The Effect of Rod-Shaped Long-Period Stacking Ordered Phases Evolution on Corrosion Behavior of Mg95.33Zn2Y2.67 Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Microstructural Analysis
2.3. Electrochemical Measurements
2.4. Immersion Tests
3. Results and Discussion
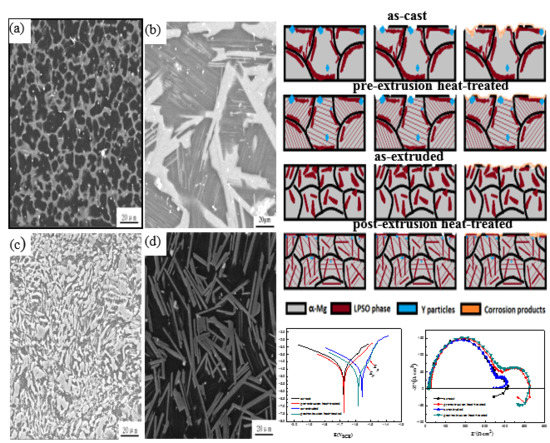
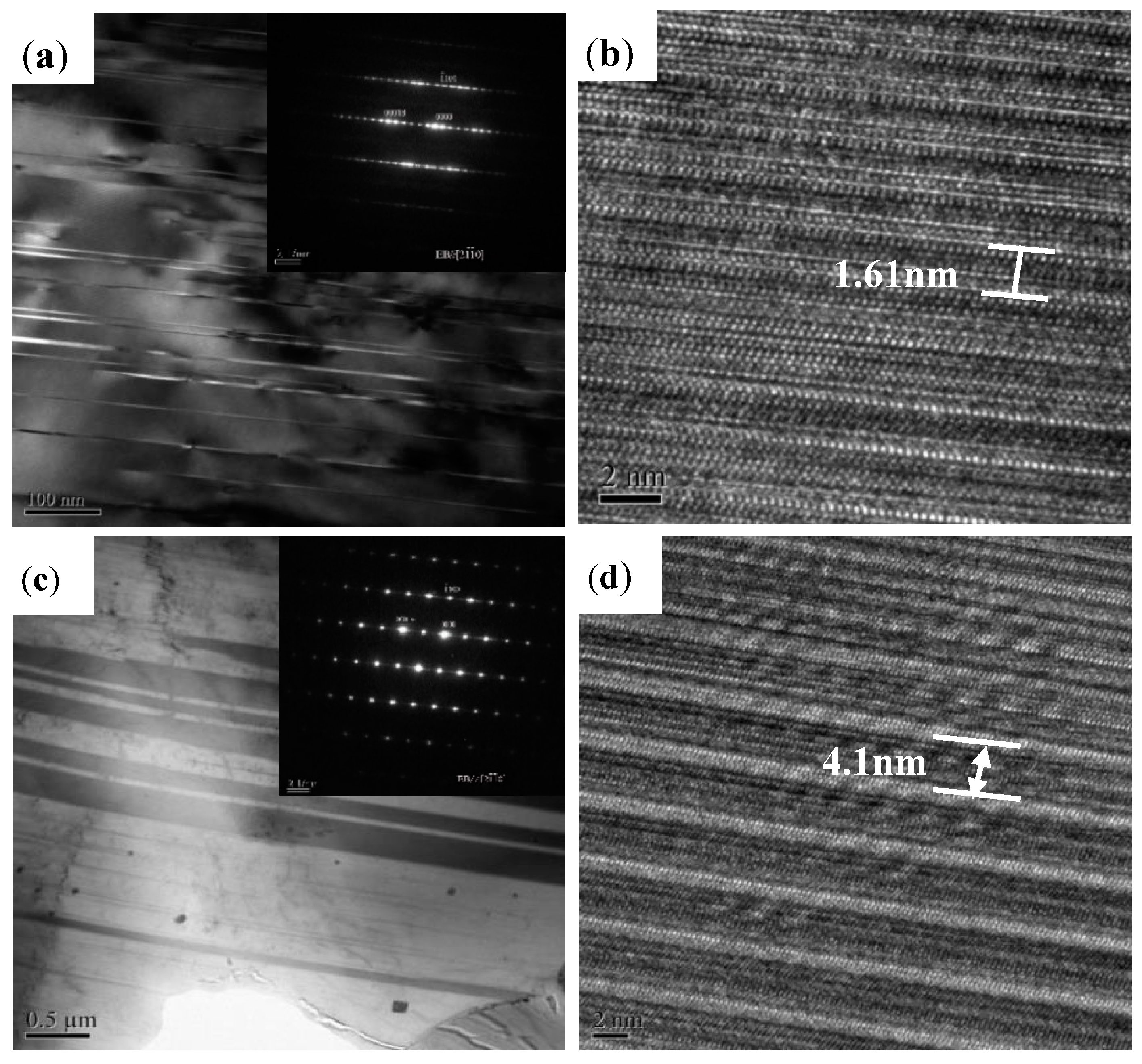
3.1. Microstructural Analysis
3.2. Corrosion Behavior
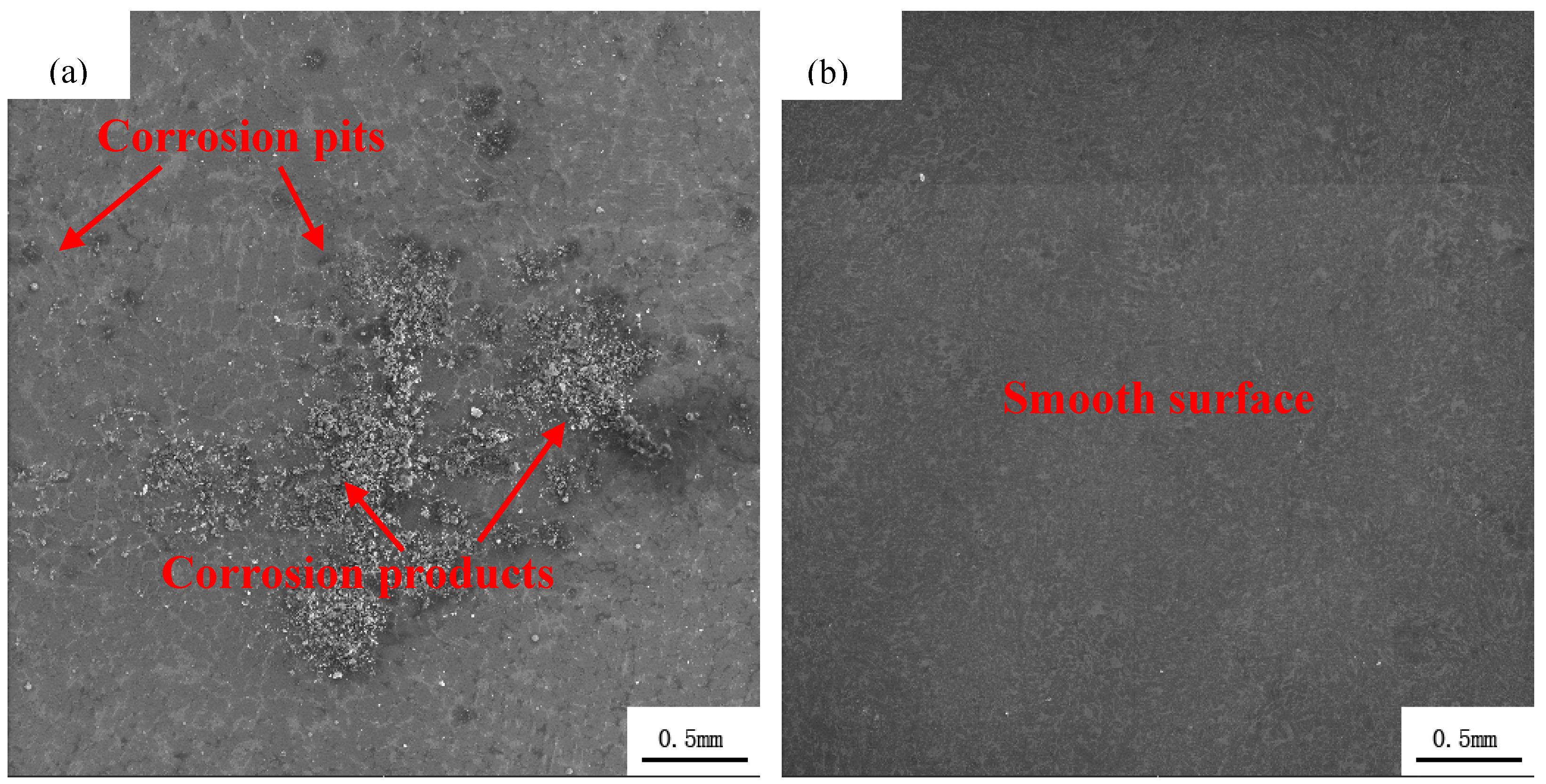
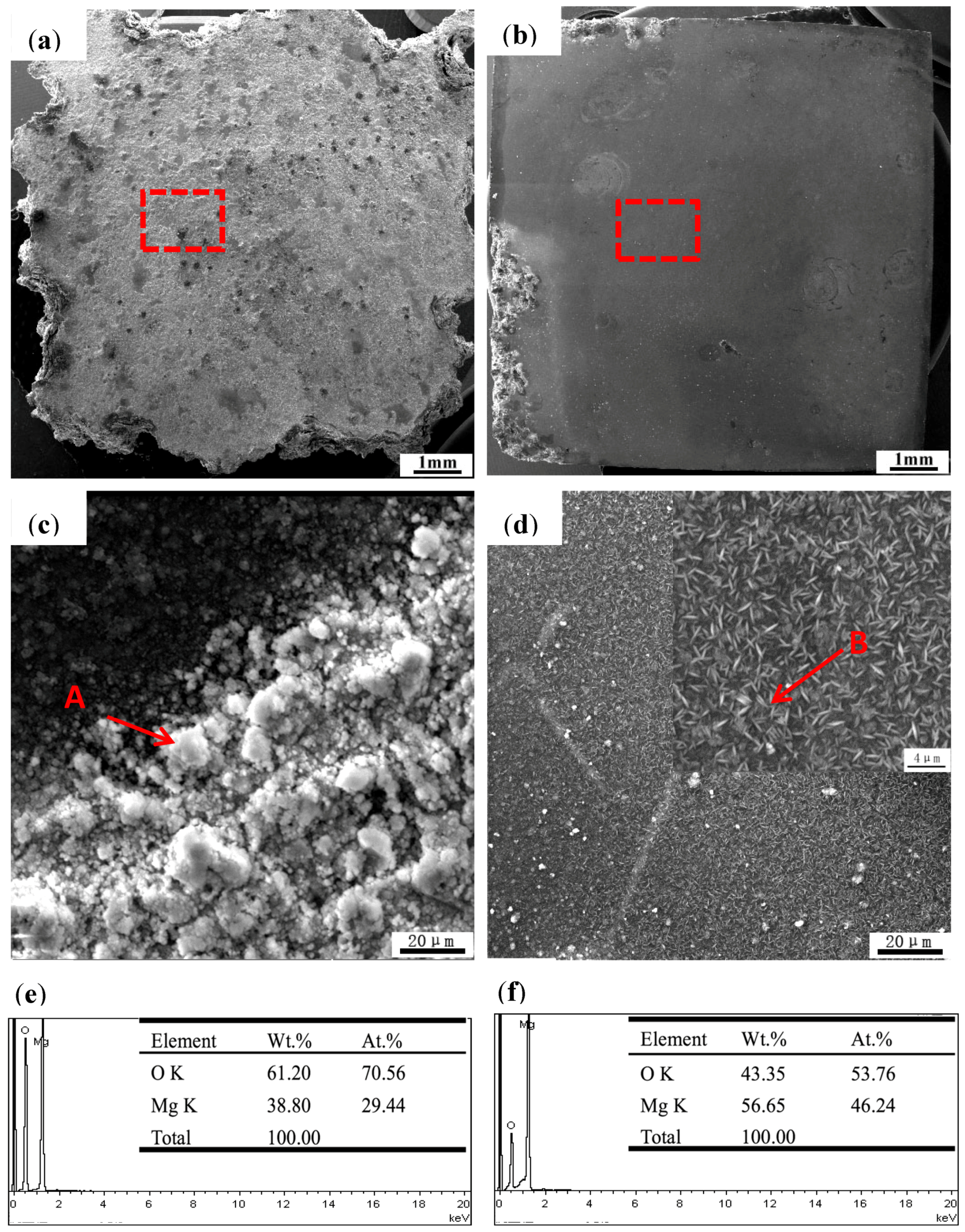
3.3. Corroded Surface Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Zhou, W.; Hu, P.H.; Zhou, Q. Microstructural characteristics and mechanical properties of Mg–Zn–Y alloy containing icosahedral quasicrystals phase treated by pulsed magnetic field. J. Alloys Compd. 2016, 688, 868–874. [Google Scholar] [CrossRef]
- Asgharzadeh, H.; Yoon, E.Y.; Chae, H.J.; Kim, T.S.; Lee, J.W.; Kim, H.S. Microstructure and mechanical properties of a Mg–Zn–Y alloy produced by a powder metallurgy route. J. Alloys Compd. 2014, 586, S95–S100. [Google Scholar] [CrossRef]
