3-[Bis(pyridin-2-ylmethyl)amino]-5-(4-carboxyphenyl)-BODIPY as Ratiometric Fluorescent Sensor for Cu2+
Abstract
:1. Introduction
2. Materials and Methods
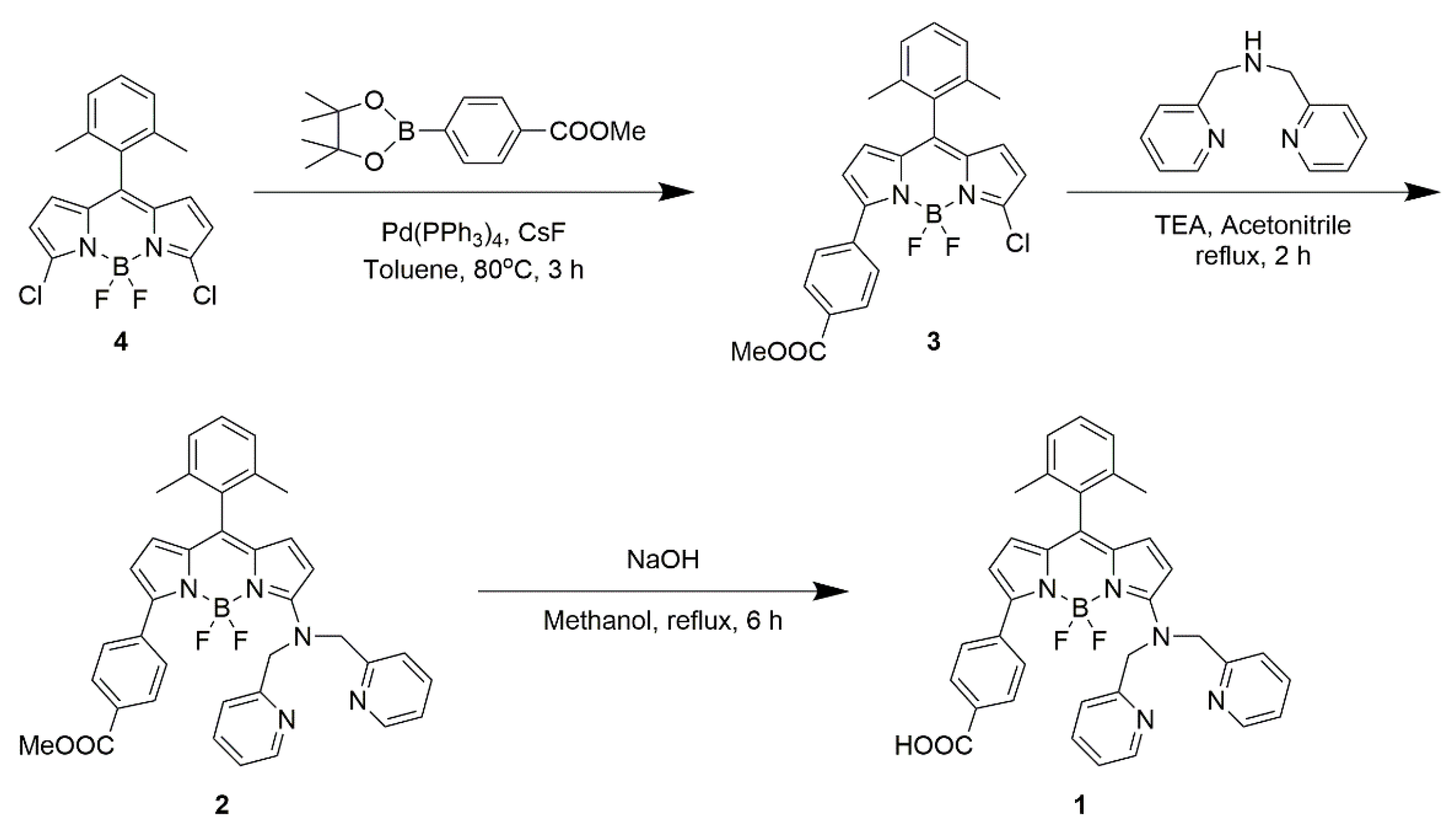
2.1. Synthesis of BODIPY 1
2.1.1. 3-Chloro-4,4-difluoro-5-[4-(methoxycarbonyl)phenyl]-8-(2,6-dimethylphenyl)-4-bora-3a,4a-diaza-s-indacene (3)
2.1.2. 3-[Bis(pyridin-2-ylmethyl)amino]-4,4-difluoro-5-[4-(methoxycarbonyl)phenyl]-8-(2,6-dimethylphenyl)-4-bora-3a,4a-diaza-s-indacene (2)
2.1.3. 3-[Bis(pyridin-2-ylmethyl)amino]-4,4-difluoro-5-(4-carboxyphenyl)-8-(2,6-dimethylphenyl)-4-bora-3a,4a-diaza-s-indacene (1)
2.2. Fluorescence and UV–Vis Spectroscopic Measurements
3. Results and Discussion
3.1. Photophysical Properties of BODIPY 1
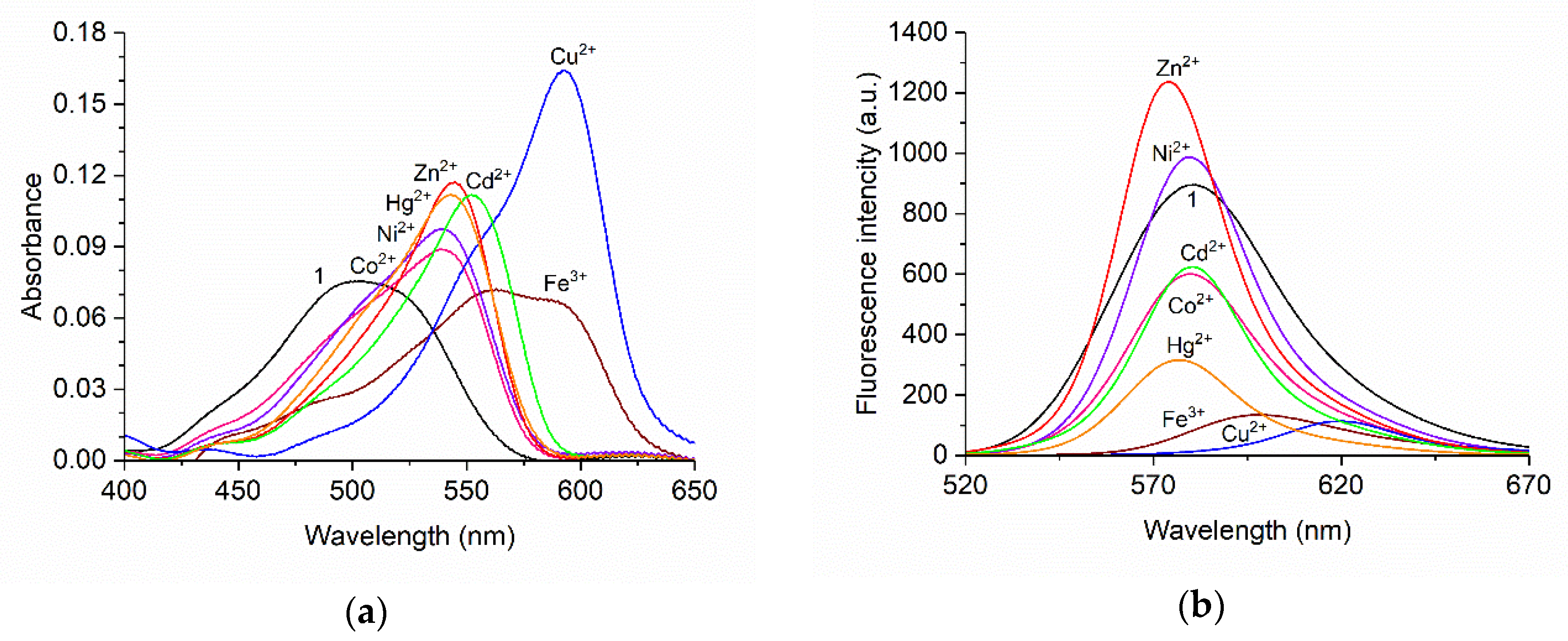
3.2. Spectroscopic Response of BODIPY 1 toward Metal Cations
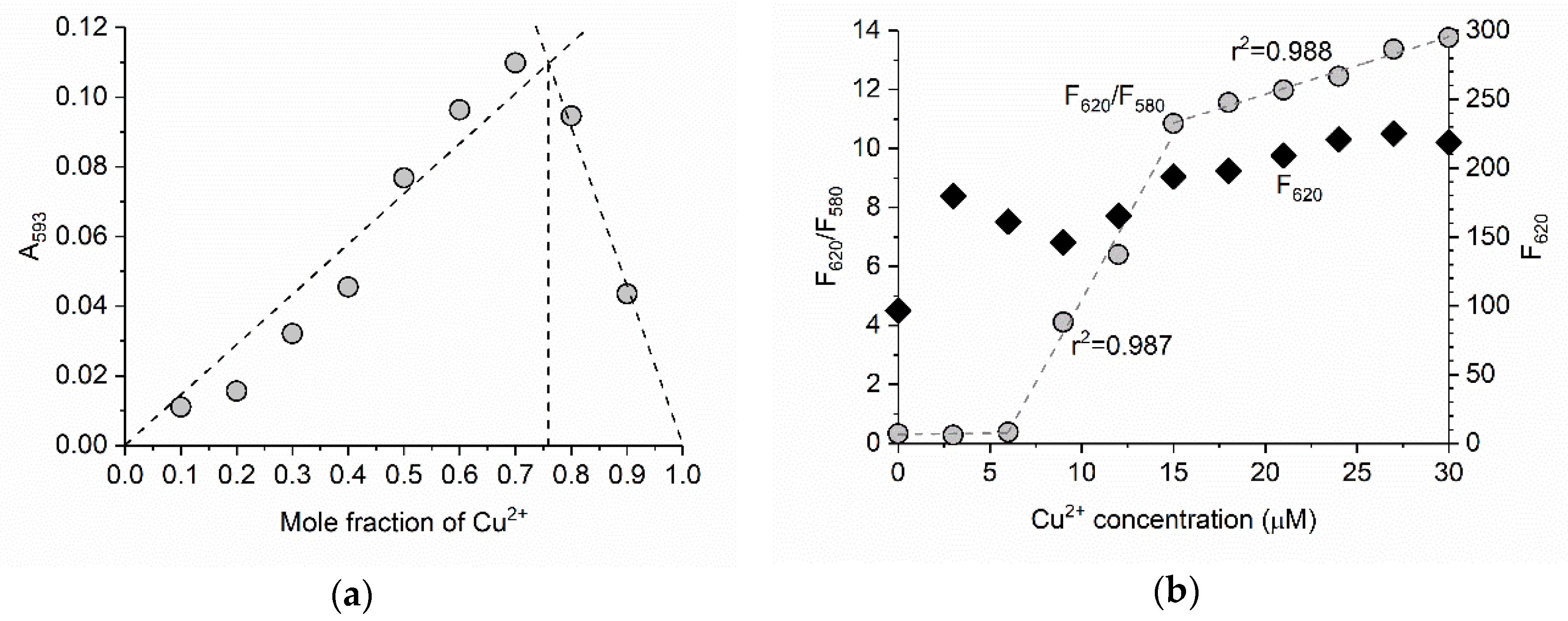
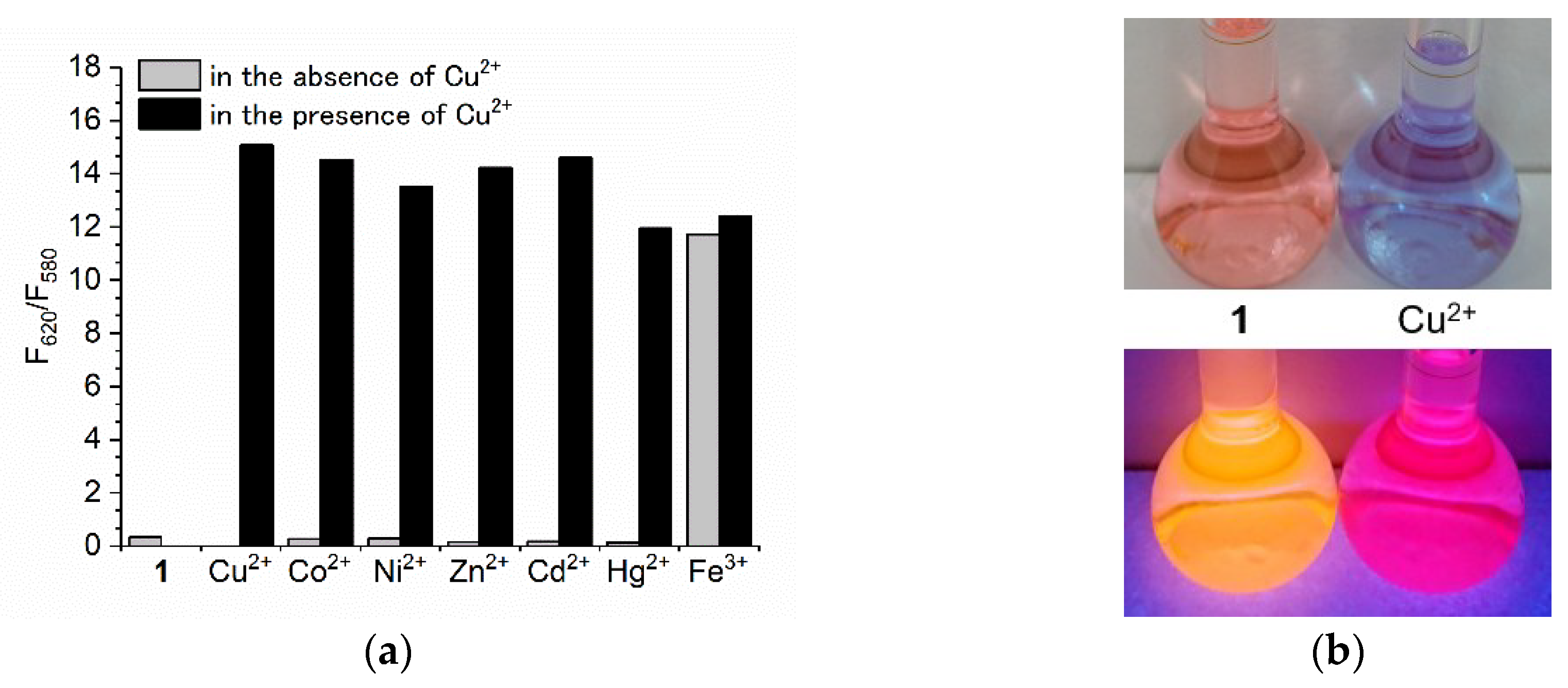
3.3. Ratiometric Determination of Cu2+ with BODIPY 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 2003, 189, 147–163. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012; ISBN 9780875530130. [Google Scholar]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; ISBN 9780387463124. [Google Scholar]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef] [PubMed]
