Dealloyed Ruthenium Film Catalysts for Hydrogen Generation from Chemical Hydrides
Abstract
:1. Introduction
2. Materials
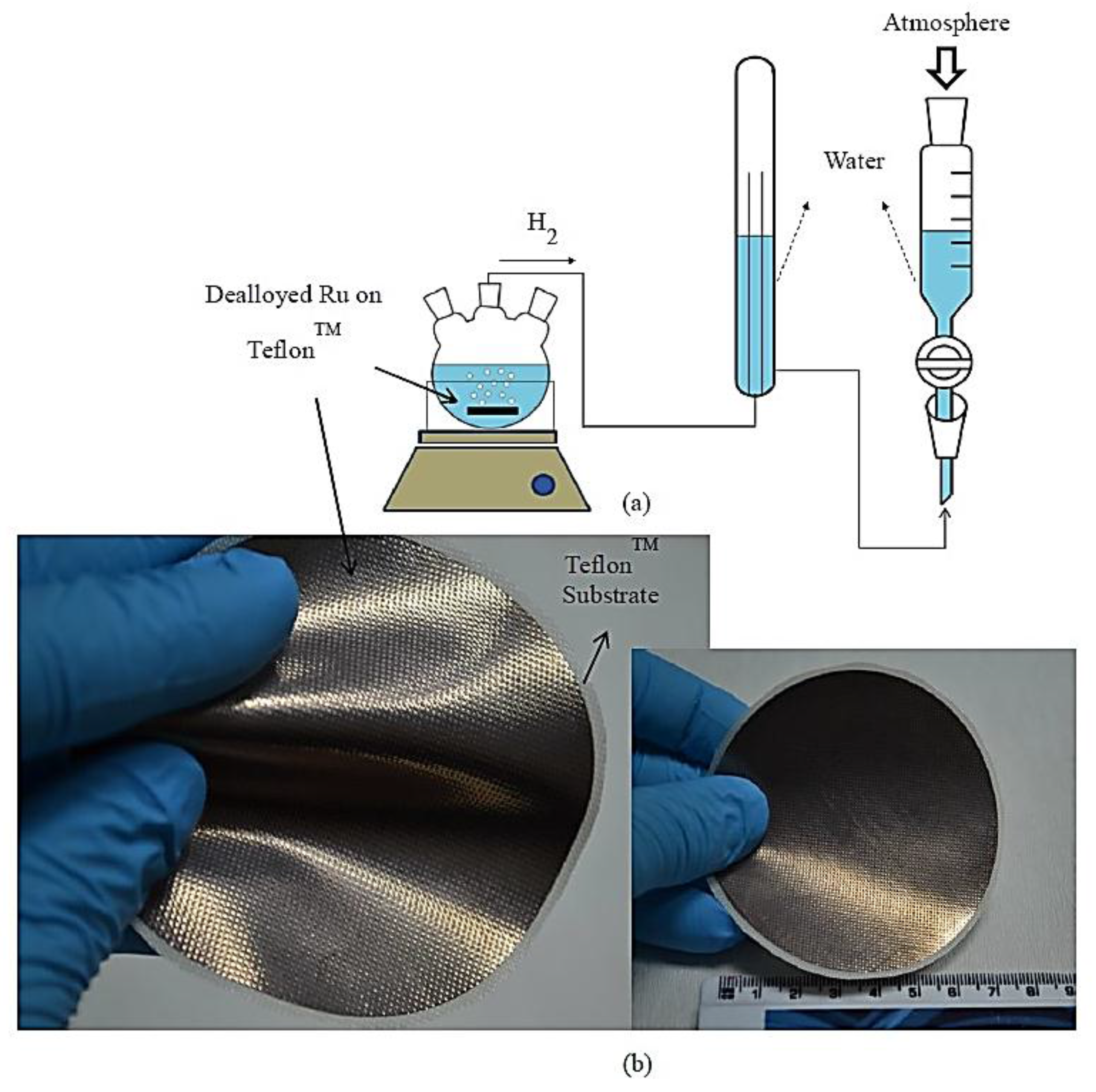
Alloying, Dealloying Methods and Hydrogen Generation and Durability Tests
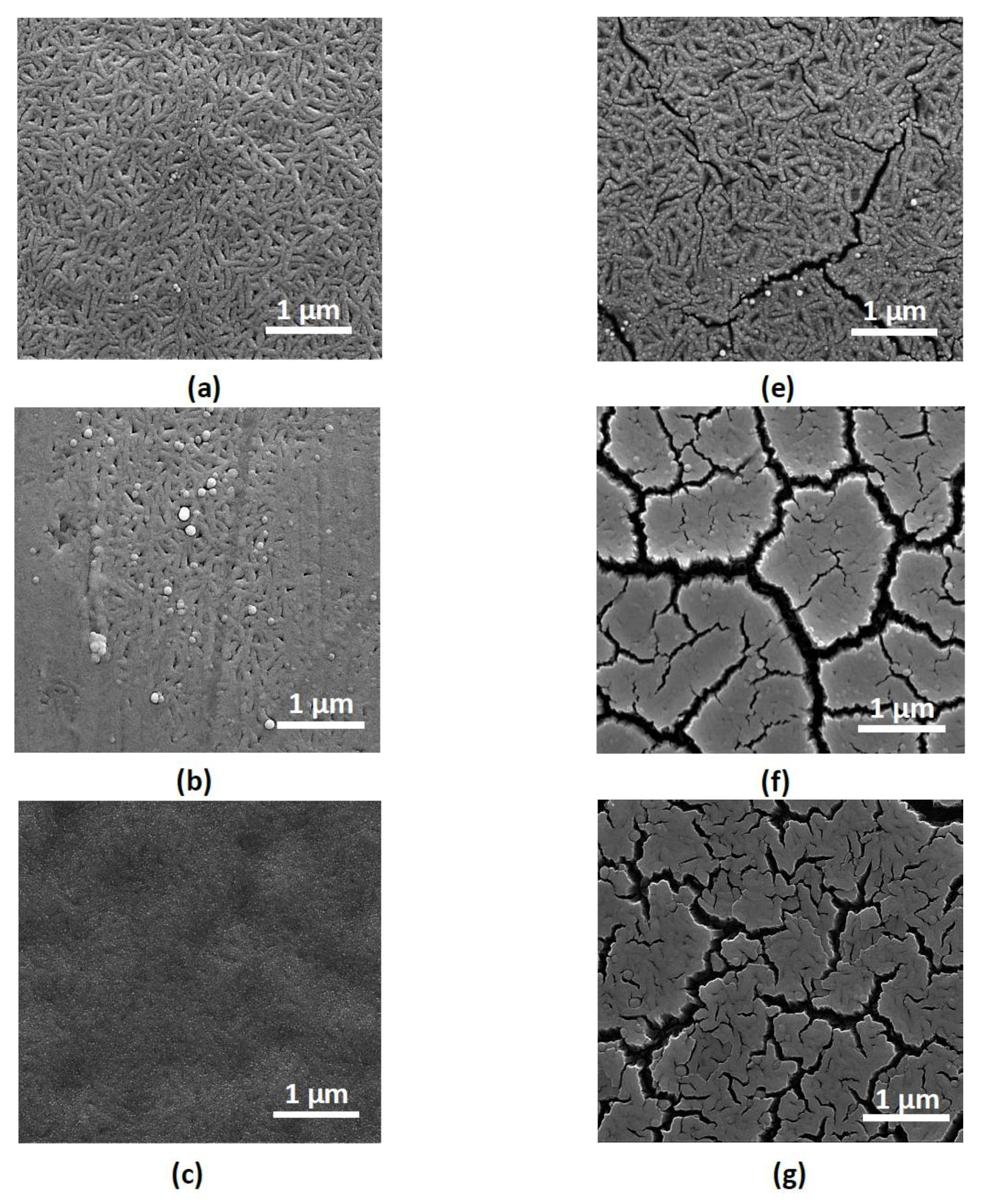
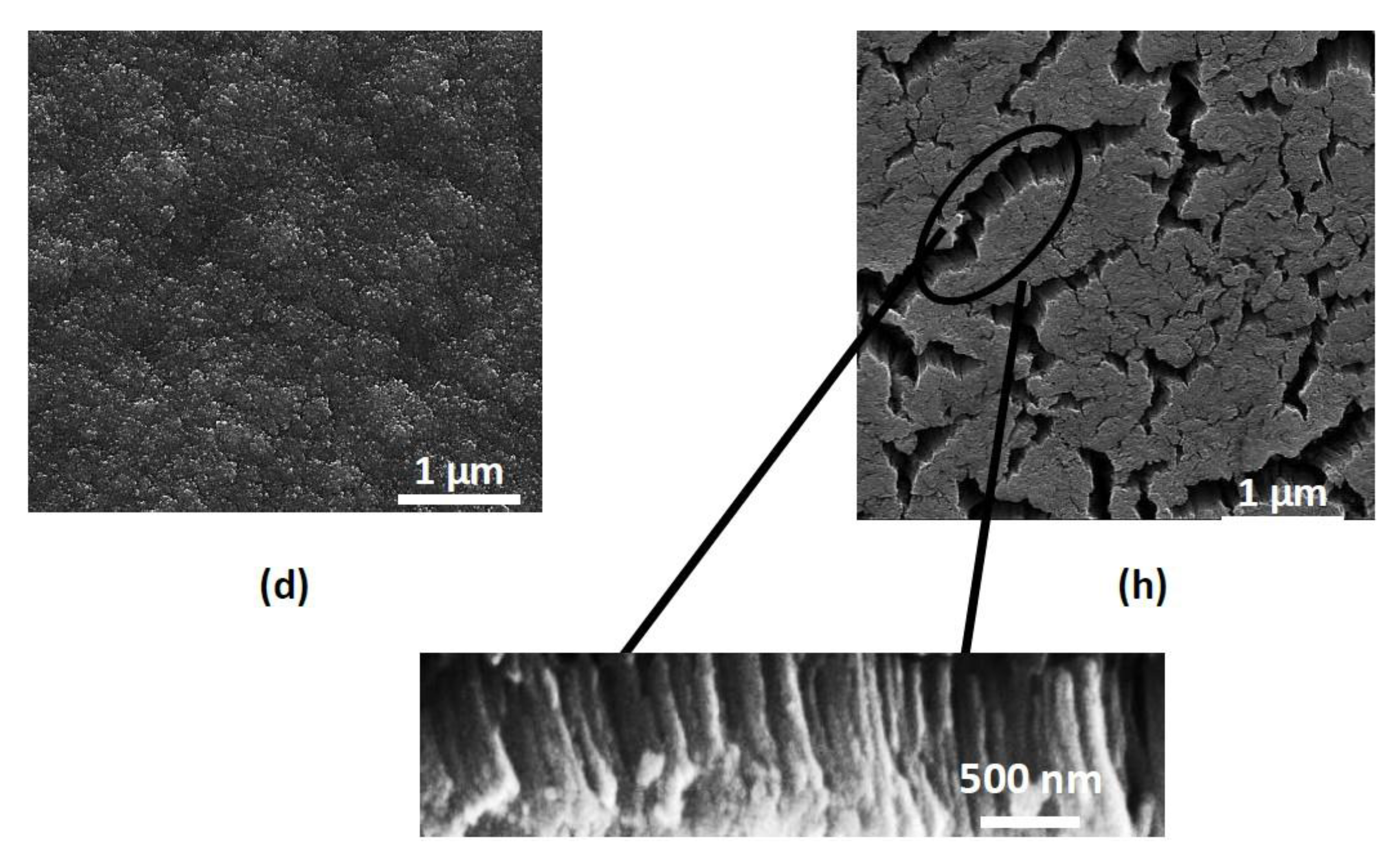
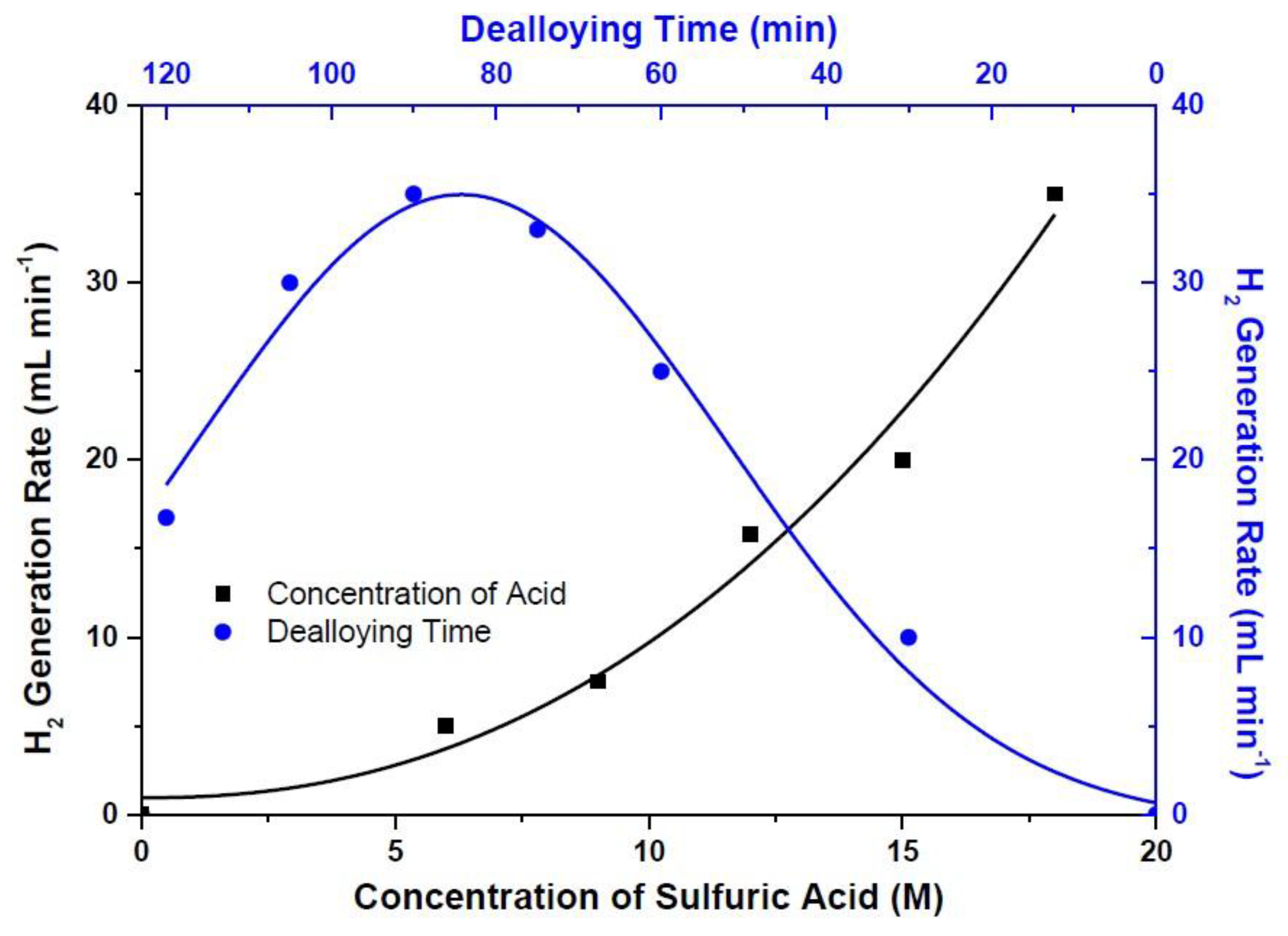
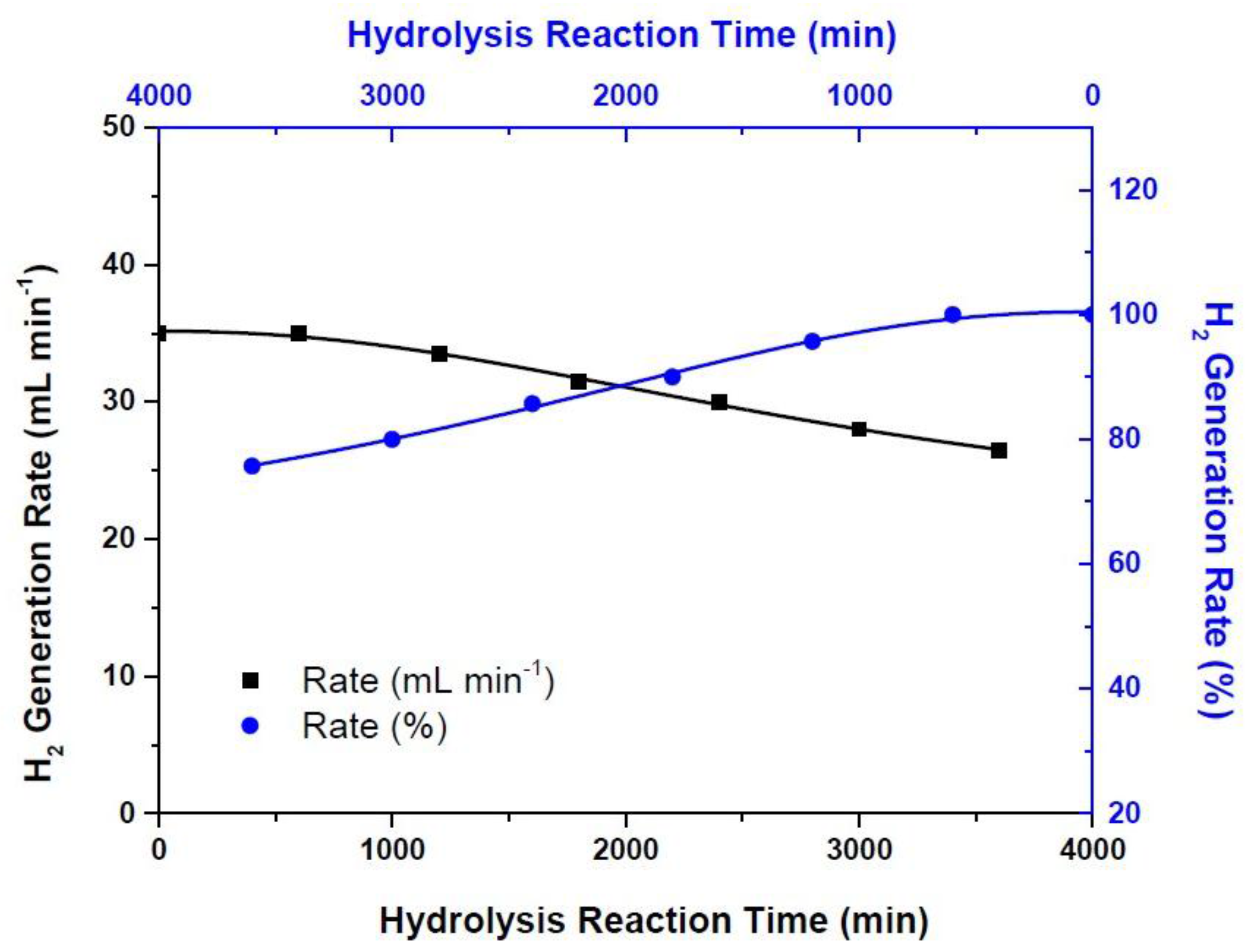
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sankir, M.; Semiz, L.; Serin, R.B.; Sankir, N.D.; Baker, D. Hydrogen Generation from Chemical Hydrides. In Advanced Catalytic Materials; Tiwari, A., Titinchi, S., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2015; pp. 145–192. ISBN 9781118998939. [Google Scholar]
- Sankir, M.; Sankir, N.D. Advances in Hydrogen Production and Storage. In Hydrogen Production Technologies; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2017; Volume 1, ISBN 978-1119283645. [Google Scholar]