- Kawamura, Y.; Hayashi, K.; Inoue, A.; Masumoto, T. Rapidly solidified powder metallurgy Mg97Zn1Y2 Alloys with excellent tensile yield strength above 600 MPa. Mater. Trans. 2001, 42, 1171–1174. [Google Scholar] [CrossRef]
- Rosalie, J.M.; Somekawa, H.; Singh, A.; Mukai, T. Effect of precipitation on strength and ductility in a Mg–Zn–Y alloy. J. Alloys Compd. 2013, 550, 114–123. [Google Scholar] [CrossRef]
- Jiang, H.; Qiao, X.; Xu, C.; Kamado, S.; Wu, K. Influence of size and distribution of W phase on strength and ductility of high strength Mg-5.1Zn-3.2Y-0.4Zr-0.4Ca alloy processed by indirect extrusion. J. Mater. Sci. Technol. 2018, 34, 277–283. [Google Scholar] [CrossRef]
- Wang, W.; Xu, R.; Hao, Y.; Wang, Q.; Wang, Q.; Yu, L.; Che, Q.; Cai, J.; Wang, K.; Ma, Z. Corrosion fatigue behavior of friction stir processed interstitial free steel. J. Mater. Sci. Technol. 2018, 34, 148–156. [Google Scholar] [CrossRef]
- Miao, H.; Huang, H.; Shi, Y.; Zhang, H.; Pei, J.; Yuan, G. Effects of solution treatment before extrusion on the microstructure, mechanical properties and corrosion of Mg–Zn–Gd alloy in vitro. Corros. Sci. 2017, 122, 90–99. [Google Scholar] [CrossRef]
