An Approach for Measuring the Dielectric Strength of OLED Materials
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
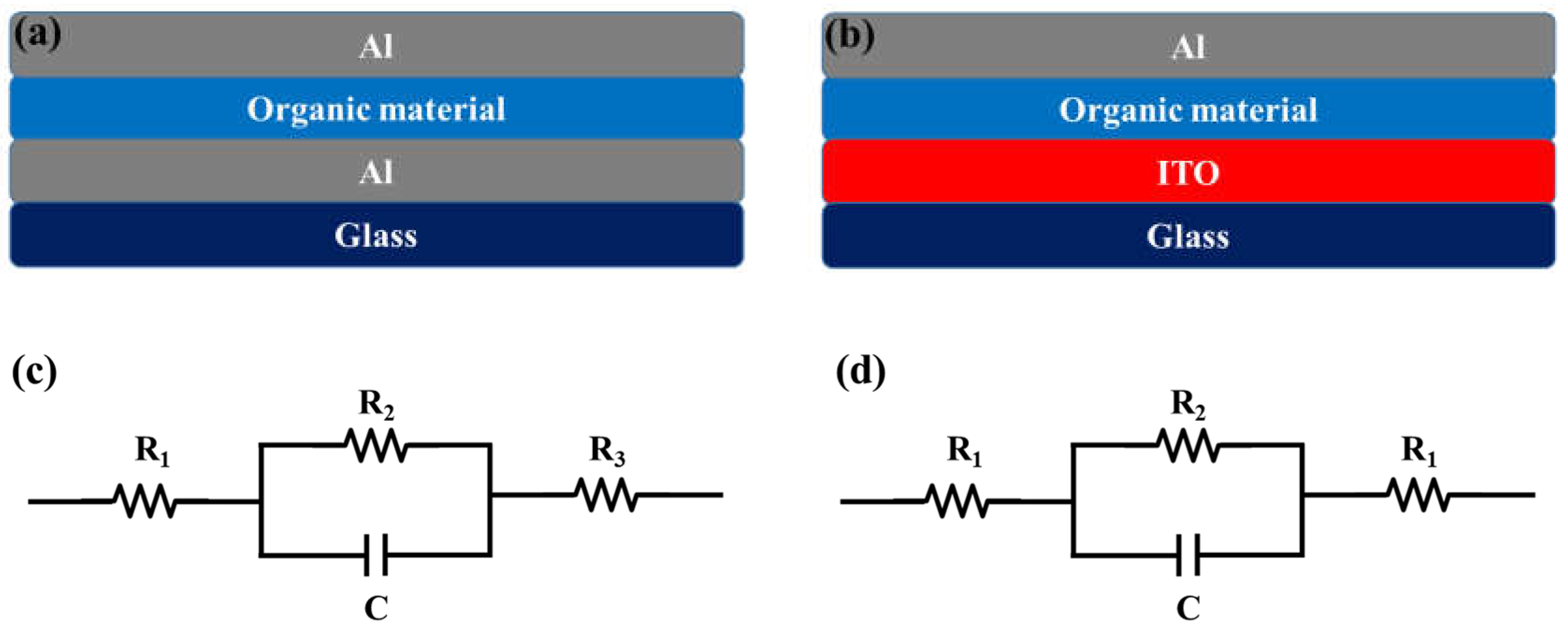
2.2. Device Fabrication
3. Measurements
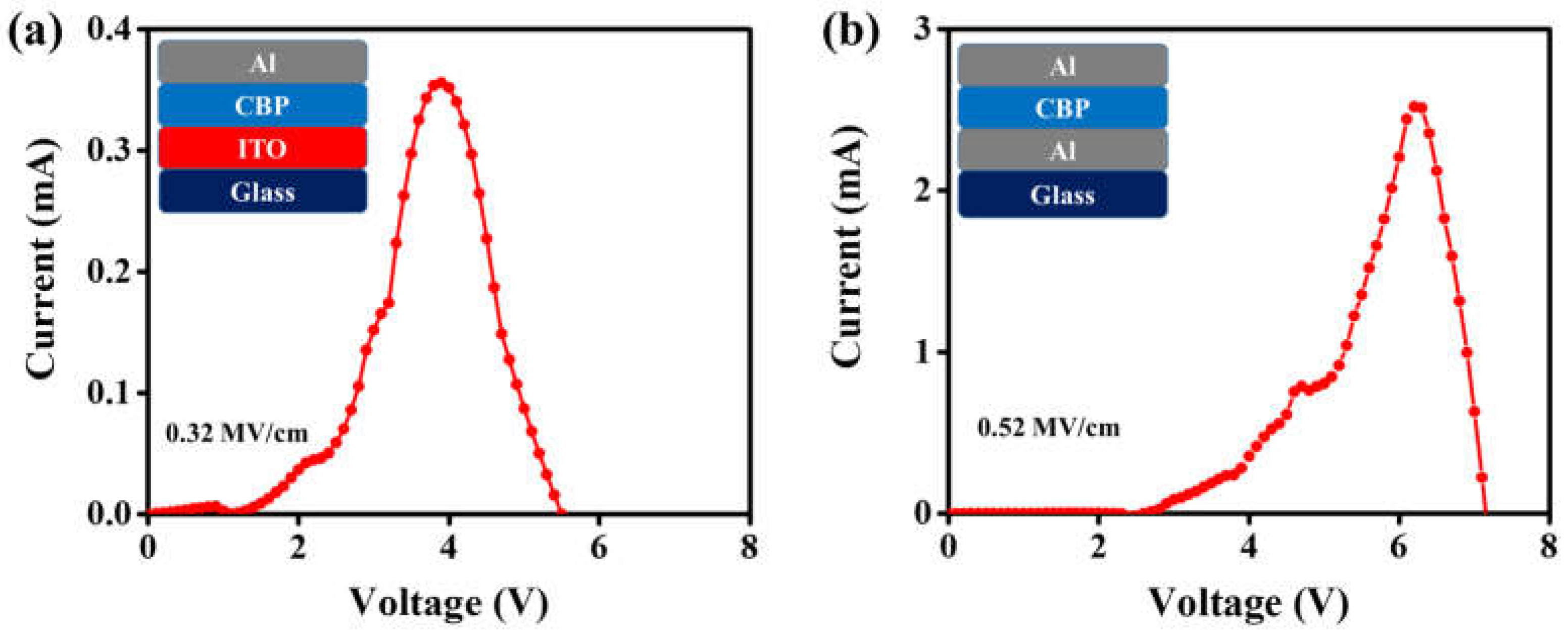
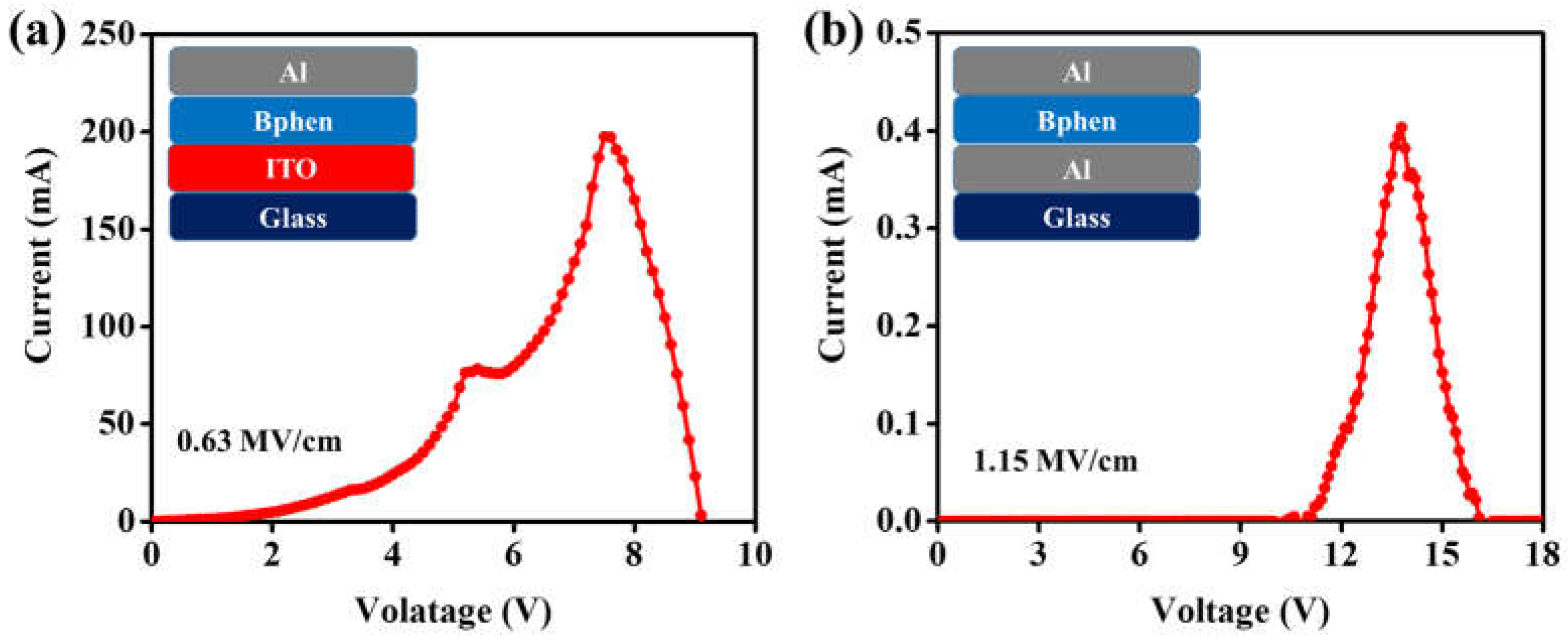
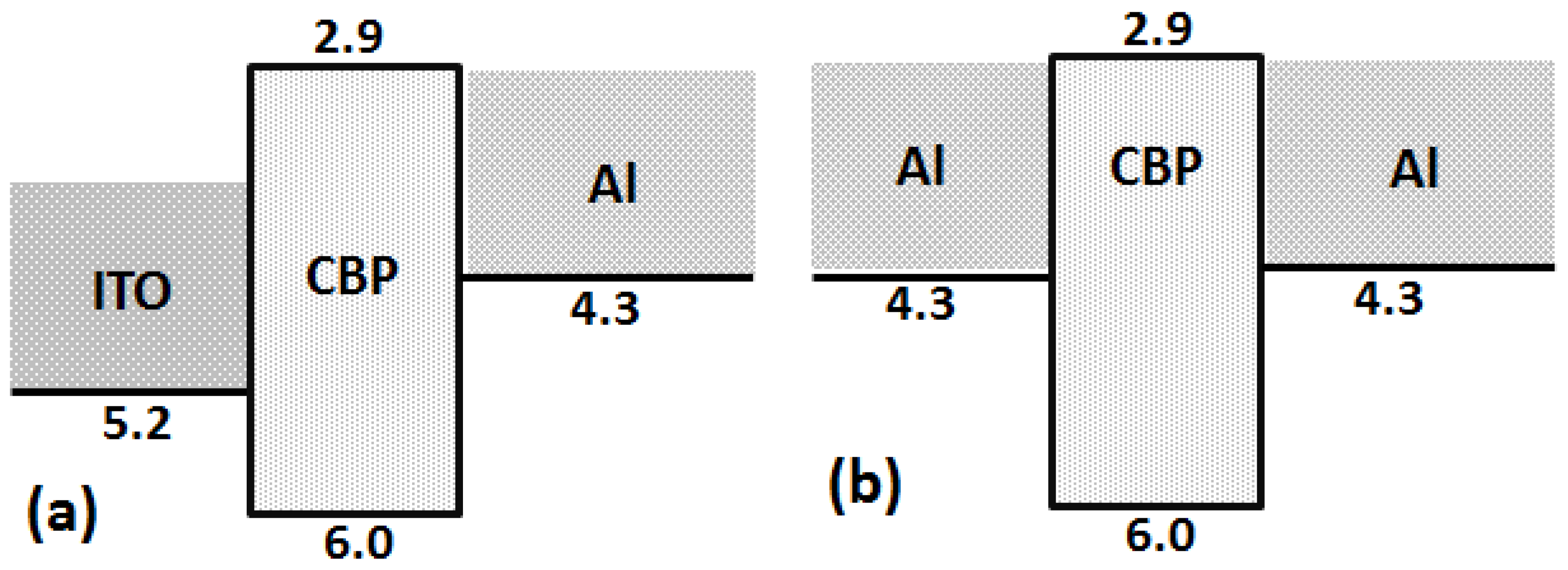
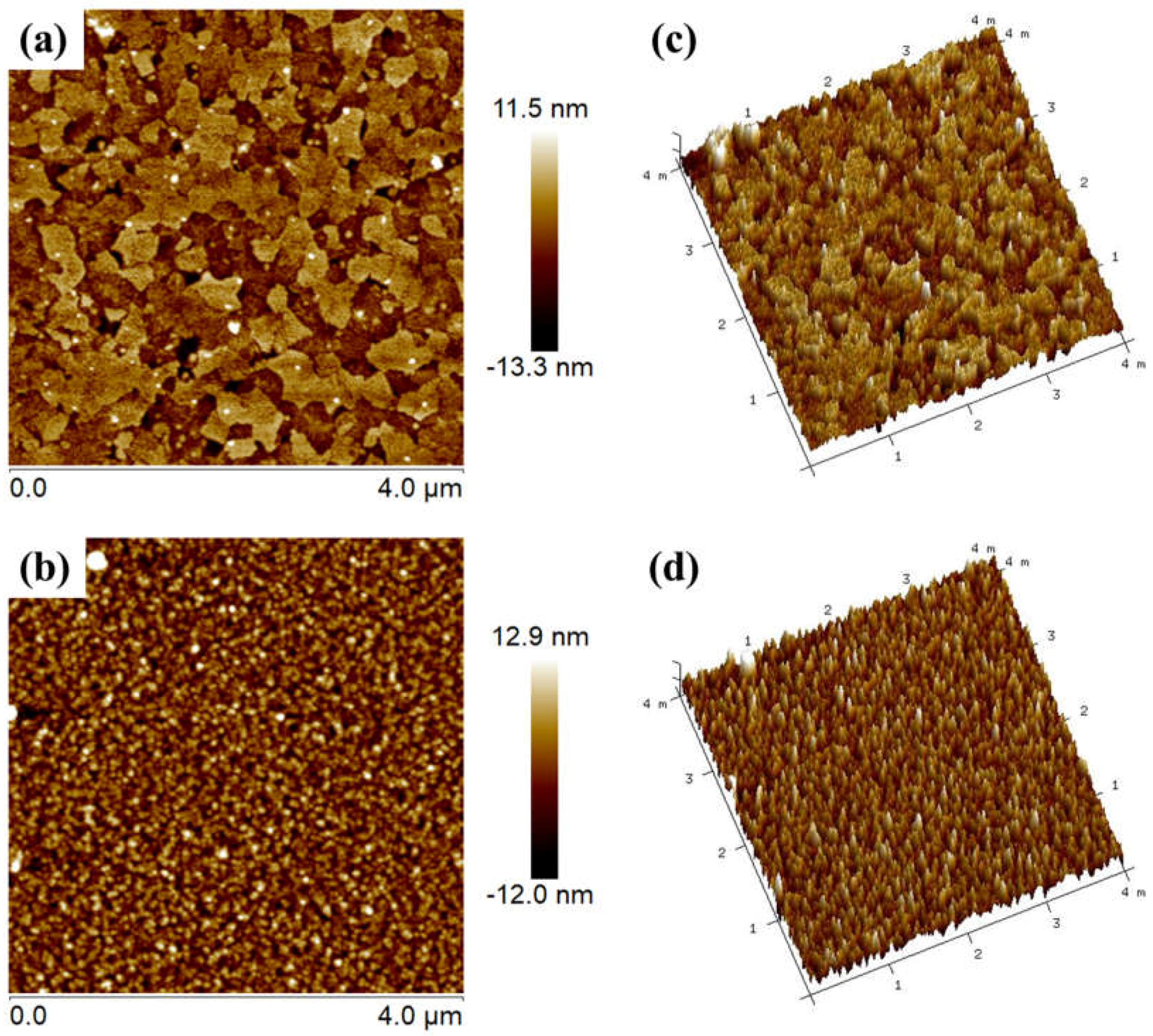
4. Result and Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tang, C.W.; VanSlyke, S.A. Organic electroluminescent diodes. Appl. Phys. Lett. 1987, 51, 913–915. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Forrest, S.R. White Organic Light-Emitting Devices for Solid-State Lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Jou, J.H.; Kumar, S.; Agrawal, A.; Li, T.H.; Sahoo, S. Approaches for fabricating high efficiency organic light emitting diodes. J. Mater. Chem. C 2015, 3, 2974–3002. [Google Scholar] [CrossRef]