- Valeur, B.; Berberan-Santos, M.N. Molecular Fluorescence: Principles and Applications, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012; ISBN 9783527328376. [Google Scholar]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Rohand, T.; Qin, W.; Boens, N.; Dehaen, W. Palladium-catalyzed coupling reactions for the functionalization of BODIPY dyes with fluorescence spanning the visible spectrum. Eur. J. Org. Chem. 2006, 2006, 4658–4663. [Google Scholar] [CrossRef]
- Domaille, D.W.; Zeng, L.; Chang, C.J. Visualizing ascorbate-triggered release of labile copper within living cells using a ratiometric fluorescent sensor. J. Am. Chem. Soc. 2010, 132, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Hafuka, A.; Taniyama, H.; Son, S.H.; Yamada, K.; Takahashi, M.; Okabe, S.; Satoh, H. BODIPY-based ratiometric fluoroionophores with bidirectional spectral shifts for the selective recognition of heavy metal ions. Bull. Chem. Soc. Jpn. 2013, 86, 37–44. [Google Scholar] [CrossRef]
- Qin, W.W.; Dou, W.; Leen, V.; Dehaen, W.; Van der Auweraer, M.; Boens, N. A ratiometric, fluorescent BODIPY-based probe for transition and heavy metal ions. RSC Adv. 2016, 6, 7806–7816. [Google Scholar] [CrossRef] [Green Version]
- Xia, S.; Shen, J.J.; Wang, J.B.; Wang, H.L.; Fang, M.X.; Zhou, H.W.; Tanasova, M. Ratiometric fluorescent and colorimetric BODIPY-based sensor for zinc ions in solution and living cells. Sens. Actuator B Chem. 2018, 258, 1279–1286. [Google Scholar] [CrossRef]