- Zhao, X.; Shi, L.; Xu, J. Mg–Zn–Y alloys with long-period stacking ordered structure: In vitro assessments of biodegradation behavior. Mater. Sci. Eng. C 2013, 33, 3627–3637. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Shan, D.; Chen, R.; Han, E.H. Effect of second phases on the corrosion behaviour of wrought Mg–Zn–Y–Zr alloy. Corros. Sci. 2010, 52, 1830–1837. [Google Scholar] [CrossRef]
- Wang, J.F.; Jiang, W.Y.; Ma, Y.; Li, Y.; Huang, S. Substantial corrosion resistance improvement in heat-treated Mg–Gd–Zn alloys with a long period stacking ordered structure. Mater. Chem. Phys. 2018, 203, 352–361. [Google Scholar] [CrossRef]
- Grobner, J.; Kozlov, A.; Schmid-Fetzer, R. Phase equilibria and transformations in ternary Mg-rich Mg–Y–Zn alloys. Acta Mater. 2012, 60, 5948–5962. [Google Scholar] [CrossRef]
- Kawamura, Y.; Yamasaki, M. Formation and Mechanical Properties of Mg97Zn1RE2 Alloys with Long-Period Stacking Ordered Structure. Mater. Trans. 2007, 48, 2986–2992. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Xue, Y.; Wang, Z.; Yang, L. Biocorrosion behavior and cytotoxicity of a Mg–Gd–Zn–Zr alloy with long period stacking ordered structure. Mater. Lett. 2012, 86, 42–45. [Google Scholar] [CrossRef]
