GPa | [80] | 13.3–20.0 | [1,80] | ||||
| β-TaN | hcp | 360(3) | [87] | ||||||
| ϵ-TaN | CoSn | 288(6) | 4.7(0.5) | [88] | 24.7 | [79] | |||
| η-TaN | US | 319(6) | [56] | 16 | [32] | ||||
| Group VI | CrN | NaCl | DFT: 340–430 | [85] | 13–17 | [49] | |||
| hp-CrN | 243(10) | [85] | |||||||
| CrN | hcp | 275(23) | 2.0(2.0) | [88] | 15.7 | in [77] | |||
| δ-MoN | FeS (2H) | 345(9) GPa | 3.5(3) | [88,89] | 38.5 | [5] | |||
| γ-MoN | NaCl | 301–304 | 4 | [55,89] | 35.7 | [77] | |||
| WN | WC | 30(5) | [50] | ||||||
| WN | NaCl | 240(10) | 11.7(1.6) | [74] | 31(3) | [50] | |||
| Group VII | MnN | IrUC | |||||||
| ReN | 395(7) | [60] | |||||||
| ReN | SMo | 401(10) | [60] | ||||||
| Group VIII | FeN | PbO | |||||||
| ϵ-FeN | hcp | 168(10) | 5.7(1.5) | [90] | |||||
| -FeN | FeN | 155(3) | [90] | ||||||
| ϵ-FeN | 172(4) | 5.7 | [91] | 7.4(10) | [91] | ||||
| OsN | o-FeS | 358(6) | 4.67 | [63] | |||||
| CoN | CFe | ||||||||
| IrN | CoSb | 428(12) | [63] | ||||||
| NiN | ORe | ||||||||
| PdN | c-FeS | ||||||||
| PtN | c-FeS | 354–372 | 4–5.26 | [65] | |||||
| Group II | ZnN | OMn | 228(2) | [92] |
5. Transition Metal Carbides
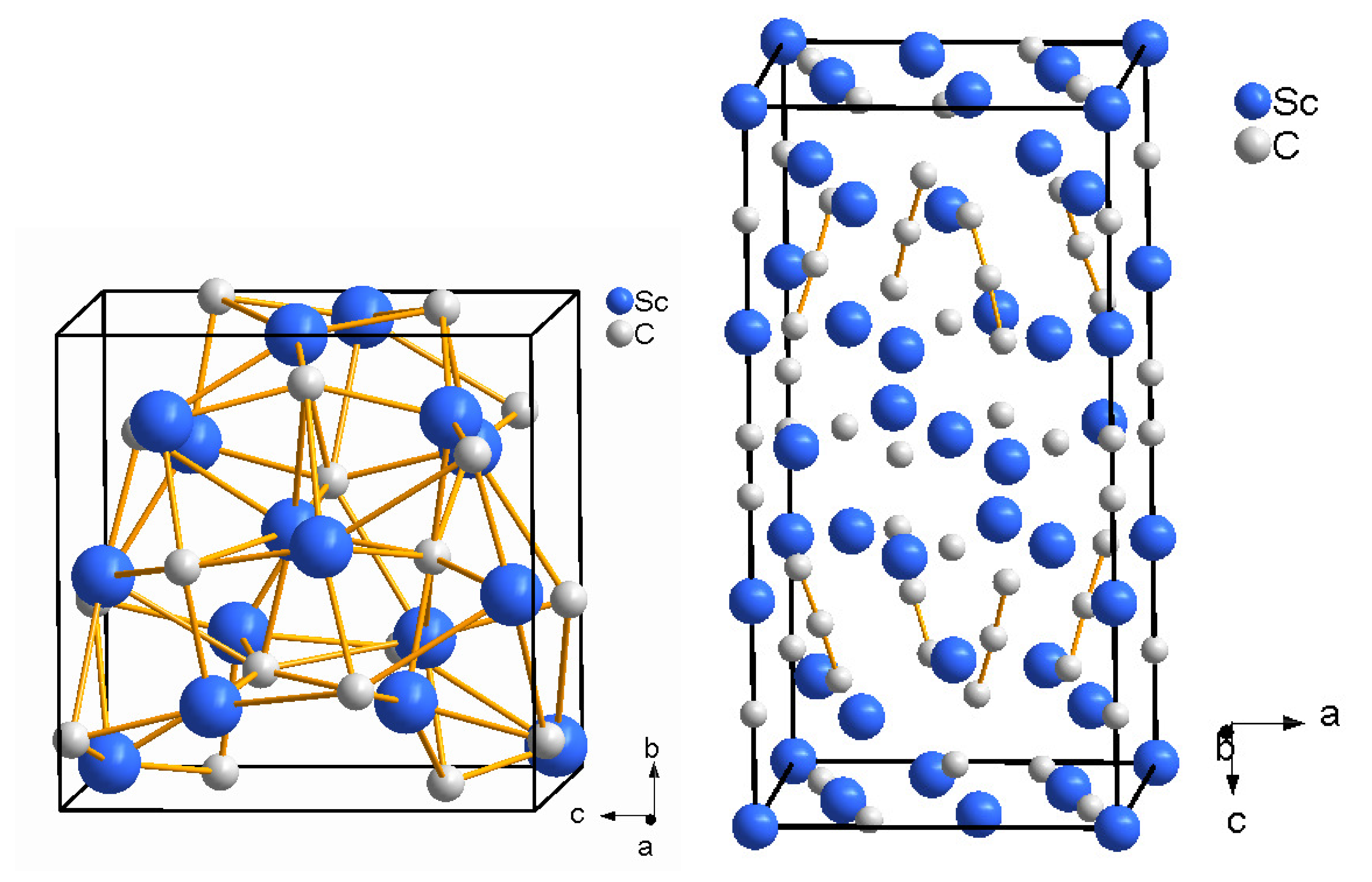
5.1. Scandium Carbides
5.2. Titanium Carbide
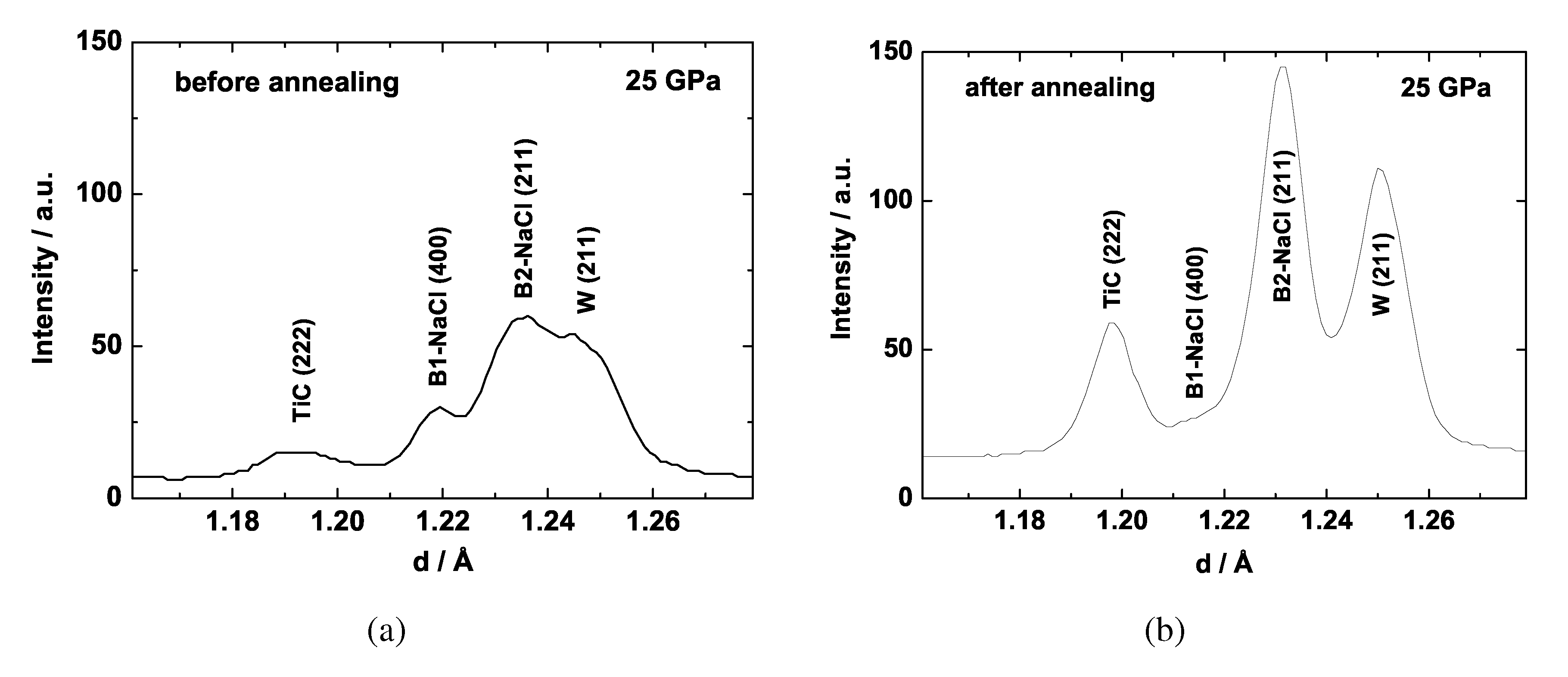
5.3. Iron Carbides
5.4. Tantalum Carbides
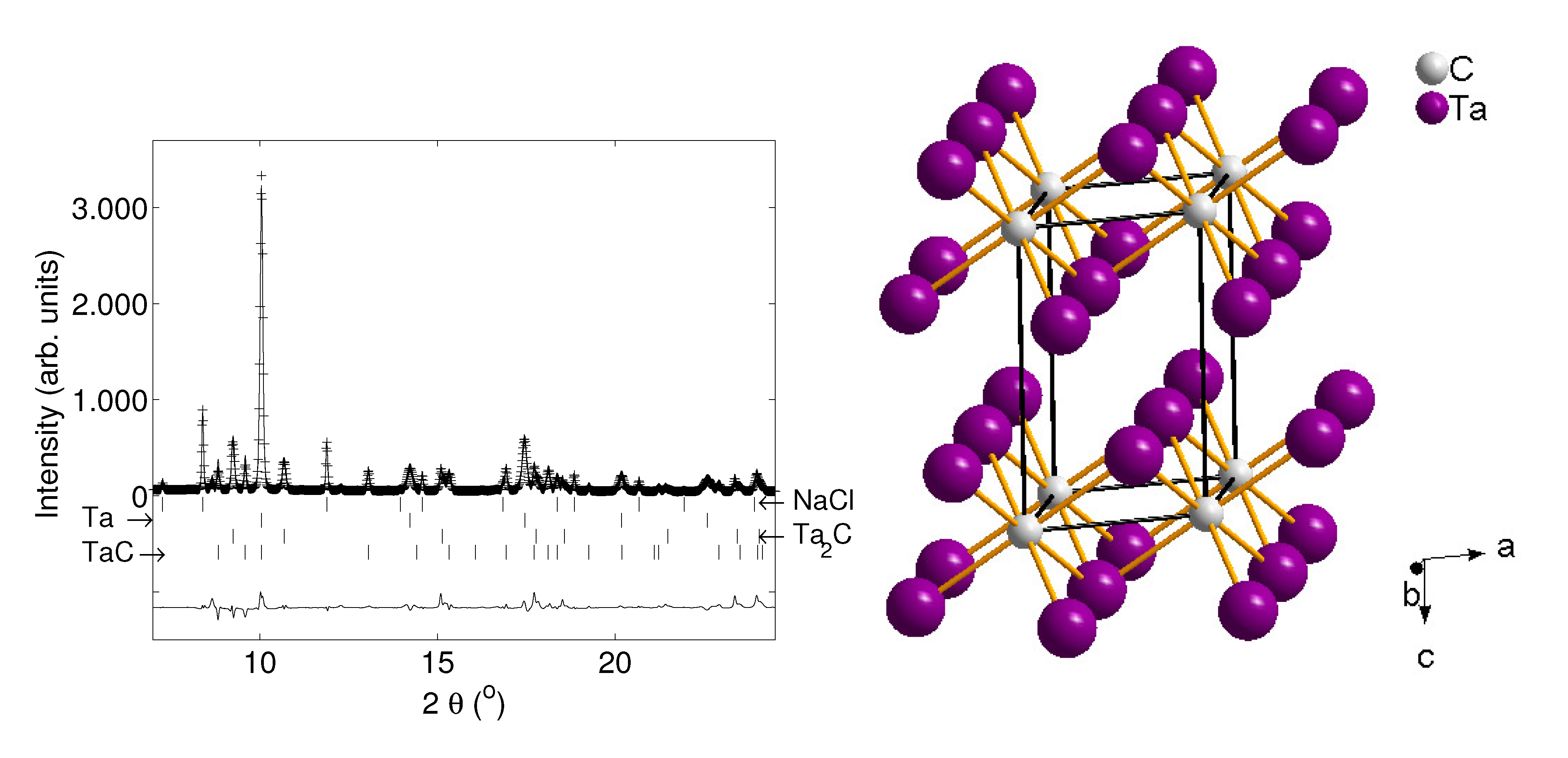
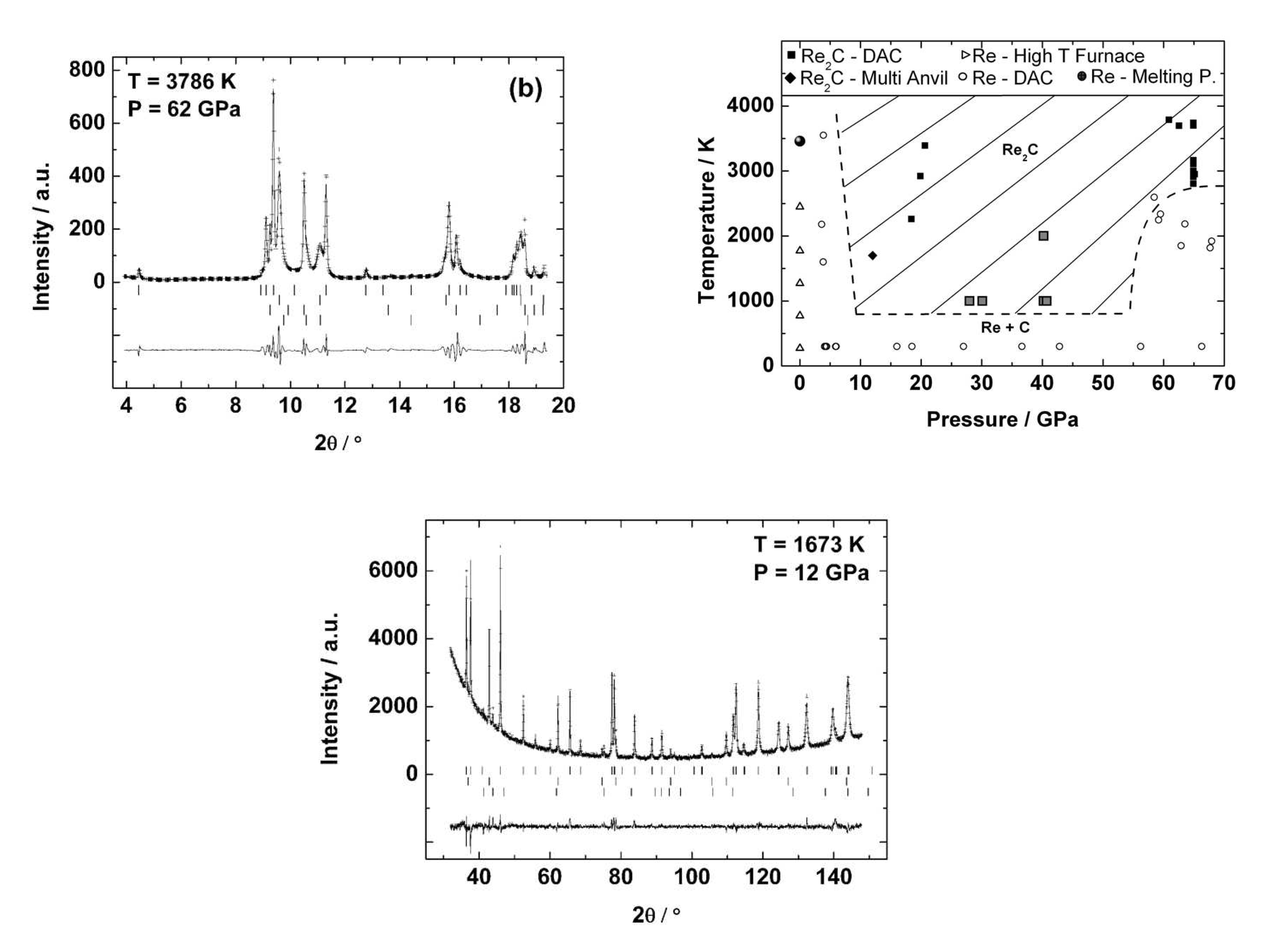
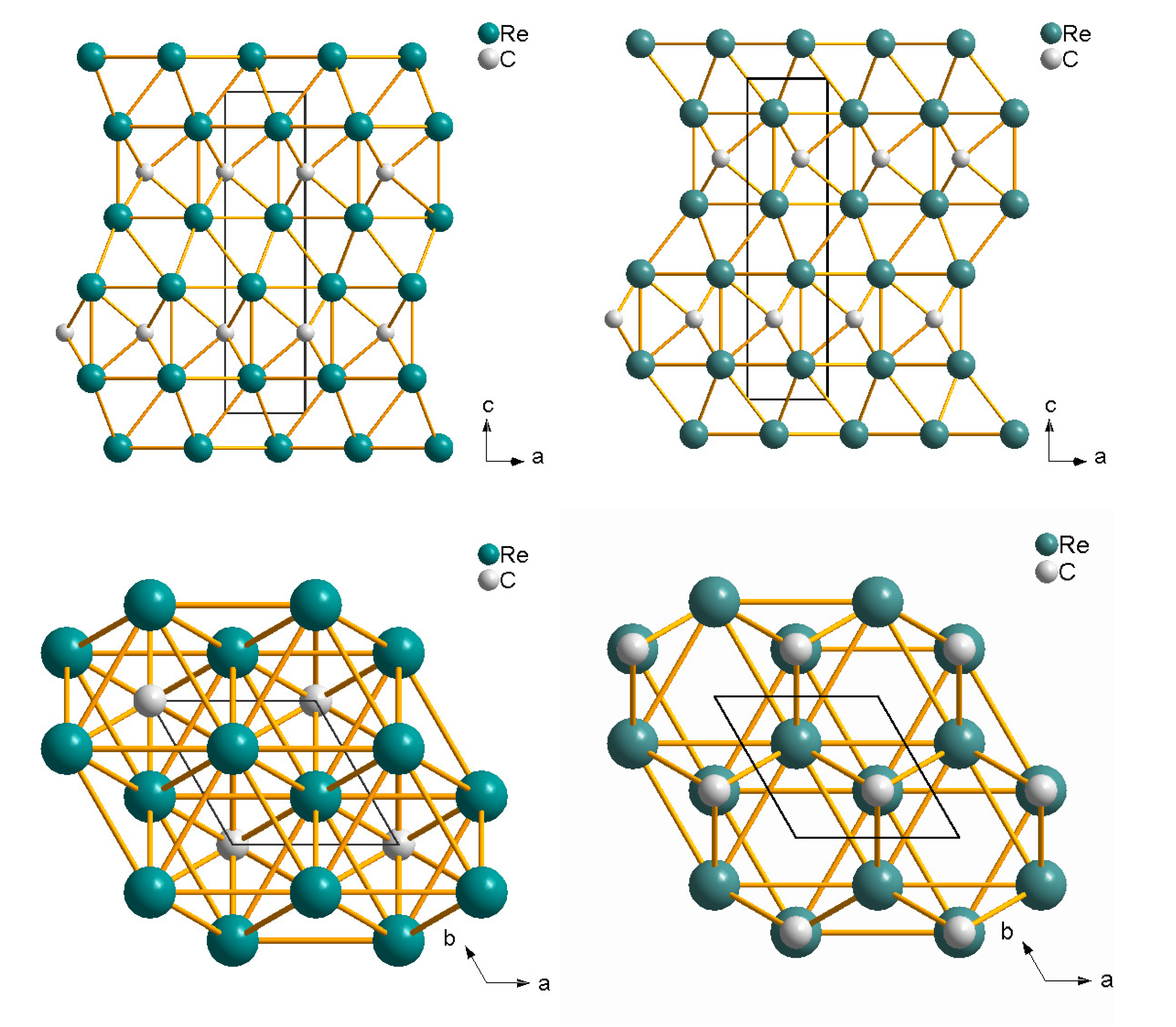
5.5. Rhenium Carbide
5.6. Platinum Carbide
5.7. Mechanical Properties of Transition Metal Carbides
| Compound | Space group | Structure | (GPa) | Ref. | Hardness (GPa) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Group III | ScOC | NaCl | 190(90) | [33] | ||||
| ScC | ThP | 157(2) | [33] | |||||
| ScC | 144(3) | [33] | ||||||
| ScC | 105(1) | [33] | ||||||
| Group IV | TiC | NaCl | 233–268 | 4–6.5 | [111,116,117,118,119,120,121,122] | 28–35 | [1] | |
| ZrC | NaCl | 207-223 | [1,116] | 26(2) | [1,77] | |||
| HfC | NaCl | 242 | [123] | 26.1 | [1] | |||
| Group V | VC | NaCl | 258(11), 390 | 4.5(6) | [1,124] | 27–29 | [77,125] | |
| NbC | NaCl | 266.7–340 | [1,123,126,127,128] | 19.6–24 | [1,77] | |||
| TaC | NaCl | 317–345 | [120,123,129,130,131] | 13.5–18 | [1,77,113] | |||