- Dubey, D.K.; Tung, F.C.; Liu, S.H.; Song, W.C.; Singh, M.; Sahoo, S.; Lai, S.H.; Jou, J.H. P–214: Flexible white organic light emitting diode via solution process. SID Symp. Dig. Tech. Pap. 2017, 48, 2025–2027. [Google Scholar] [CrossRef]
- Dubey, D.K.; Singh, M.; Sahoo, S.; Jou, J.H. Simple-structured efficient white organic light emitting diode via solution process. Microelectron. Reliab. 2018, 83, 293–296. [Google Scholar] [CrossRef]
- Dubey, D.K.; Yadav, R.A.K.; Tavgeniene, D.; Grigalevicius, S.; Jou, J.H. Crosslinkable hole-transporting small molecule as a mixed host for efficient solution-processed red organic light emitting diodes. Thin Solid Films 2018. [Google Scholar] [CrossRef]
- Wu, Z.; Ma, D. Recent advances in white organic light-emitting diodes. Mater. Sci. Eng. R Rep. 2016, 107, 1–42. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, D. Approaches to high performance white organic light-emitting diodes for general lighting. Mater. Chem. Front. 2017, 1, 1933–1950. [Google Scholar] [CrossRef]
- Jou, J.H.; Swayamprabha, S.S.; Yadav, R.A.K.; Dubey, D.K. Nano-Structures Enabling Sunlight and Candlelight-Style OLEDs. J. Nanomater. Mol. Nanotechnol. 2018, 7. [Google Scholar] [CrossRef]
- Borchardt, J.K. Developments in organic displays. Mater. Today 2004, 7, 42–46. [Google Scholar] [CrossRef]
- Thejokalyani, N.; Dhoble, S.J. Importance of Eco-friendly OLED Lighting. Defect Diffus. Forum 2014, 357, 1–27. [Google Scholar] [CrossRef]
- Scholz, S.; Kondakov, D.; Lüssem, B.; Leo, K. Degradation Mechanisms and Reactions in Organic Light-Emitting Devices. Chem. Rev. 2015, 115, 8449–8503. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, J.H. Heat dissipation property of cover glasses with heat sink films for transparent organic light-emitting diodes. Semicond. Sci. Technol. 2014, 29. [Google Scholar] [CrossRef]
- Liu, G.; Kerr, J.B.; Johnson, S. Dark spot formation relative to ITO surface roughness for polyfluorene devices. Synth. Matter 2004, 144, 1–6. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tran, V.H.; Massardier, V. Electrical conduction in poly (phenyleneviny1ene) thin films. J. Phys. Condens. Matter 1993, 5, 6243–6252. [Google Scholar] [CrossRef]
- Li, Y.; Gao, J.; Yu, G.; Cao, Y.; Heeger, A.J. ac impedance of polymer light-emitting electrochemical cells and light-emitting diodes: A comparative study. Chem. Phys. Lett. 1998, 287, 83–88. [Google Scholar] [CrossRef]
- Wang, F.; Liu, S.; Zhang, C. The dielectric constant of materials effect the property of the OLED. Microelectron. J. 2007, 38, 259–261. [Google Scholar] [CrossRef]
- Ohta, S.; Chuman, T.; Miyaguchi, S.; Satoh, H.; Tanabe, T.; Okuda, Y.; Tsuchida, M. Active Matrix Driving Organic Light-Emitting Diode Panel Using Organic Thin-Film Transistors. Jpn. J. Appl. Phys. 2005, 44, 3678–3681. [Google Scholar] [CrossRef] [Green Version]
- Ahn, J.H.; Lee, H.S.; Seo, D.S.; Jang, K.U.; Lee, W.J.; Kim, T.W. Dielectric properties depending on frequency in organic light-emitting diodes. Thin Solid Films 2008, 516, 2626–2629. [Google Scholar] [CrossRef]
- Kim, H.K.; Shi, F.G. Thickness Dependent Dielectric Strength of a Low-permittivity Dielectric Film. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 248–252. [Google Scholar] [CrossRef]
- Calculating the Dielectric Strength of Material. Available online: https://www.buckleysinternational.com/pages/calculating-dielectric-stength (accessed on 8 June 2018).