- Zhang, X.; Ba, Z.; Wang, Q.; Wu, Y.; Wang, Z.; Wang, Q. Uniform corrosion behavior of GZ51K alloy with long period stacking ordered structure for biomedical application. Corros. Sci. 2014, 88, 1–5. [Google Scholar] [CrossRef]
- Li, C.Q.; Xu, D.K.; Zeng, Z.R.; Wang, B.J.; Sheng, L.Y.; Chen, X.B.; Han, E.H. Effect of volume fraction of LPSO phases on corrosion and mechanical properties of Mg–Zn–Y alloys. Mater. Des. 2017, 121, 430–441. [Google Scholar] [CrossRef]
- Izumi, S.; Yamasaki, M.; Kawamura, Y. Relation between corrosion behavior and microstructure of Mg–Zn–Y alloys prepared by rapid solidification at various cooling rates. Corros. Sci. 2009, 51, 395–402. [Google Scholar] [CrossRef]
- Pérez, P.; Onofre, E.; Caheza, S.; Llorente, I.; del Valle, J.A.; GarcÍa-Alonso, M.C.; Adeva, P.; Escudero, M.L. Corrosion behaviour of Mg–Zn–Y–Mischmetal alloys in phosphate buffer saline solution. Corros. Sci. 2013, 69, 226–235. [Google Scholar] [CrossRef]