| TaC | NaCl | 217 | [132] | |||||
| Group VI | CrC | CrC | DFT: 300–315 | 4.26 | in [133] | 16 | [134] | |
| CrC | CrC | DFT: 282–300 | in [133] | 14.5 | [134] | |||
| CrC | CrC | DFT: 313–333 | in [133] | 10–18 | [1] | |||
| MoC | FeN | 307(5) | 6.2(3) | [135] | 14–24.5 | [1,135,136] | ||
| WC | WC | 329-439 | 4-4.7 | [1,123,137,138] | 22–28 | [1,29,139] | ||
| Group VII | ReC | SMo | 405(30) | 4.6 | [114] | 17.5 | [115] | |
| Group VIII | FeC | FeC | 174–175 | 4.8–5.2 | [140,141] | 8–11 | [134] | |
| FeC | CrC | 253 | 3.6 | in [142] | ||||
| PtC | NaCl | 301-339 | 4–5.2 | [97] |
6. Transition Metal Borides
6.1. Titanium Borides
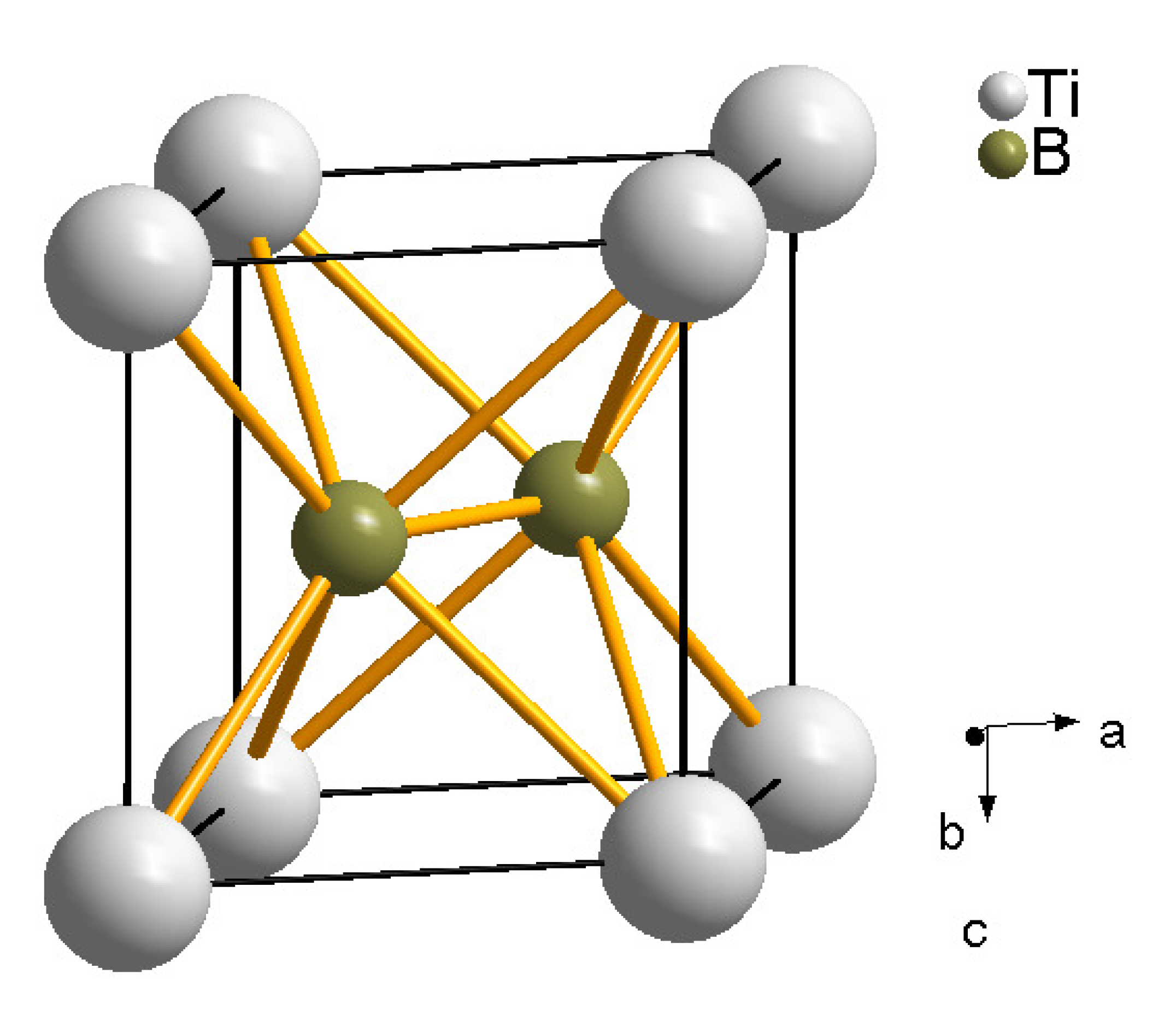
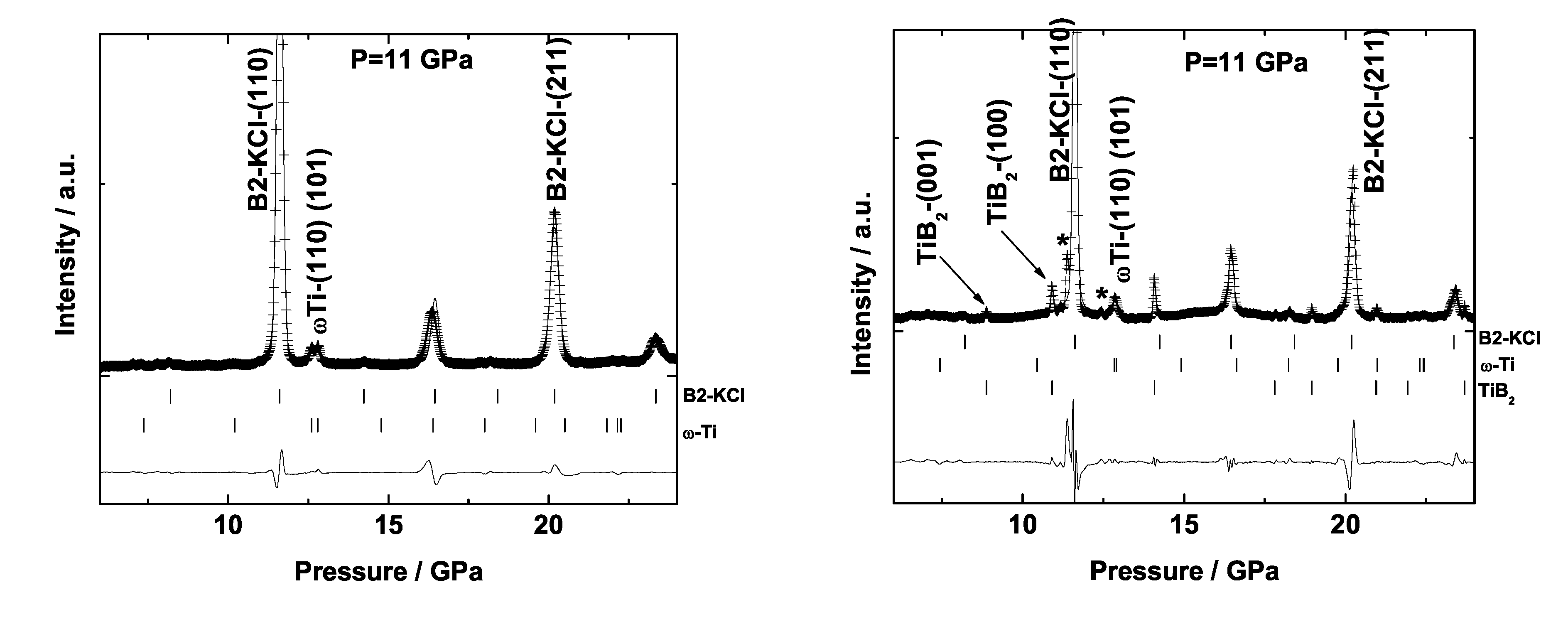
6.2. Tantalum Boride
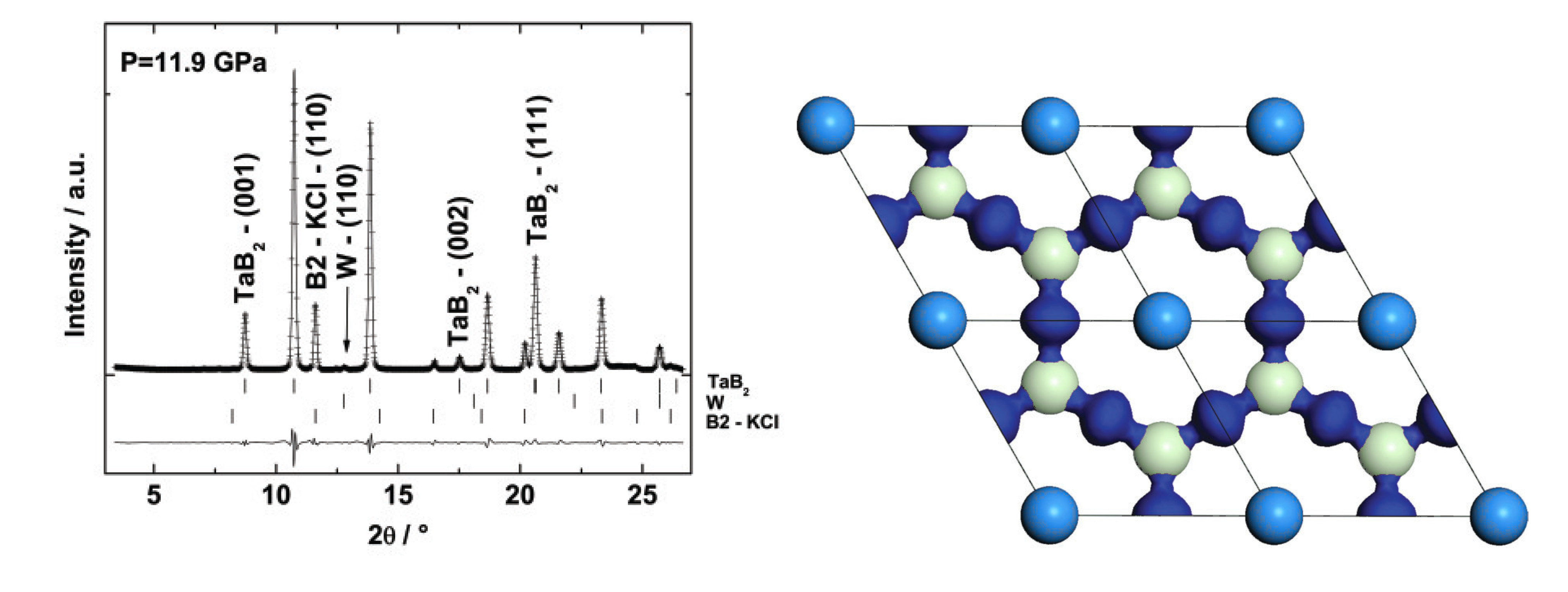
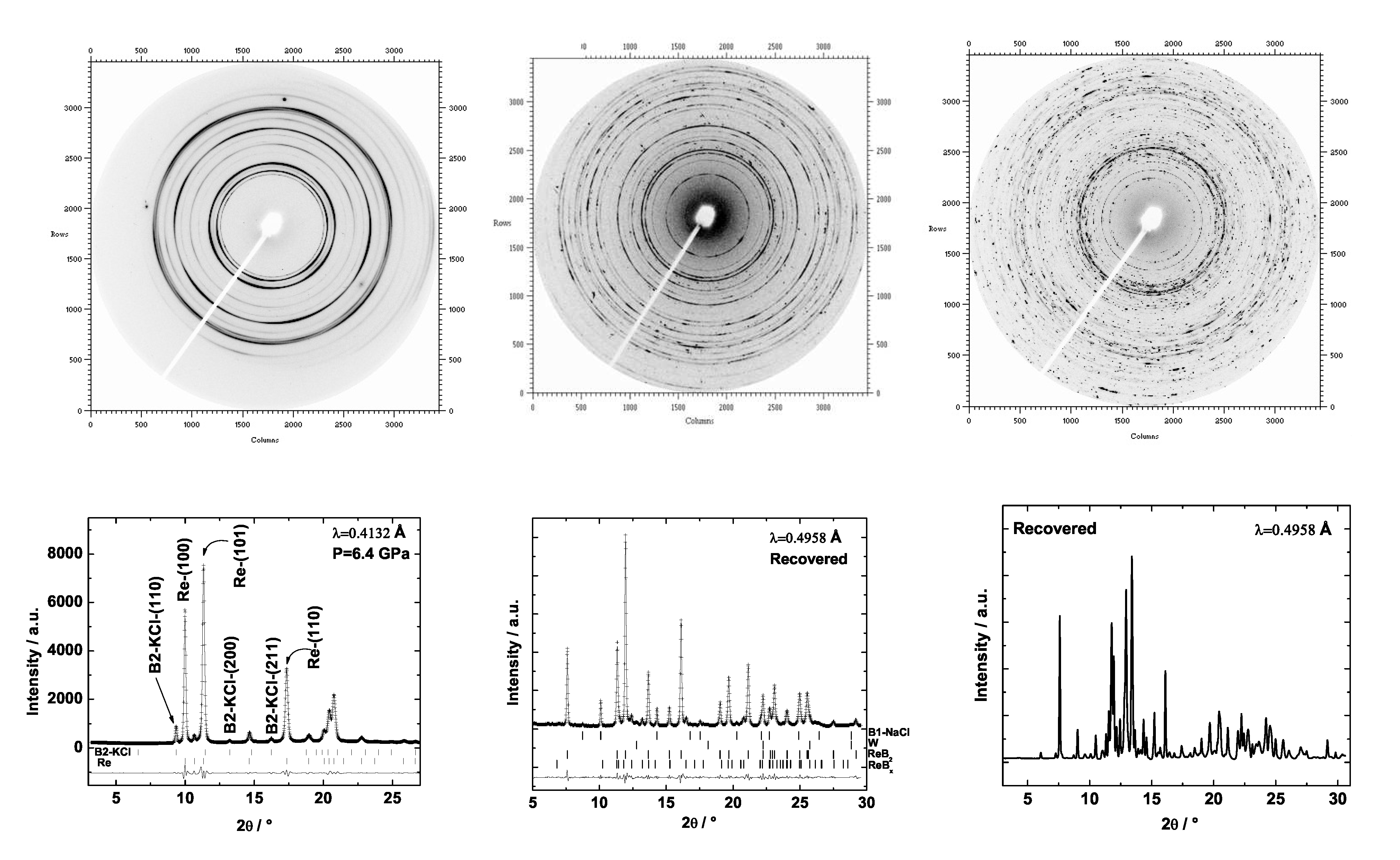
6.3. Rhenium Borides
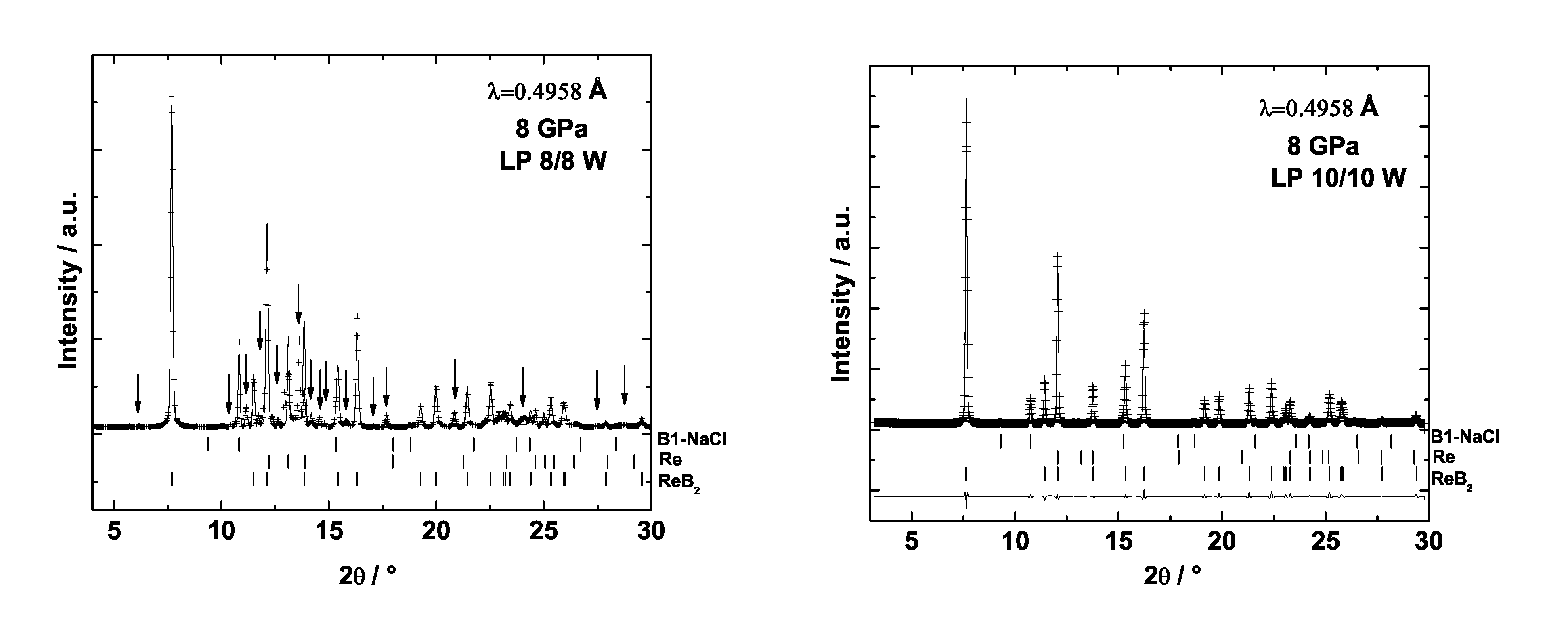
6.4. Mechanical Properties of Transition Metal Borides
7. Conclusions
7.1. Crystal Chemistry
| Compound | Space group | Structure | (GPa) | Ref. | Hardness (GPa) | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Group IV | TiB | AlB | 240, DFT: 292 | DFT: 3.34 | [6,159] | 15–45 | [1,29] | |
| ZrB | AlB | 317 | 4 | [159] | 22.5–35 | [1,29] | ||
| HfB | AlB | 29.0 | [1] | |||||
| Group V | VB | AlB | 322 | 4 | [159] | 20.9 | [1] | |
| NbB | AlB | 20.9 | [1] | |||||
| TaB | AlB | 336.3(5) | 4 | [147] | 22.6–25.6 | [1,160] | ||
| Group VI | CrB | AlB | 20.5 | [1] | ||||
| MoB | 23.0 | [1] | ||||||
| MoB | 31.8 | [115] | ||||||
| WB | MoB | 26.1 | [1] | |||||
| WB | 341–372 | 4–6.4 | [2] | 38.4(14)/27.7(6) | [2] | |||
| WB | 304–325 | [2,161] | 46.2(12)/31.8(12) | [2] | ||||
| Group VII | ReB | ReB | 360–382 | [3,162,163] | 30–48 | [2,3,4,29,162] | ||
| Group VIII | bct-FeB | AlCu | 164(14) | 4.4(5) | [164] | 14 | [134] | |
| RuB | WC | 261–275 | 4–5.2 | [2] | 13.6 | [158] | ||
| RuB | RuB | 242–303 | 4–9.7 | [2,162] | 19(2) | [162,163] | ||
| OsB | WC | 431–453 | 4–5.8 | [2] | 14.4(1.1)/10.6(1.3) | [2] | ||
| OsB | RuB | 396–443 | 4–7.1 | [2] | 21.8(1.5)/14.7(8) | [2] | ||
| OsB | RuB | 342–395, 297(25) | 4–4.4 | [2,158,162,163,165,166] | 17–37 | [2,158,162,163] | ||