- Yin, S.; Leen, V.; Van Snick, S.; Boens, N.; Dehaen, W. A highly sensitive, selective, colorimetric and near-infrared fluorescent turn-on chemosensor for Cu2+ based on BODIPY. Chem. Commun. 2010, 46, 6329–6331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.L.; Han, Z.; Wang, M.J.; Yang, Z.H.; Ran, X.Q.; He, W.J. A new BODIPY-derived ratiometric senor with internal charge transfer (ICT) effect: Colorimetric/fluorometric sensing of Ag+. Dalton Trans. 2018, 47, 2285–2291. [Google Scholar] [CrossRef] [PubMed]
- Chopin, N.; Medebielle, M.; Maury, O.; Novitchi, G.; Pilet, G. Quenching of fluorescence in bodipy-derived trifluoromethyl enaminone ligands upon coordination to copper(II). Eur. J. Inorg. Chem. 2014, 2014, 6185–6195. [Google Scholar] [CrossRef]
- Liu, Z.C.; Yang, Z.Y.; Li, T.R.; Wang, B.D.; Li, Y.; Qin, D.D.; Wang, M.F.; Yan, M.H. An effective Cu(II) quenching fluorescence sensor in aqueous solution and 1D chain coordination polymer framework. Dalton Trans. 2011, 40, 9370–9373. [Google Scholar] [CrossRef] [PubMed]
- Razi, S.S.; Srivastava, P.; Ali, R.; Gupta, R.C.; Dwivedi, S.K.; Misra, A. A coumarin-derived useful scaffold exhibiting Cu2+ induced fluorescence quenching and fluoride sensing (On-Off-On) via copper displacement approach. Sens. Actuator B Chem. 2015, 209, 162–171. [Google Scholar] [CrossRef]