- Wang, S.D.; Xu, D.K.; Chen, X.B.; Han, E.H.; Dong, C. Effect of heat treatment on the corrosion resistance and mechanical properties of an as-forged Mg–Zn–Y–Zr alloy. Corros. Sci. 2015, 92, 228–236. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.H.; Shan, D.; Yim, C.D.; You, B.S. The effect of Zn concentration on the corrosion behavior of Mg–xZn alloys. Corros. Sci. 2012, 65, 322–330. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A.; StJohn, D. An hydrogen evolution method for the estimation of the corrosion rate of magnesium alloys. Magnes. Technol. 2001, 2001, 255–262. [Google Scholar]
- Abidin, N.I.Z.; Atrens, A.D.; Martin, D.; Atrens, A. Corrosion of high purity Mg, Mg2Zn0.2Mn, ZE41 and AZ91 in Hank’s solution at 37 °C. Corros. Sci. 2011, 53, 3542–3556. [Google Scholar] [CrossRef]
- ASTM-G31-72. Standard Practice for Laboratory Immersion Corrosion Testing of Metals; Annual Book of ASTM Standards; American Society for Testing and Materials: Philadelphia, PA, USA, 2004; Available online: https://wenku.baidu.com/view/fe282d63caaedd3383c4d3b5.html (accessed on 1 May 2004).