| IrB | AlB | DFT: 324 | 4.3 | [167] | ||||
| IrB | DFT: 346 | 4.58 | [167] |
7.2. Non-Stoichiometry
7.3. Compressibility
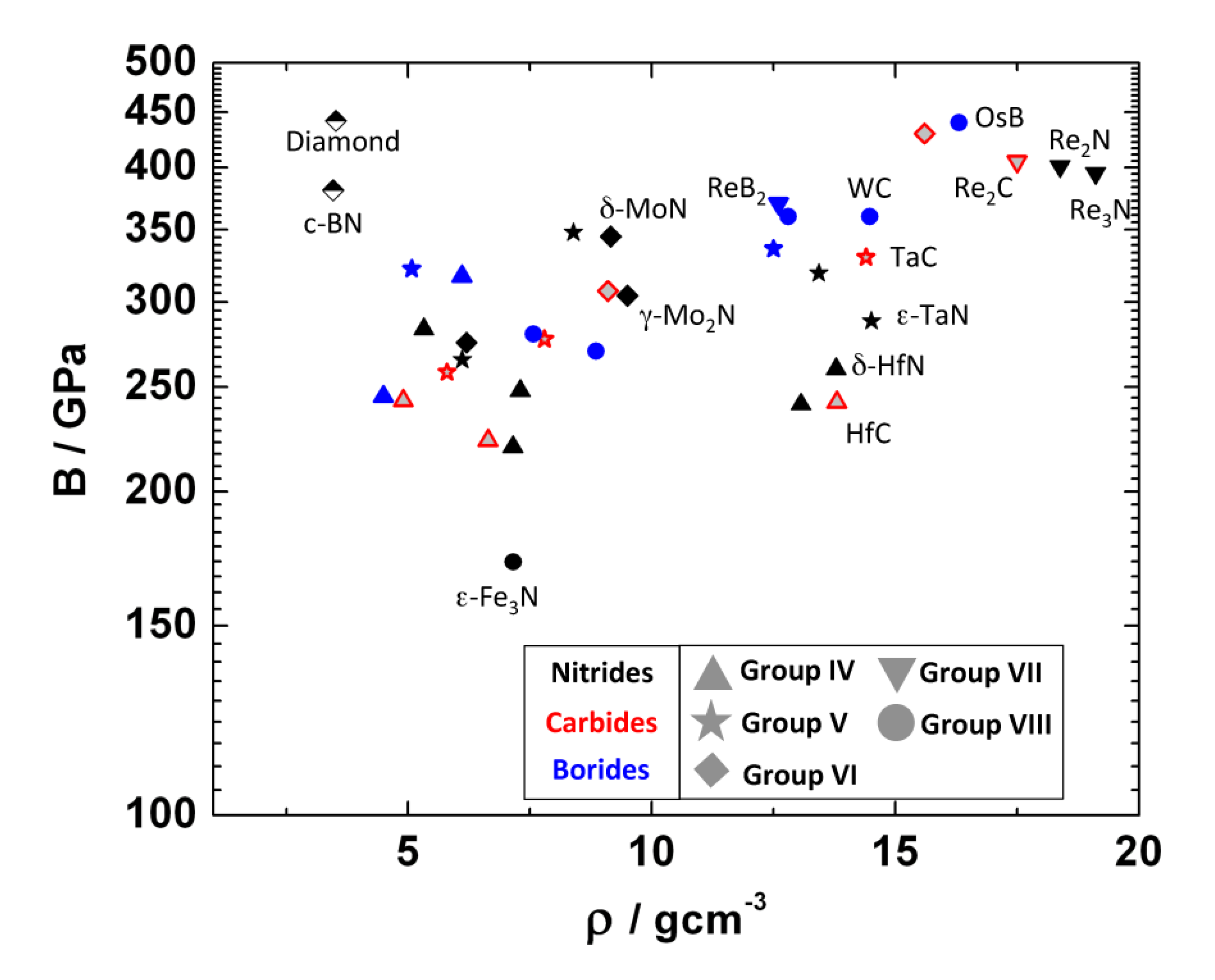

7.4. Hardness
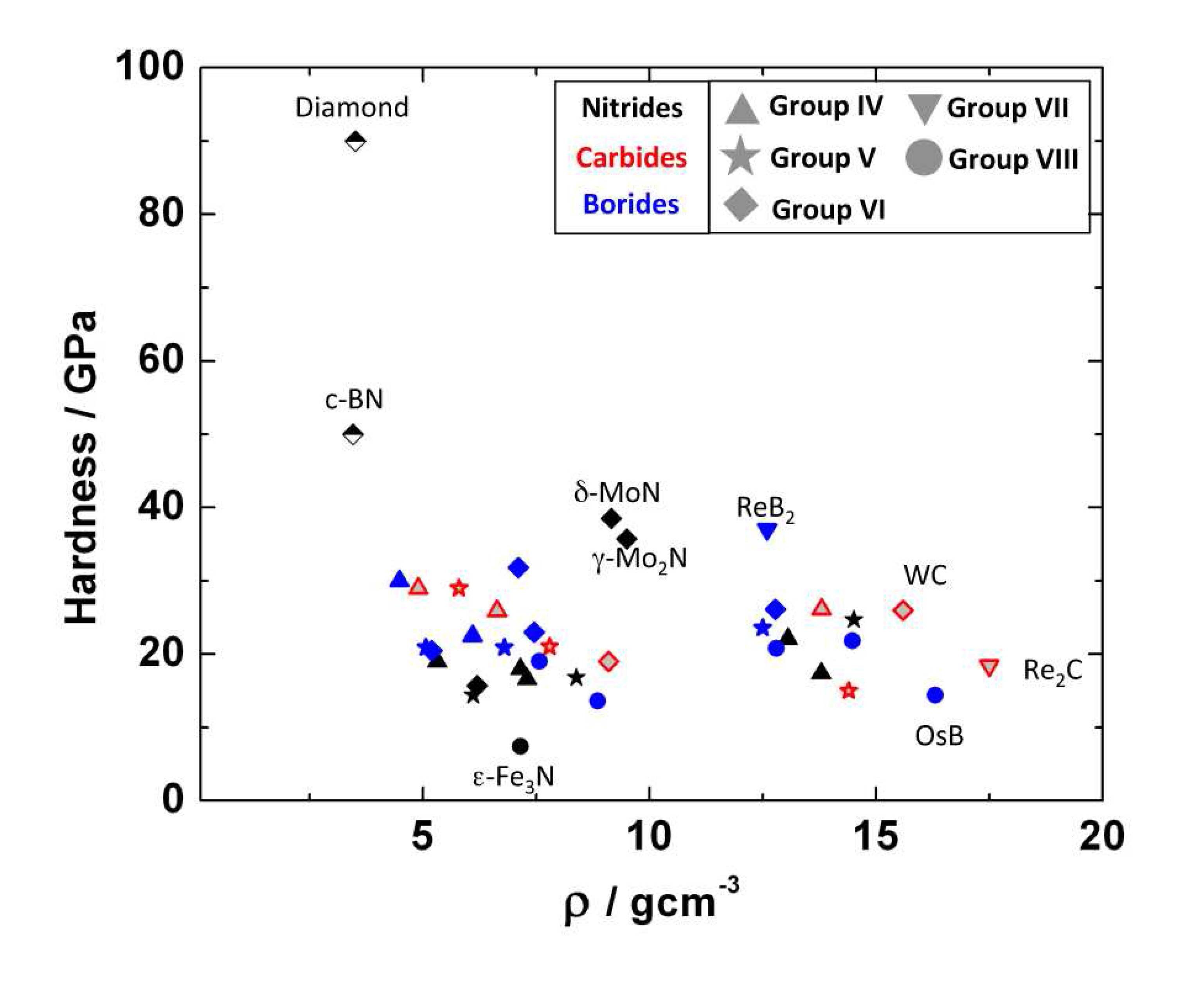
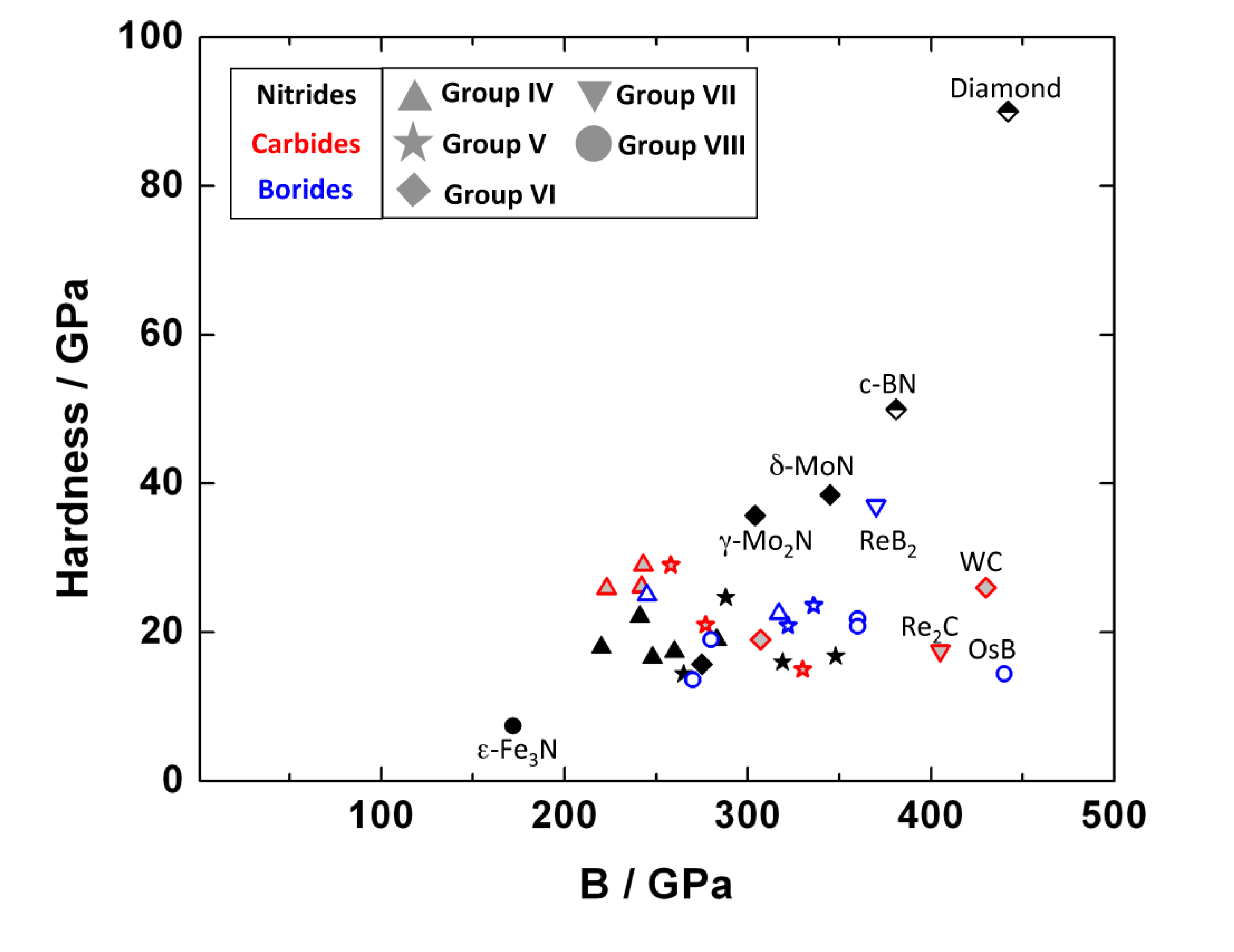
Acknowledgements
References
- Pierson, H.O. Handbook of Refractory Carbides and Nitrides; Noyes Publication: Park Ridge, UT, USA, 1996. [Google Scholar]
- Gu, Q.; Krauss, G.; Steurer, W. Transition metal borides: Superhard versus Ultra-incompressible. Adv. Mater. 2008, 20, 3620–3626. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure. Science 2007, 316, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Otani, S.; Korsukova, M.M.; Aizawa, T. High-temperature hardness of ReB2 single crystals. J. Alloy Compd. 2009, 477, L28–L29. [Google Scholar] [CrossRef]
- Ürgen, M.; Eryilmaz, O.L.; Cakir, A.F.; Kayali, E.S.; Nilüfer, B.; Isik, Y. Characterization of molybdenum nitride coatings produced by arc-PVD technique. Surf. Coat. Tech. 1997, 94–95, 501–506. [Google Scholar] [CrossRef]
- Munro, R.G. Material properties of titanium diboride. J. Res. Natl. Inst. Stan. 2000, 105, 709–720. [Google Scholar] [CrossRef]
- Opila, E.; Levine, S.; Lorincz, J. Oxidation of ZrB2- and HfB2-based ultra-high temperature ceramics: Effect of Ta additions. J. Mater. Sci. 2004, 39, 5969–5977. [Google Scholar] [CrossRef]
- Blum, Y.D.; Marschall, J.; Hui, D.; Adair, B.; Vestel, M. Hafnium reactivity with boron and carbon sources under non-self-propagating high-temperature synthesis conditions. J. Am. Ceram. Soc. 2008, 91, 1481–1488. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Serghiou, G.; Schwarz, M.; Kroke, E.; Riedel, R.; Fueß, H.; Kroll, P.; Boehler, R. Synthesis of cubic silicon nitride. Nature 1999, 400, 340–342. [Google Scholar]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. High melting points of tantalum in a laser-heated diamond anvil cell. Phys. Rev. Lett. 2010, 104, 255701:1–255701:4. [Google Scholar] [CrossRef] [PubMed]
- Narygina, O.; Dubrovinsky, L.S.; Miyajima, N.; McCammon, C.A.; Kantor, I.Y.; Mezouar, M.; Prakapenka, V.B.; Dubrovinskaia, N.A.; Dmitriev, V. Phase relations in Fe-Ni-C system at high pressures and temperatures. Phys. Chem. Miner. 2011, 38, 203–214. [Google Scholar] [CrossRef]