- Akay, T.E.; Abdullayeva, N.; Sankir, M.; Sankir, N.D. On-Board Hydrogen Powered Proton Exchange Membrane Fuel Cells. ECS Trans. 2016, 75, 511–513. [Google Scholar] [CrossRef]
- Sankir, M.; Serin, R.B.; Semiz, L.; Sankir, N.D. Unusual behavior of dynamic hydrogen generation from sodium borohydride. Int. J. Hydrogen Energy 2014, 39, 2608–2613. [Google Scholar] [CrossRef]
- Huang, Z.M.; Su, A.; Liu, Y.C. Hydrogen generation with sodium borohydride solution by Ru catalyst. Int. J. Energy Res. 2013, 37, 1187–1195. [Google Scholar] [CrossRef]
- Sankir, M.; Semiz, L.; Serin, R.B.; Sankir, N.D. Hydrogen generation from nanoflower platinum films. Int. J. Hydrogen Energy 2015, 40, 8522–8529. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, R.; Meng, L.; Wang, Y.; Li, G.; Xin, S.; Zhao, X.; Zhang, K. Hydrogen generation from alkaline NaBH4 solution using a dandelion-like Co–Mo–B catalyst supported on carbon cloth. Int. J. Hydrogen Energy 2017, 42, 9945–9951. [Google Scholar] [CrossRef]