- Hafuka, A.; Kando, R.; Ohya, K.; Yamada, K.; Okabe, S.; Satoh, H. Substituent effects at the 5-position of 3- bis(pyridine-2-ylmethyl)amino -BODIPY cation sensor used for ratiometric quantification of Cu2+. Bull. Chem. Soc. Jpn. 2015, 88, 447–454. [Google Scholar] [CrossRef]
- Magde, D.; Wong, R.; Seybold, P.G. Fluorescence quantum yields and their relation to lifetimes of rhodamine 6G and fluorescein in nine solvents: Improved absolute standards for quantum yields. Photochem. Photobiol. 2002, 75, 327–334. [Google Scholar] [CrossRef]
- Connars, K.A. Binding Constants, 1st ed.; Wiley-Interscience: New York, NY, USA, 1987; ISBN 9780471830832. [Google Scholar]
- Huang, J.L.; Wang, B.; Ye, J.G.; Liu, B.; Qiu, H.Y.; Yin, S.C. A Highly Copper-Selective Ratiometric Fluorescent Sensor Based on BODIPY. Chin. J. Chem. 2012, 30, 1857–1861. [Google Scholar] [CrossRef]
- Bricks, J.L.; Kovalchuk, A.; Trieflinger, C.; Nofz, M.; Buschel, M.; Tolmachev, A.I.; Daub, J.; Rurack, K. On the development of sensor molecules that display FeIII-amplified fluorescence. J. Am. Chem. Soc. 2005, 127, 13522–13529. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, Z. A fluorescent sensor for Cu2+ and Fe3+ based on multiple mechanisms. RSC Adv. 2016, 6, 59073–59080. [Google Scholar] [CrossRef]