- Caldwell, W.; Kunz, M.; Celestre, R.; Domning, E.E.; Walter, M.J.; Walker, D.; Glossinger, J.; MacDowell, A.A.; Padmore, H.A.; Jeanloz, R.; Clark, S.M. Laser-heated diamond anvil cell at the advanced light source beamline 12.2.2. Nuc. Instrum. Methods 2007, 582, 221–225. [Google Scholar] [CrossRef]
- Mezouar, M.; Crichton, W.A.; Bachau, S.; Thurel, F.; Witsch, H.; Torrecillas, F.; Blattmann, G.; Marion, P.; Dabin, Y.; Chavanne, J.; Hignette, O.; Morawe, C.; Borel, C. Development of a new state-of-the-art beamline optimized for monochromatic single-crystal and powder X-ray diffraction under extreme conditions at the ESRF. J. Synchrotron Rad. 2005, 12, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Prakapenka, V.B.; Eng, P.J.; Rivers, M.L.; Sutton, S.R. Facilities for high-pressure research with the diamond anvil cell at GSECARS. J. Synchrotron Rad. 2005, 12, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Kondo, T.; Watanuki, T.; Shimomura, O.; Kikegawa, T. Laser heated diamond anvil apparatus at the Photon Factory and SPring-8: Problems and improvements. Rev. Sci. Instrum. 2001, 72, 1293–1297. [Google Scholar] [CrossRef]
- Liermann, H.P.; Morgenroth, W.; Berghäuser, A.; Ehnes, E.; Winkler, B.; Franz, H.; Weckert, E. The extrem conditions beamline at PETRA III, DESY: Possibilities to conduct time resolved monochromatic and pink beam diffraction experiments in laser heated DAC. In Abstract of the Joint AIRAPT-22 & HPCJ-50; Tokyo, Japan, 2009; No. 28P94. [Google Scholar]
- Mao, H.; Bell, P.; Shaner, J.; Steinberg, D. Specific volume measurements of Cu, Mo, Pd, and Ag and calibration of the ruby R1 fluorescence pressure gauge from 0.06 to 1 Mbar. J. Appl. Phys. 1978, 49, 3276–3283. [Google Scholar] [CrossRef]
- Mao, H.K.; Xu, J.; Bell, P.M. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Benedetti, L.R.; Loubeyre, P. Temperature gradients, wavelength-dependent emissivity, and accuracy of high and very-high temperatures measured in the laser-heated diamond cell. High Pressure Res. 2004, 4, 423–445. [Google Scholar] [CrossRef]