- Wang, J.; Song, P.; Zhou, X.; Huang, X.; Pan, F. Influence of the morphology of long-period stacking ordered phase on the mechanical properties of as-extruded Mg-5Zn-5Y-0.6Zr magnesium alloy. Mater. Sci. Eng. A 2012, 556, 68–75. [Google Scholar] [CrossRef]
- Lu, R.; Wang, J.; Chen, Y.; Qin, D.; Yang, W. Effects of heat treatment on the morphology of long-period stacking ordered phase, the corresponding damping capacities and mechanical properties of Mg–Zn–Y alloys. J. Alloys Compd. 2015, 639, 541–546. [Google Scholar] [CrossRef]
- Mandal, M.; Moon, A.P.; Deo, G.; Mendis, C.L.; Mondal, K. Corrosion behavior of Mg–2.4Zn alloy micro-alloyed with Ag and Ca. Corros. Sci. 2014, 78, 172–182. [Google Scholar] [CrossRef]
- Song, G. Effect of tin modification on corrosion of AM70 magnesium alloy. Corros. Sci. 2009, 51, 2063–2070. [Google Scholar] [CrossRef]
- Yamasaki, M.; Izumi, S.; Kawamura, Y.; Habazaki, H. Corrosion and passivation behavior of Mg–Zn–Y–Al alloys prepared by cooling rate-controlled solidification. Appl. Surf. Sci. 2011, 257, 8258–8267. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, J.; Cheng, W.; Chen, C.; Kang, J. Corrosion Behavior of Mg–Zn–Y alloy with Long-period stacking ordered structure. J. Mater. Sci. Technol. 2012, 28, 1157–1162. [Google Scholar] [CrossRef]
- Zhang, X.; Kairy, S.K.; Dia, J.; Birbilis, N. A Closer Look at the Role of Nanometer Scale Solute-Rich Stacking Faults in the Localized Corrosion of a Magnesium Alloy GZ31K. J. Electrochem. Soc. 2018, 165, C310–C316. [Google Scholar] [CrossRef]



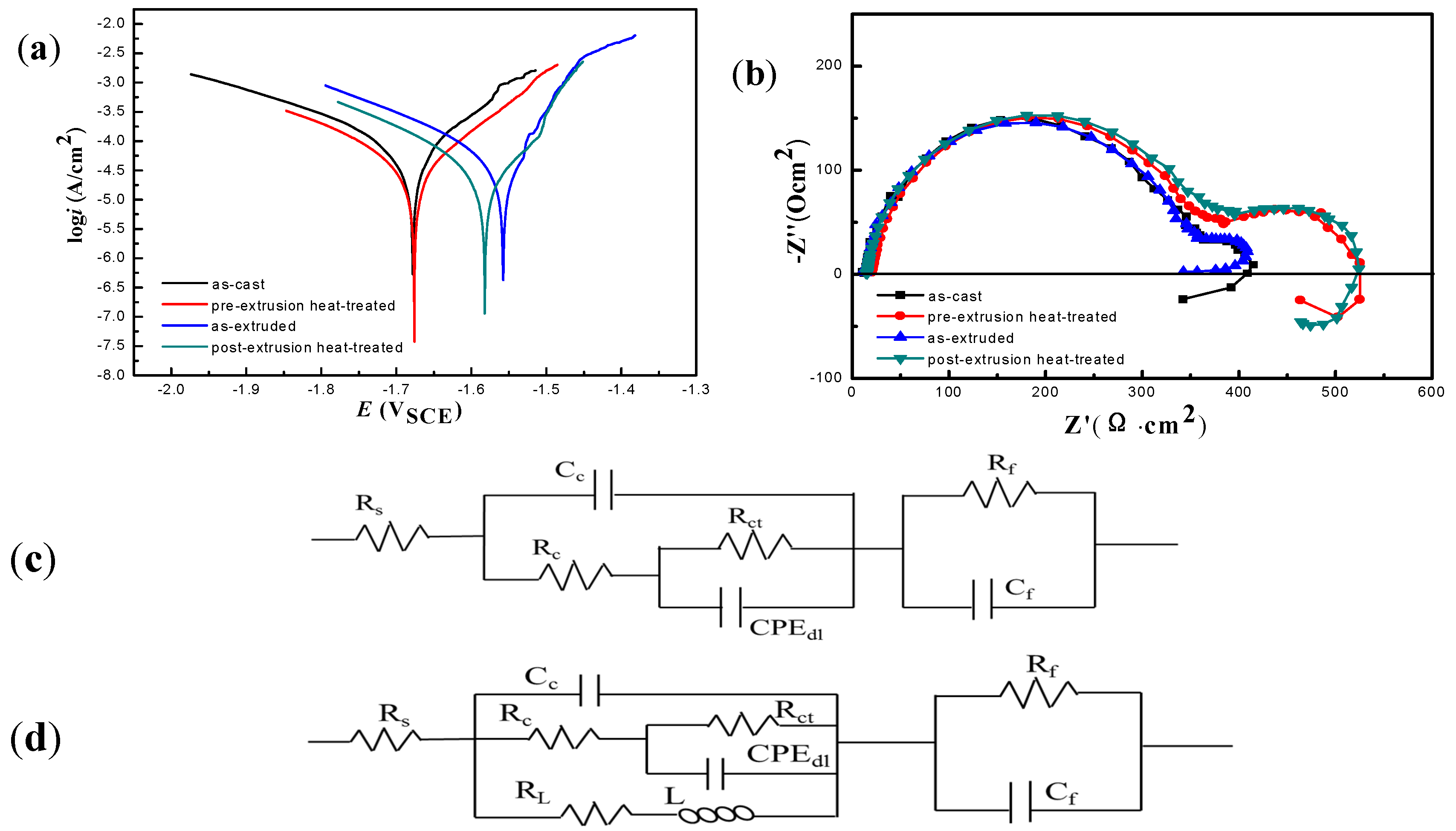



| Alloy | Ecorr (V) | icorr (A/cm2) | Rp | |
|---|---|---|---|---|
| as-cast | −1.6792 | 5.4 × 10−5 | 480 | Present work |
| pre-extrusion heat-treated | −1.6768 | 3.40 × 10−5 | 770.7 | Present work |