- Joshi, S.; Kumari, S.; Sarmah, A.; Sakhuja, R.; Pant, D.D. Solvatochromic shift and estimation of dipole moment of synthesized coumarin derivative: Application as sensor for fluorogenic recognition of Fe3+ and Cu2+ ions in aqueous solution. J. Mol. Liq. 2016, 222, 253–262. [Google Scholar] [CrossRef]


| λabs | λflu | ε503 | Ф | Stokes Shift |
|---|---|---|---|---|
| (nm) | (nm) | (M−1 cm−1) | (-) | (cm−1) |
| 503 | 580 | 25,000 | 0.34 | 2600 |
| λabs | λflu | ε593 | Ф |
|---|---|---|---|
| (nm) | (nm) | (M−1 cm−1) | (-) |
| 593 | 620 | 59,000 | 0.07 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafuka, A.; Satoh, H.; Yamada, K.; Takahashi, M.; Okabe, S. 3-[Bis(pyridin-2-ylmethyl)amino]-5-(4-carboxyphenyl)-BODIPY as Ratiometric Fluorescent Sensor for Cu2+. Materials 2018, 11, 814. https://doi.org/10.3390/ma11050814
Hafuka A, Satoh H, Yamada K, Takahashi M, Okabe S. 3-[Bis(pyridin-2-ylmethyl)amino]-5-(4-carboxyphenyl)-BODIPY as Ratiometric Fluorescent Sensor for Cu2+. Materials. 2018; 11(5):814. https://doi.org/10.3390/ma11050814
Chicago/Turabian StyleHafuka, Akira, Hisashi Satoh, Koji Yamada, Masahiro Takahashi, and Satoshi Okabe. 2018. "3-[Bis(pyridin-2-ylmethyl)amino]-5-(4-carboxyphenyl)-BODIPY as Ratiometric Fluorescent Sensor for Cu2+" Materials 11, no. 5: 814. https://doi.org/10.3390/ma11050814