- Bauer, J.D.; Bayarjargal, L.; Winkler, B. The fluorescence life time of ruby at high pressures and temperatures. In Abstract of the XXII International Congress and General Assembly of the IUCr; Madrid, Spain, 2011. [Google Scholar]
- Wilke, M.; Appel, K.; Vincze, L.; Schmidt, C.; Borchert, M.; Pascarelli, S. A confocal set-up for micro-XRF and XAFS experiments using diamond-anvil cells. J. Synchrotron Rad. 2010, 17, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Tamura, N.; Chen, K.; MacDowell, A.A.; Celestre, R.S.; Church, M.M.; Fakra, S.; Domning, E.E.; Glossinger, J.M.; Kirschman, J.L.; Morrison, G.Y.; Plate, D.W.; Smith, B.V.; Warwick, T.; Yashchuk, V.V.; Padmore, H.A.; Ustundag, E. A dedicated superbend X-ray microdiffraction beamline for materials, geo-, and environmental sciences at the advanced light source. Rev. Sci. Instrum. 2009, 80, 035108:1–035108:10. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, Y.; Yang, W.; Liu, W.; Cai, Z.; Kung, J.; Shu, J.; Hemley, R.J.; Mao, W.L.; Mao, H.K. Nanoprobe measurements of materials at megabar pressures. Proc. Natl. Acad. Sci. USA 2010, 107, 6140–6145. [Google Scholar] [CrossRef] [PubMed]
- Kolb, U.; Gorelik, T.; Kübel, C.; Otten, M.T.; Hubert, D. Towards automated diffraction tomography: Part I-Data acquisition. Ultramicroscopy 2007, 107, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Kolb, U.; Gorelik, T.; Otten, M.T. Towards automated diffraction tomography: Part II-Cell parameter determination. Ultramicroscopy 2008, 108, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Mugnaioli, E.; Gorelik, T.; Kolb, U. “Ab initio” structure solution from electron diffraction data obtained by a combination of automated diffraction tomography and precession technique. Ultramicroscopy 2009, 109, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Speziale, S.; Marquardt, H.; Wirth, R.; Schreiber, A.; Marquardt, K.; Neusser, G.; Reichmann, H.J. Combining focused ion beam and electron microscopy to prepare and analyze starting and recovered materials of high pressure and temperature diamond-anvil cell experiments. In Proceedings of AGU 2010 Fall Meeting, San Francisco, CA, USA, 2010.