| as-extruded | −1.5582 | 4.4 × 10−5 | 586.6 | Present work |
| post-extrusion heat-treated | −1.5823 | 2.9 × 10−5 | 905.2 | Present work |
| as-cast Mg97Zn1Y2 | −1.488 | 7.6 × 10−5 | - | [9] |
| as-extruded AZ31 | −1.518 | 8.7 × 10−5 | - | [9] |
| as-cast WE43 | −1.69 | 5.9 × 10−5 | - | [9] |
| as-cast ZK60 | −1.544 | 2.8 × 10−4 | - | [9] |
| Parameters | As-Cast | Pre-Extrusion Heat Treated | As-Extruded | Post-Extrusion Heat Treated |
|---|---|---|---|---|
| Rs (Ω·cm2) | 8.68 | 16.52 | 8.857 | 13.36 |
| Rc (Ω·cm2) | 4.74 | 4.27 | 5.50 | 0.098 |
| Cc (F·cm2) | 3.38 × 10−7 | 2.32 × 10−7 | 2.90 × 10−7 | 1.85 × 10−13 |
| CPE-T (μΩ−1·cm−2·s−1) | 2.55 × 10−5 | 2.32 × 10−5 | 3.00 × 10−5 | 2.18 × 10−5 |
| CPE-P | 0.91279 | 0.90695 | 0.91894 | 0.91298 |
| Rct (Ω·cm2) | 339.8 | 350.8 | 343 | 548.2 |
| RL (Ω·cm2) | 1110 | 640.6 | - | 1716 |
| L (H·cm2) | 9989 | 9261 | - | 22,845 |
| Rf (Ω·cm2) | 63.3 | 153.2 | 50.3 | 133.7 |
| Cf (F·cm2) | 0.0074 | 0.0038 | 0.0073 | 0.0034 |
| chisq | 0.0020 | 0.0014 | 0.0033 | 0.0011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jiang, W.; Guo, S.; Li, Y.; Ma, Y. The Effect of Rod-Shaped Long-Period Stacking Ordered Phases Evolution on Corrosion Behavior of Mg95.33Zn2Y2.67 Alloy. Materials 2018, 11, 815. https://doi.org/10.3390/ma11050815
Wang J, Jiang W, Guo S, Li Y, Ma Y. The Effect of Rod-Shaped Long-Period Stacking Ordered Phases Evolution on Corrosion Behavior of Mg95.33Zn2Y2.67 Alloy. Materials. 2018; 11(5):815. https://doi.org/10.3390/ma11050815
Chicago/Turabian StyleWang, Jingfeng, Weiyan Jiang, Shengfeng Guo, Yang Li, and Yao Ma. 2018. "The Effect of Rod-Shaped Long-Period Stacking Ordered Phases Evolution on Corrosion Behavior of Mg95.33Zn2Y2.67 Alloy" Materials 11, no. 5: 815. https://doi.org/10.3390/ma11050815