- Juhasz, P.; Nevrela, J.; Micjan, M.; Novota, M.; Uhrik, J.; Stuchlikova, L.; Jakabovic, J.; Harmatha, L.; Weis, M. Charge injection and transport properties of an organic light-emitting diode. Beilstein J. Nanotechnol. 2016, 7, 47–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naka, S.; Okada, H.; Onnagawa, H.; Tsutsui, T. High electron mobility in bathophenanthroline. Appl. Phys. Lett. 2000, 76, 197–199. [Google Scholar] [CrossRef]
- Pereira, A.; Conte, G.; Gallardo, H.; Zucco, C.; Quirino, W.G.; Legnani, C.; Cremona, M.; Bechtold, I.H. OLEDs based on an europium(III) complex: {Tris(thenoyltrifluoroacetonate)-[1,2,5]-thiadiazolo[3,4-f][1,10]phenanthroline} europium(III). J. SID 2011, 793–797. [Google Scholar] [CrossRef]
- Jou, J.H.; Kumar, S.; Singh, M.; Chen, Y.H.; Chen, C.C.; Lee, M.T. Carrier Modulation Layer-Enhanced Organic Light-Emitting Diodes. Molecules 2015, 20, 13005–13030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, S.; Dubey, D.K.; Singh, M.; Joseph, V.; Thomas, K.R.J.; Jou, J.H. Highly efficient deep-blue organic light emitting diode with a carbazole based fluorescent emitter. Jpn. J. Appl. Phys. 2018, 57. [Google Scholar] [CrossRef]
- Ulmeanu, M.; Serghei, A.; Mihailescu, I.N.; Budau, P.; Enachescu, M. C Ni amorphous multilayers studied by atomic force microscopy. Appl. Surf. Sci. 2000, 165, 109–115. [Google Scholar] [CrossRef]


| Organic Material | Anode | Breakdown Voltage (V) | Calculated Dielectric Strength (MV/cm) |
|---|---|---|---|
| CBP | ITO | 3.90 | 0.32 |
| CBP | Al | 6.20 | 0.52 |
| Bphen | ITO | 7.52 | 0.63 |
| Bphen | Al | 13.80 | 1.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudheendran Swayamprabha, S.; Kumar Dubey, D.; Song, W.-C.; Lin, Y.-T.; Ashok Kumar Yadav, R.; Singh, M.; Jou, J.-H. An Approach for Measuring the Dielectric Strength of OLED Materials. Materials 2018, 11, 979. https://doi.org/10.3390/ma11060979
Sudheendran Swayamprabha S, Kumar Dubey D, Song W-C, Lin Y-T, Ashok Kumar Yadav R, Singh M, Jou J-H. An Approach for Measuring the Dielectric Strength of OLED Materials. Materials. 2018; 11(6):979. https://doi.org/10.3390/ma11060979
Chicago/Turabian StyleSudheendran Swayamprabha, Sujith, Deepak Kumar Dubey, Wei-Chi Song, You-Ting Lin, Rohit Ashok Kumar Yadav, Meenu Singh, and Jwo-Huei Jou. 2018. "An Approach for Measuring the Dielectric Strength of OLED Materials" Materials 11, no. 6: 979. https://doi.org/10.3390/ma11060979