- Marquardt, H.; Marquardt, K. Focused Ion Beam preparation and characterization of single-crystal samples for high-pressure experiments in the diamond-anvil cell. Am. Mineral. 2011. in submit. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Dubrovinsky, L.; Solozhenko, V.L. Comment on “Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure”. Science 2007, 318, 1550. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Weinberger, M.B.; Levine, J.B.; Cumberland, R.W.; Kavner, A.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Response to comment on “Synthesis of ultra-incompressible superhard rhenium diboride at ambient pressure”. Science 2007, 318, 1550d. [Google Scholar] [CrossRef]
- Brazhkin, V.; Dubrovinskaia, N.; Nicol, M.; Novikov, N.; Riedel, R.; Solzhenko, V.; Zhao, Y. What does “harder than diamond” mean? Nat. Mater. 2004, 3, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Zerr, A.; Miehe, G.; Li, J.; Dzivenko, D.A.; Bulatov, V.K.; Höfer, H.; Bolfan-Casanova, N.; Fialin, M.; Brey, G.; Watanabe, T.; Yoshimura, M. High-pressure synthesis of tantalum nitride having orthorhombic U2S3 structure. Adv. Funct. Mater. 2009, 19, 2282–2288. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Bayarjargal, L.; Friedrich, A.; Milman, V.; Kammler, D.R.; Clark, S.M.; Yan, J.; Koch-Müller, M.; Schröder, F.; Avalos-Borja, M. Formation of scandium carbides and scandium oxycarbide from the elements at high-(P,T) conditions. J. Solid State Chem. 2010, 183, 975–983. [Google Scholar] [CrossRef]
- Jiang, C.; Lin, Z.; Zhao, Y. Thermodynamic and mechanical stabilities of tantalum nitride. Phys. Rev. Lett. 2009, 103, 185501:1–185501:4. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M. Electronic Structure: Basic Theory and Methods; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Gonze, X.; Amadon, B.; Anglade, P.M.; Beuken, J.M.; Bottin, F.; Boulanger, P.; Bruneval, F.; Caliste, D.; Caracas, R.; Cote, M.; et al. ABINIT: First-principles approach to material and nanosystem properties. Comput. Phys. Commun. 2009, 180, 2582–2615. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys.-Condens. Mat. 2009, 21, 395502:1–395502:19. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Kristallogr. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Von Barth, U.; Hedin, L. Local exchange-correlation potential for spin polarized case 1. J. Phys. C 1972, 5, 1629–1642. [Google Scholar] [CrossRef]
- Perdew, J.P. Generalized gradient aproximations for exchange and correlation: A look backward and forward. Phys. B 1991, 172, 1–6. [Google Scholar] [CrossRef]
- Barone, V. Validation of self-consistent hybrid approaches for the study of transition metal complexes. NiCO and CuCO as case studies. Chem. Phys. Lett. 1995, 233, 129–133. [Google Scholar] [CrossRef]
- Milman, V.; Winkler, B.; White, J.A.; Pickard, C.J.; Payne, M.C.; Akhmatskaya, E.V.; Nobes, R.H. Electronic structure, properties, and phase stability of inorganic crystals: A pseudopotential plane-wave study. Int. J. Quantum Chem. 2000, 77, 895–910. [Google Scholar] [CrossRef]
- Winkler, B.; Hytha, M.; Warren, M.C.; Milman, V.; Gale, J.D.; Schreuer, J. Calculation of the elastic constants of the Al2SiO5 polymorphs andalusite, sillimanite and kyanite. Z. Kristallogr. 2001, 216, 67–70. [Google Scholar] [CrossRef]
- Mori-Sanchez, P.; Cohen, A.J.; Yang, W. Localization and delocalization errors in density functional theory and implications for band-gap prediction. Phys. Rev. Lett. 2008, 100, 146401:1–146401:4. [Google Scholar] [CrossRef] [PubMed]
- Baroni, S.; de Gironcoli, S.; Dal Corso, A.; Giannozzi, P. Phonons and related crystal properties from density-functional perturbation theory. Rev. Mod. Phys. 2001, 73, 515–562. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational density-functional perturbation theory for dielectrics and lattice dynamics. Phys. Rev. B 2006, 73, 1–12. [Google Scholar] [CrossRef]
- Anisimov, V.; Aryasetiawan, F.; Lichtenstein, A.I. First-principles calculations of the electronic structure and spectra of strongly correlated systems: the LDA+ U method. J. Phys.-Condens. Mat. 1997, 9, 767–808. [Google Scholar] [CrossRef]
- Grant, W.K.; Koomis, C.; Moore, J.J.; Olson, D.L.; Mishra, B.; Perry, A.J. Characterization of hard chromium nitride coatings deposited by cathodic arc vapor deposition. Surf. Coat. Tech. 1996, 86–87, 788–796. [Google Scholar] [CrossRef]
- Hones, P.; Martin, N.; Regula, M.; Levy, F. Structural and mechanical properties of chromium nitride, molybdenum nitride, and tungsten nitride thin films. J. Phys. D Appl. Phys. 2003, 36, 1023–1029. [Google Scholar] [CrossRef]
- ICSD. organic Crystal Structure Database ICSD Release 2010/2; National Institute of Standards and Technology Gaitersburg: Karlsruhe, Germany, 2010. [Google Scholar]
- Horvath-Bordon, E.; Riedel, R.; Zerr, A.; McMillan, P.F.; Auffermann, G.; Prots, Y.; Bronger, W.; Kniep, R.; Kroll, P. High-pressure chemistry of nitride-based materials. Chem. Soc. Rev. 2006, 35, 987–1014. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Yagi, T. Systematic study of formation and crystal structure of 3d-transition metal nitrides synthesized in a supercritical nitrogen fluid under 10 GPa and 1800 K using diamond anvil cell and YAG laser heating. J. Alloy. Compd. 2005, 403, 131–142. [Google Scholar] [CrossRef]
- Zerr, A.; Miehe, G.; Riedel, R. Synthesis of cubic zirconium and hafnium nitride having Th3P4 structure. Nat. Mater. 2003, 2, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Machon, D.; Daisenberger, D.; Soignard, E.; Shen, G.; Kawashima, T.; Takayama-Muromachi, E.; McMillan, P.F. High pressure-high temperature studies and reactivity of γ-Mo2N and δ-MoN. Phys. Status Solidi A 2006, 203, 831–836. [Google Scholar] [CrossRef]
- Friedrich, A.; Winkler, B.; Bayarjargal, L.; Juarez-Arellano, E.A.; Morgenroth, W.; Biehler, J.; Schröder, F.; Yan, J.; Clark, S.M. In situ observation of the reaction of tantalum with nitrogen in a laser heated diamond anvil cell. J. Alloy Compd. 2010, 502, 5–12. [Google Scholar] [CrossRef]
- Schönberg, N. X-ray study of the tantalum-nitrogen system. Acta Chem. Scand. 1954, 8, 199–203. [Google Scholar] [CrossRef]
- Kroll, P.; Schröter, T.; Peters, M. Predicition of novel phases of tantalum (V) nitride and tungsten (VI) nitride that can be synthesized under high pressure and high temperature. Angew. Chem. Int. Edit. 2005, 44, 4249–4254. [Google Scholar] [CrossRef] [PubMed]
- Boiko, L.G.; Popova, S.V. Crystal structure and superconducting properties of tantalum nitride obtained at high pressures. JETP Lett. USSR 1970, 12, 70–71. [Google Scholar]
- Friedrich, A.; Winkler, B.; Bayarjargal, L.; Morgenroth, W.; Juarez-Arellano, E.A.; Milman, V.; Refson, K.; Kunz, M.; Chen, K. Novel rhenium nitrides. Phys. Rev. Lett. 2010, 105, 085504:1–085504:4. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, A.; Winkler, B.; Refson, K.; Milman, V. Vibrational properties of Re3N from experiment and theory. Phys. Rev. B 2010, 82, 224106:1–224106:5. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Friedrich, A.; Wilson, D.J.; Koch-Müller, M.; Knorr, K.; Vogel, S.C.; Wall, J.J.; Reiche, H.; Crichton, W.; Ortega-Aviles, M.; Avalos-Borja, M. Reaction of rhenium and carbon at high pressures and temperatures. Z. Kristallogr. 2008, 223, 492–501. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.k. Synthesis of novel transition metal nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501:1–155501:4. [Google Scholar] [CrossRef] [PubMed]
- Crowhurst, J.C.; Goncharov, A.F.; Sadigh, B.; Evans, C.L.; Morrall, P.G.; Ferreira, J.L.; Nelson, A.J. Synthesis and characterization of the nitrides of platinum and iridium. Science 2006, 311, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Gregoryanz, E.; Sanloup, C.; Somayazulu, M.; Badro, J.; Fiquet, G.; Mao, H.k.; Hemley, R.J. Synthesis and characterization of a binary noble metal nitride. Nat. Mater. 2004, 3, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Crowhurst, J.C.; Goncharov, A.F.; Sadigh, B.; Zaug, J.M.; Aberg, D.; Meng, Y.; Prakapenka, V.B. Synthesis and characterization of nitrides of iridium and palladium. J. Mater. Res. 2008, 23, 1–5. [Google Scholar] [CrossRef]
- Åberg, D.; Erhart, P.; Crowhurst, J.; Zaug, J.M.; Goncharov, A.F.; Sadigh, B. Pressure-induced phase transition in the electronic structure of palladium nitride. Phys. Rev. B 2010, 82, 104116:1–104116:9. [Google Scholar] [CrossRef]
- Montoya, J.A.; Hernandez, A.D.; Sanloup, C.; Gregoryanz, E.; Scandolo, S. OsN2: Crystal structure and electronic properties. Appl. Phys. Lett. 2007, 90, 011909:1–011909:3. [Google Scholar] [CrossRef]
- Yu, R.; Zhan, Q.; De Jonghe, L.C. Crystal structures of and displacive transitions in OsN2, IrN2, RuN2, and RhN2. Angew. Chem. Int. Edit. 2007, 46, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Tse, J.S.; Jiang, J.Z. An ab initio study of 5d noble metal nitrides: OsN2, IrN2, PtN2 and AuN2. Solid State Commun. 2010, 150, 181–186. [Google Scholar] [CrossRef]
- Von Appen, J.; Lumey, M.W.; Dronskowski, R. Mysterious platinum nitride. Angew. Chem. Int. Edit. 2006, 45, 4365–4368. [Google Scholar] [CrossRef] [PubMed]
- Kjekshus, A. On the properties of binary compounds with the CoSb2 type crystal structure. Acta Chem. Scand. 1971, 25, 411–422. [Google Scholar] [CrossRef]
- Schmidt, T.; Lutz, H.D.; Hönle, W. Verfeinerung der Kristallstruktur von p-PtP2. Z. Kristallogr. 1990, 190, 143–146. [Google Scholar] [CrossRef]
- Ma, Y.; Cui, Q.; Shen, L.; He, Z. X-ray diffraction study of nanocrystalline tungsten nitride and tungsten to 31 GPa. J. Appl. Phys. 2007, 102, 013525:1–013525:4. [Google Scholar] [CrossRef]
- Mancera, L.; Rodriguez, J.A.; Takeuchi, N. First principles calculations of the ground state properties and structural phase transformation in YN. J. Phys.-Condens. Mat. 2003, 15, 2625–2633. [Google Scholar] [CrossRef]
- Yang, Q.; Lengauer, W.; Koch, T.; Scheerer, M.; Smid, I. Hardness and elastic properties of Ti(CxN1-x), Zr(CxN1-x) and Hf(CxN1-x). J. Alloy. Compd. 2000, 309, L5–L9. [Google Scholar] [CrossRef]
- Shebanova, O.; Soignard, E.; McMillan, P.F. Compressibilities and phonon spectra of high-hardness transition metal-nitride materials. High Pressure Res. 2006, 26, 87–97. [Google Scholar] [CrossRef]
- Chen, H.; Peng, F.; Mao, H.k.; Shen, G.; Liermann, H.P.; Li, Z.; Shu, J. Strength and elastic moduli of TiN from radial X-ray diffraction under nonhydrostatic compression up to 45 GPa. J. Appl. Phys. 2010, 107, 113503:1–113503:5. [Google Scholar] [CrossRef]
- Musil, J. Hard and superhard nanocomposite coatings. Surf. Coat. Tech. 2000, 125, 322–330. [Google Scholar] [CrossRef]
- Chen, X.J.; Struzhkin, V.V.; Wu, Z.; Somayazulu, M.; Qian, J.; Kung, S.; Christensen, A.N.; Zhao, Y.; Cohen, R.E.; Mao, H.k.; Hemley, R.J. Hard superconducting nitrides. Proc. Natl. Acad. Sci. USA 2005, 102, 3198–3201. [Google Scholar] [CrossRef] [PubMed]
- Dzivenko, D.A.; Zerr, A.; Schweitzer, E.; Göken, M.; Boehler, R.; Riedel, R. Elastic moduli and hardness of c-Zr2.86(N0.88O0.12)4 having Th3P4-type structure. Appl. Phys. Lett. 2007, 90, 191910:1–191910:3. [Google Scholar] [CrossRef]
- Zerr, A.; Chigarev, N.; Brenner, R.; Dzivenko, D.A.; Gusev, V. Elastic moduli of hard c-Zr3N4 from laser ultrasonic measurements. Phys. Status Solidi-R 2010, 4, 353–355. [Google Scholar] [CrossRef]
- Dzivenko, D.A.; Zerr, A.; Boehler, R.; Riedel, R. Equation of state of cubic hafnium(IV) nitride having the Th3P4 structure. Solid State Commun. 2006, 139, 255–258. [Google Scholar] [CrossRef]
- Mattesini, M.; Ahuja, R.; Johansson, B. Cubic Hf3N4 and Zr3N4: A class of hard materials. Phys. Rev. B 2003, 68, 184108:1–184108:5. [Google Scholar] [CrossRef]
- Rivadulla, F.; Banobre-Lopez, M.; Quintela, C.X.; Pineiro, A.; Pardo, V.; Baldomir, D.; Lopez-Quintela, M.A.; Rivas, J.; Ramos, C.A.; Salva, H.; Zhou, J.S.; Goodenough, J.B. Reduction of the bulk modulus at high pressure in CrN. Nat. Mater. 2009, 8, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.E.; Gan, B.K.; McKenzie, D.R.; Bilek, M.M.M.; Taylor, M.B.; McCulloch, D.G.; Latella, B.A. Correlation between stress and hardness in pulsed cathodic arc deposited titantium/vanadium nitride alloys. J. Phys.-Condens. Mat. 2004, 16, 7947–7954. [Google Scholar] [CrossRef]
- Lei, W.W.; Liu, D.; Li, X.F.; Zhang, J.; Zhou, Q.; Cui, Q.L.; Zou, G.T. High-pressure study of low-compressibility Ta2N. J. Phys.-Condens. Mat. 2007, 19, 425233:1–425233:6. [Google Scholar] [CrossRef]
- Soignard, E.; Shebanova, O.; McMillan, P.F. Compressibility measurements and phonon spectra of hexagonal transition-metal nitrides at high pressure: ε-TaN, δ-MoN, and Cr2N. Phys. Rev. B 2007, 75, 014104:1–014104:9. [Google Scholar] [CrossRef]
- Soignard, E.; McMillan, P.F.; Chaplin, T.D.; Farag, S.M.; Bull, C.L.; Somayazulu, M.S.; Leinenweber, K. High-pressure synthesis and study of low-compressibility molybdenum nitride (MoN and MoN1-x) phases. Phys. Rev. B 2003, 68, 132101:1–132101:4. [Google Scholar] [CrossRef]
- Adler, J.F.; Williams, Q. A high-pressure X-ray diffraction study of iron nitrides: Implications for Earth’s core. J. Geophys. Res. 2005, 110, B01203:1–B01203:11. [Google Scholar] [CrossRef]
- Niewa, R.; Rau, D.; Wosylus, A.; Meier, K.; Hanfland, M.; Wessel, M.; Dronskowski, R.; Dzivenko, D.A.; Riedel, R.; Schwarz, U. High-pressure, high-temperature single-crystal growth, ab initio electronic structure calculations, and equation of state of ϵ-Fe3N1+x. Chem. Mater. 2009, 21, 392–398. [Google Scholar] [CrossRef]
- Zhao, J.G.; Yang, L.X.; You, S.J.; Li, F.Y.; Jin, C.Q.; Liu, J. Structural stability of Zn3N2 under high pressure. Physica B 2010, 405, 1836–1838. [Google Scholar] [CrossRef]
- Kempter, C.P.; Nadler, M.R. Preparation and crystal structure of RuC and OsC. J. Chem. Phys. 1960, 33, 1580–1581. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, M.; Sun, C.; Zhang, X.; Liu, R. Ultrastiff carbides uncovered in first principles. Appl. Phys. Lett. 2007, 91, 061905:1–061905:3. [Google Scholar] [CrossRef]
- Guo, X.; Xu, B.; He, J.; Yu, D.; Liu, Z.; Tian, Y. Structure and mechanical properties of osmium carbide: First principles calculations. Appl. Phys. Lett. 2008, 93, 041904:1–041904:3. [Google Scholar] [CrossRef]
- Zemzemi, M.; Hebbache, M. Ab initio investigation of osmium carbide. Int. J. Refract. Met. H. 2008, 26, 61–67. [Google Scholar] [CrossRef]
- Ono, S.; Kikegawa, T.; Ohishi, Y. A high-pressure and high-temperature synthesis of platinum carbide. Solid State Commun. 2005, 133, 55–59. [Google Scholar] [CrossRef]
- Nowotny, H.; Auer-Welsbach, H. Über das Scandiumcarbid. Monatsh. Chem. 1961, 92, 789–793. [Google Scholar] [CrossRef]
- Auer-Welsbach, H.; Nowotny, H. Zur Frage des Scandiumcarbids. Monatsh. Chem. 1961, 92, 198–201. [Google Scholar] [CrossRef]
- Rassaerts, H.; Nowotny, H.; Vinek, G.; Benesovsky, F. Zum System Scandium-Kohlenstoff, 1. Mitt. Monatsh. Chem. 1967, 98, 460–468. [Google Scholar] [CrossRef]
- Krikorian, N.H.; Bowman, A.L.; Krupka, M.C.; Arnold, G.P. The preparation and crystal structure of Sc4C3. High Temp. Sci. 1969, 1, 360–366. [Google Scholar]
- Krikorian, N.H.; Giorgi, A.L.; Szklarz, E.G.; Krupka, M.C. Preparation and superconductivity of germanium-stabilized Sc13C10. J. Less-Common Met. 1969, 19, 253–257. [Google Scholar] [CrossRef]
- Jedlicka, H.; Nowotny, H.; Benesovsky, F. Zum System Scandium-Kohlenstoff, 2. Mitt.: Kristallstruktur des C-reichen Carbids. Monatsh. Chem. 1971, 102, 389–403. [Google Scholar] [CrossRef]
- Pöttgen, R.; Jeitschko, W. Sc3C4, a carbide with C3 units derived from propadine. Inorg. Chem. 1991, 30, 427–431. [Google Scholar] [CrossRef]
- Nowotny, H.; Neckel, A. Chemical bonding in interstitial compounds. J. I. Met. 1969, 97, 161. [Google Scholar]
- Karen, P.; Hájek, B.; Valvoda, V. Determination of composition limits of scandium carbide-oxide Sc(C,O) by the combined diffractometric and hydrolysis method. J. Less-Common Met. 1986, 120, 337–344. [Google Scholar] [CrossRef]
- Dufek, V.; Petru̇, F.; Broz̆ek, V. Über Sauerstoff-haltige Verbindungen vom Strukturtyp B1 der ersten vier Übergangsmetalle. Monatsh. Chem. 1967, 98, 2424–2430. [Google Scholar] [CrossRef]
- Dufek, V.; Broz̆ek, V.; Petru̇, F. Zur Existenz des Scandiummonoxides. Monatsh. Chem. 1969, 100, 1628–1630. [Google Scholar] [CrossRef]
- Tashmetov, M.Y.; Em, V.T.; Lee, C.H.; Shim, H.S.; Choi, Y.N.; Lee, J.S. Neutron diffraction study of the ordered structures of nonstoichiometric titanium carbide. Physica B 2002, 311, 318–325. [Google Scholar] [CrossRef]
- Winkler, B.; Wilson, D.J.; Vogel, S.C.; Brown, D.W.; Sisneros, T.A.; Milman, V. In situ observation of the formation of TiC from the elements by neutron diffraction. J. Alloy Compd. 2007, 441, 374–380. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.A.; Dubrovinsky, L.S.; Saxena, S.K.; Ahuja, R.; Johansson, B. High-pressure study of titanium carbide. J. Alloy Compd. 1999, 289, 24–27. [Google Scholar] [CrossRef]
- Winkler, B.; Juarez-Arellano, E.A.; Friedrich, A.; Bayarjargal, L.; Yan, J.; Clark, S.M. Reaction of titanium with carbon in a laser heated diamond anvil cell and reevaluation of a proposed pressure-induced structural phase transition of TiC. J. Alloy Compd. 2008, 478, 392–397. [Google Scholar] [CrossRef]
- Hackett, K.; Verhoef, S.; Cutler, R.A.; Shettyw, D.K. Phase constitution and mechanical properties of carbides in the Ta-C system. J. Am. Ceram. Soc. 2009, 92, 2404–2407. [Google Scholar] [CrossRef]
- Juarez-Arellano, E.A.; Winkler, B.; Friedrich, A.; Bayarjargal, L.; Milman, V.; Yan, J.; Clark, S.M. Stability field of the high-(P,T) Re2C phase and properties of an analogous osmium carbide phase. J. Alloy Compd. 2009, 481, 577–581. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, L.; Wang, L.M.; Xu, B.; Liu, Z.; Yu, D.; He, J.; Zhou, X.F.; Wang, H.T.; Tian, Y. Bulk Re2C: Crystal structure, hardness, and ultra-incompressibility. Cryst. Growth Des. 2010, 10, 5024–5026. [Google Scholar] [CrossRef]
- Chang, R.; Graham, L.J. Low-temperature elastic properties of ZrC and TiC. J. Appl. Phys. 1966, 37, 3778–3783. [Google Scholar] [CrossRef]
- Gilman, J.J.; Roberts, B.W. Elastic constants of TiC and TiB2. J. Appl. Phys. 1961, 32, 1405. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Murray, M.J. Elastic-moduli measurements of some cubic transition-metal carbides and alloyed carbides. J. Mater. Sci. 1974, 9, 223–228. [Google Scholar] [CrossRef]
- Pintschovius, L.; Reichardt, W.; Scheerer, B. Lattice dynamics of TiC. J. Phys. C Solid State 1978, 11, 1557–1562. [Google Scholar] [CrossRef]
- Dodd, S.P.; Cankurtaran, M.; James, B. Ultrasonic determination of the elastic and nonlinear acoustic properties of transition-metal carbide ceramics: TiC and TaC. J. Mater. Sci. 2003, 38, 1107–1115. [Google Scholar] [CrossRef]
- Gu, Q.F.; Krauss, G.; Gramm, F.; Steurer, W. On the compressibility of TiC in microcrystalline and nanoparticulate form. J. Phys.-Condens. Mat. 2008, 20, 445226:1–445226:6. [Google Scholar] [CrossRef]
- Yu, X.; Lin, Z.; Zhang, J.; Wang, L.; Ding, Z.; Jin, C.; Zhao, Y. Thermal equation of state of TiC: A synchrotron X-ray diffraction study. J. Appl. Phys. 2010, 107, 113517:1–113517:4. [Google Scholar] [CrossRef]
- Brown, H.L.; Armstrong, P.E.; Kempter, P. Elastic properties of some polycrystalline transition-metal monocarbides. J. Chem. Phys. 1966, 45, 547–549. [Google Scholar] [CrossRef]
- Liermann, H.P.; Singh, A.K.; Manoun, B.; Saxena, S.K.; Prakapenka, V.B.; Shen, G. Compression behavior of VC0.85 up to 53 GPa. Int. J. Refract. Met. H. 2004, 22, 129–132. [Google Scholar] [CrossRef]
- Simunek, A.; Vackar, J. Hardness of covalent and ionic crystals: First-principles calculations. Phys. Rev. Lett. 2006, 96, 085501:1–085501:4. [Google Scholar] [CrossRef] [PubMed]
- Ledbetter, H.M.; Chevacharoenkul, S.; Davis, R.F. Monocrystal elastic constants of NbC. J. Appl. Phys. 1986, 60, 1614–1617. [Google Scholar] [CrossRef]
- Liermann, H.P.; Singh, A.K.; Somayazulu, M.; Saxena, S.K. Compression behavior of NbC under nonhydrostatic conditions to 57 GPa. Int. J. Refract. Met. H. 2007, 25, 386–391. [Google Scholar] [CrossRef]
- Chen, J.; Boyer, L.L.; Krakauer, H.; Mehl, M.J. Elastic constants of NbC and MoN: Instability of B1-MoN. Phys. Rev. B 1988, 37, 3295–3298. [Google Scholar] [CrossRef]
- Rowcliffe, D.J.; Hollox, G.E. Hardness anisotropy, deformation mechanisms and brittle-to-ductile transition in carbides. J. Mater. Sci. 1971, 6, 1270–1276. [Google Scholar] [CrossRef]
- Jun, C.K.; Shaffer, P.T.B. Elastic moduli of niobium carbide and tantalum carbide at high temperature. J. Less-Common Met. 1971, 23, 367–373. [Google Scholar] [CrossRef]
- Liermann, H.P.; Singh, A.K.; Manoun, B.; Saxena, S.K.; Zha, C.S. Compression behavior of TaC0.98 under nonhydrostatic and quasi-hydrostatic pressures up to 76 GPa. Int. J. Refract. Met. H. 2005, 23, 109–114. [Google Scholar] [CrossRef]
- Bartlett, R.W.; Smith, C.W. Elastic constants of tantalum monocarbide, TaC0.90. J. Appl. Phys. 1967, 38, 5428–5429. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Xiao, B.; Min, T.; Yang, Y.; Ma, S.; Yi, D. The electronic, mechanical properties and theoretical hardness of chromium carbides by first-principles calculations. J. Alloy Compd. 2011, 509, 5242–5249. [Google Scholar] [CrossRef]
- Xiao, B.; Xing, J.D.; Feng, J.; Zhou, C.T.; Li, Y.F.; Su, W.; Xie, X.J.; Cheng, Y.H. A comparative study of Cr7C3, Fe3C and Fe2B in cast iron both from ab initio calculations and experiments. J. Phys. D Appl. Phys. 2009, 42, 115415:1–115415:16. [Google Scholar] [CrossRef]
- Haines, J.; Leger, J.M.; Chateau, C.; Lowther, J.E. Experimental and theoretical investigation of Mo2C at high pressure. J. Phys.-Condens. Mat. 2001, 13, 2447–2454. [Google Scholar] [CrossRef]
- Thümmler, F.; Holleck, H.; Prakash, L. New results in the field of cemented carbides. High Temp. High Press. 1982, 14, 129–144. [Google Scholar]
- Lee, M.; Gilmore, R.S. Single crystal elastic constants of tungsten monocarbide. J. Mater. Sci. 1982, 17, 2657–2660. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Fei, Y.; Suzuki, A.; Ohtani, E.; Funakoshi, K. Pressure-volume-temperature equation of state of tungsten carbide to 32 GPa and 1673 K. J. Appl. Phys. 2010, 108, 053513:1–053513:7. [Google Scholar] [CrossRef]
- Lee, M. High temperature hardness of tungsten carbide. Metall. Trans. A 1983, 14A, 1625–1629. [Google Scholar] [CrossRef]
- Scott, H.P.; Williams, Q.; Knittle, E. Stability and equation of state of Fe3C to 73 GPa: Implications for carbon in the Earth’s core. Geophys. Res. Lett. 2001, 28, 1875–1878. [Google Scholar] [CrossRef]
- Li, J.; Mao, H.k.; Fei, Y.; Gregoryanz, E.; Eremets, M.; Zha, C.S. Compression of Fe3C to 30 GPa at room temperature. Phys. Chem. Miner. 2002, 29, 166–169. [Google Scholar] [CrossRef]
- Mookherjee, M.; Nakajima, Y.; Steinle-Neumann, G.; Glazyrin, K.; Wu, X.; Dubrovinsky, L.; McCammon, C.; Chumakov, A. High-pressure behavior of iron carbide (Fe7C3) at inner core conditions. J. Geophys. Res. 2011, 116, B04201:1–B04201:13. [Google Scholar] [CrossRef]
- Wald, F.; Stormont, R.W. Investigations on constitution of certain binary boron-metal systems. J. Less-Common Met. 1965, 9, 423–433. [Google Scholar] [CrossRef]
- Musa, C.; Orru, R.; Licheri, R.; Cao, G. On the controversial formation of silver diboride: Processing of Ag + 2B powders by spark plasma sintering. Physica C 2009, 469, 1991–1995. [Google Scholar] [CrossRef]
- Obrowski, W. Die Struktur der Diboride von Gold und Silber. Naturwissenschaften 1961, 48, 428. [Google Scholar] [CrossRef]
- Kwon, S.K.; Youn, S.J.; Kim, K.S.; Min, B.I. New High Temperature Diboride Superconductors: AgB2 and AuB2; Cornell Unversity Labrary: New York, NY, USA, 23 June 2001. [Google Scholar]
- Winkler, B.; Juarez-Arellano, E.A.; Friedrich, A.; Bayarjargal, L.; Schröder, F.; Biehler, J.; Milman, V.; Clark, S.M.; Yan, J. In situ synchrotron X-ray diffraction study of the formation of TaB2 from the elements in a laser heated diamond anvil cell. Solid State Sci. 2010, 12, 2059–2064. [Google Scholar] [CrossRef]
- Yamamoto, A.; Takao, C.; Masui, T.; Izumi, M.; Tajima, S. High-pressure synthesis of superconducting Nb1-xB2 (x = 0-0.48) with the maximum Tc = 9.2 K. Physica C 2002, 383, 197–206. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Koizumi, M.; Yamada, O. High-pressure self-combustion sintering for ceramics. J. Am. Ceram. Soc. 1984, 67, c222–c225. [Google Scholar] [CrossRef]
- Bhaumik, S.K.; Divakar, C.; Singh, A.K.; Upadhyaya, G.S. Synthesis and sintering of TiB2 and TiB2-TiC composite under high pressure. Mater. Sci. Eng. A-Struct 2000, 279, 275–281. [Google Scholar] [CrossRef]
- Lepakova, O.K.; Raskolenko, L.G.; Maksimov, Y.M. Titanium borides prepared by self-propagating high-temperature synthesis. Inorg. Mater. 2000, 36, 568–575. [Google Scholar] [CrossRef]
- La Placa, S.; Post, B. The crystal structure of rhenium diboride. Acta Crystallogr. 1962, 15, 97–99. [Google Scholar] [CrossRef]
- Otani, S.; Aizawa, T.; Ishizawa, Y. Preparation of ReB2 single crystals by the floating zone method. J. Alloy Compd. 1997, 252, L19–L21. [Google Scholar] [CrossRef]
- Levin, J.B.; Nguyen, S.J.; Rasool, H.I.; Wright, J.A.; Brown, S.E.; Kaner, R.B. Preparation and properties of metallic, superhard rhenium diboride crystals. Adv. Funct. Mater. 2008, 130, 16953–16958. [Google Scholar] [CrossRef] [PubMed]
- Latini, A.; Rau, J.V.; Ferro, D.; Teghil, R.; Albertini, V.R.; Barinov, S.M. Superhard rhenium diboride films: Preparation and characterization. Chem. Mater. 2008, 20, 4507–4511. [Google Scholar] [CrossRef]
- Kawano, A.; Mizuta, Y.; Takagiwa, H.; Muranaka, T.; Akimitsu, J. The superconductivity in Re–B system. J. Phys. Soc. Jpn. 2003, 72, 1724–1728. [Google Scholar] [CrossRef]
- Takagiwa, H.; Kawano, A.; Mizuta, Y.; Yamamoto, T.; Yamada, M.; Ohishi, K.; Muranaka, T.; Akimitsu, J.; Higemoto, W.; Kadono, R. Magnetic penetration depth of a new boride superconductor Re3B. Phys. B 2003, 326, 355–358. [Google Scholar] [CrossRef]
- Ivanovskii, A.L. The search for novel superhard and incompressible materials on the basis of higher borides of s, p, d metals. J. Superhard Mater. 2011, 33, 73–87. [Google Scholar] [CrossRef]
- Pereira, A.S.; Perottponi, C.A.; da Jornada, J.A.H.; Léger, J.M.; Haines, J. Compressibility of AlB2-type transition metal diborides. J. Phys.-Condens. Mat. 2002, 14, 10615–10618. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, M.; Cui, T.; Ma, Y.; Niu, Y.; Zou, G. Electronic structure, phase stability, and hardness of the osmium borides, carbides, nitrides, and oxides: First principles calculations. J. Phys. Chem. Solids 2008, 69, 2096–2102. [Google Scholar] [CrossRef]
- Liu, C.J.; Peng, F.; Tan, N.; Liu, J.; Li, F.J.; Qin, J.G.; Wang, J.H.; Wang, Q.M.; He, D.W. Low-compressibility of tungsten tetraboride: A high pressure X-ray diffraction study. High Pressure Res. 2011, 31, 275–282. [Google Scholar] [CrossRef]
- Chung, H.Y.; Weinberger, M.B.; Yang, J.M.; Tolbert, S.H.; Kaner, R.B. Correlation between hardness and elastic moduli of the ultraincompressible transition metal diborides RuB2, OsB2, and ReB2. Appl. Phys. Lett. 2008, 92, 261904:1–261904:3. [Google Scholar] [CrossRef]
- Levine, J.B.; Tolbert, S.H.; Kaner, R.B. Advancements in the search for superhard Ultra-Incompressible metal borides. Adv. Funct. Mater. 2009, 19, 3519–3533. [Google Scholar] [CrossRef]
- Chen, B.; Penwell, D.; Nguyen, J.H.; Kruger, M.B. High pressure X-ray diffraction study of Fe2B. Solid State Commun. 2004, 129, 573–575. [Google Scholar] [CrossRef]
- Hebbache, M.; Zˇivkovic´, D. Boron Rich Solids: Sensors, Ultra High Temperature Ceramics, Thermoelectrics, Armor; Springer: Berlin, Germany, 2011; chapter Investigation of Hard Boron Rich Solids: Osmium Diboride and β-Rhombohedral Boron; pp. 115–130. [Google Scholar]
- Cumberland, R.W.; Weinberger, M.B.; Gilman, J.J.; Clark, S.M.; Tolbert, S.H.; Kaner, R.B. Osmium diboride, an ultra-incompressible, hard material. J. Am. Chem. Soc. 2005, 127, 7264–7265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.J.; Wang, Y.X. Structural, mechanical, and electronic properties of TaB2, TaB, IrB2, and IrB: First principles calculations. J. Solid State Chem. 2009, 182, 2880–2886. [Google Scholar] [CrossRef]
- Gatterer, J.; Dufek, G.; Ettmayer, P.; Kieffer, H. Das kubische Tantalmononitrid (B1-Typ) und seine Mischbarkeit mit den isotypen Übergangsmetallnitriden und -carbiden. Monatsh. Chem. 1975, 106, 1137–1147. [Google Scholar] [CrossRef]
- Mashimo, T.; Tashiro, S.; Toya, T.; Nishida, M.; Yamazaki, H.; Yamaya, S.; Oh-Ishi, K.; Syono, S. Synthesis of the B1-type tantalum nitride by shock compression. J. Mater. Sci. 1993, 28, 3439–3443. [Google Scholar] [CrossRef]
- McSkimin, H.J.; Andreatch, P., Jr. Elastic moduli of diamond as a function of pressure and temperature. J. Appl. Phys. 1972, 43, 2944–2948. [Google Scholar] [CrossRef]
- Vogelgesang, R.; Ramdas, A.K.; Rodriguez, S.; Grimsditch, M.; Anthony, T.R. Brillouin and Raman scattering in natural and isotopically controlled diamond. Phys. Rev. B 1996, 54, 3989–3999. [Google Scholar] [CrossRef]
- Occelli, F.; Loubeyre, P.; LeToullec, R. Properties of diamond under hydrostatic pressures up to 140 GPa. Nat. Mater. 2003, 2, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Birch, F. The velocity of compressional waves in rocks to 10 kilobars, Part 2. J. Geophys. Res. 1961, 66, 2199–2224. [Google Scholar] [CrossRef]
- Birch, F. Composition of the Earth’s mantle. Geophys. J. Roy. Astr. S. 1961, 4, 295–311. [Google Scholar] [CrossRef]
- Anderson, O.L.; Nafe, J.E. The bulk modulus-volume relationship for oxide compounds and related geophysical problems. J. Geophys. Res. 1965, 70, 3951–3963. [Google Scholar] [CrossRef]
- Brookes, C.A. Plastic deformation and anisotropy in the hardness of diamond. Nature 1970, 228, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Savvides, N.; Bell, T.J. Microhardness and Young’s modulus of diamond and diamondlike carbon films. J. Appl. Phys. 1992, 72, 2791–2796. [Google Scholar] [CrossRef]
- Dubrovinskaia, N.; Solozhenko, V.L.; Miyajima, N.; Dmitriev, V.; Kurakevych, O.O.; Dubrovinsky, L. Superhard nanocomposite of dense polymorphs of boron nitride: Nanocarbon material has reached diamond hardness. Appl. Phys. Lett. 2007, 90, 101912:1–101912:3. [Google Scholar] [CrossRef]
- Knittle, E.; Wentzcovitch, R.M.; Jeanloz, R.; Cohen, M.L. Experimental and theoretical equation of state of cubic boron nitride. Nature 1989, 337, 349–352. [Google Scholar] [CrossRef]
- Cynn, H.; Klepeis, J.E.; Yoo, C.S.; Young, D.A. Osmium has the lowest experimentally determined compressibility. Phys. Rev. Lett. 2002, 88, 135701:1–135701:4. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.B.; Tolbert, S.H.; Kavner, A. Osmium metal studied under high pressure and nonhydrostatic stress. Phys. Rev. Lett. 2008, 100, 045506:1–045506:4. [Google Scholar] [CrossRef] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/.)
Share and Cite
Friedrich, A.; Winkler, B.; Juarez-Arellano, E.A.; Bayarjargal, L. Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations. Materials 2011, 4, 1648-1692. https://doi.org/10.3390/ma4101648
Friedrich A, Winkler B, Juarez-Arellano EA, Bayarjargal L. Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations. Materials. 2011; 4(10):1648-1692. https://doi.org/10.3390/ma4101648
Chicago/Turabian StyleFriedrich, Alexandra, Björn Winkler, Erick A. Juarez-Arellano, and Lkhamsuren Bayarjargal. 2011. "Synthesis of Binary Transition Metal Nitrides, Carbides and Borides from the Elements in the Laser-Heated Diamond Anvil Cell and Their Structure-Property Relations" Materials 4, no. 10: 1648-1692. https://doi.org/10.3390/ma4101648