- Jeon, H.; Chung, Y. Hydrogen production from formic acid dehydrogenation over Pd/C catalysts: Effect of metal and support properties on the catalytic performance. Appl. Catal. B 2017, 210, 212–222. [Google Scholar] [CrossRef]
- Ma, M.; Duan, R.; Ouyang, L.; Zhu, X.; Chen, Z.; Peng, C.; Zhu, M. Hydrogen storage and hydrogen generation properties of CaMg2-based alloys. J. Alloys Compd. 2017, 691, 929–935. [Google Scholar] [CrossRef]
- Santra, S.; Das, D.; Das, N.S.; Nanda, K.K. An efficient on-board metal-free nanocatalyst for controlled room temperature hydrogen production. Chem. Sci. 2017, 8, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Sankir, M.; Semiz, L.; Sankir, N.D. Catalyst free hydrogen generation from directly disulfonated poly(arylene ether sulfone) copolymer membranes. J. Membr. Sci. 2015, 496, 318–324. [Google Scholar] [CrossRef]
- Li, F.; Zhu, B.; Sun, Y.; Tao, W. Hydrogen generation by means of the combustion of aluminum powder/sodiumborohydride in steam. Int. J. Hydrogen Energy 2017, 42, 3804–3812. [Google Scholar] [CrossRef]
- Loghmani, M.H.; Shojaei, A.F.; Khakzad, M. Hydrogen generation as a clean energy through hydrolysis of sodium borohydride over Cu-Fe-B nano powders: Effect of polymers and surfactants. Energy 2017, 126, 830–840. [Google Scholar] [CrossRef]
- Guo, Y.; Qian, J.; Iqbal, A.; Zhang, L.; Liu, W.; Qin, W. Pd nanoparticles immobilized on magnetic carbon dots@Fe3O4 nanocubes as a synergistic catalyst for hydrogen generation. Int. J. Hydrogen Energy 2017, 42, 15167–15177. [Google Scholar] [CrossRef]
- Huang, Z.M.; Su, A.; Liu, Y.C. Hydrogen generator system using Ru catalyst for PEMFC (proton exchange membrane fuel cell) applications. Energy 2013, 51, 230–236. [Google Scholar] [CrossRef]
- Sousa, T.; Fernandes, V.R.; Pinto, P.J.R.; Slavkov, Y.; Bosukov, L.; Rangel, C.M. A sodium borohydride hydrogen generation reactor for stationary applications: Experimental and reactor simulation studies. Chem. Eng. Sci. 2012, 84, 70–79. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wu, S.; Wei, Y.; Meng, W.; Xie, Y.; Cui, Y.; Lian, X.; Chen, Y.; Zhang, X. Hydrogen Generation from Alkaline NaBH4 Solution Using Nanostructured Co-Ni-P catalysts. Int. J. Hydrogen Energy 2017, 42, 16529–16537. [Google Scholar] [CrossRef]
- Ma, Y.; Li, X.; Zhang, Y.; Chen, L.; Wu, J.; Gao, D.; Bi, J.; Fan, G. Ruthenium nanoparticles supported on TiO2 (B) nanotubes: Effective catalysts in hydrogen evolution from the hydrolysis of ammonia borane. J. Alloys Compd. 2017, 708, 270–277. [Google Scholar] [CrossRef]
- Bandal, H.A.; Jadhav, A.R.; Kim, H. Cobalt impregnated magnetite-multiwalled carbon nanotube nanocomposite as magnetically separable efficient catalyst for hydrogen generation by NaBH4 hydrolysis. J. Alloys Compd. 2017, 699, 1057–1067. [Google Scholar] [CrossRef]
- Du, X.; Yang, C.; Zeng, X.; Wu, T.; Zhou, Y.; Cai, P.; Cheng, G.; Luo, W. Amorphous NiP supported on rGO for superior hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2017, 42, 14181–14187. [Google Scholar] [CrossRef]
- Semiz, L. Development and Testing of Advanced Energy Materials for Use in Fuel Cell Systems and Flow Batteries. Ph.D. Thesis, TOBB University of Economics and Technology, Ankara, Turkey, June 2016. [Google Scholar]
- Serin, R.B. High Kinetic Hydrogen Generation from Chemical Hydrides. M.Sc. Thesis, TOBB University of Economics and Technology, Ankara, Turkey, December 2014. [Google Scholar]
- Tuan, N.T.; Park, J.; Lee, J.; Gwak, J.; Lee, D. Synthesis of nanoporous Cu films by dealloying of electrochemically deposited Cu–Zn alloy films. Corros. Sci. 2014, 80, 7–11. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, J.B.; Nguyen, Q.N.; Lee, J.M.; Park, S.; Chung, T.D.; Byun, J.Y. Nanoporous platinum thin films synthesized by electrochemical dealloying for nonenzymatic glucose detection. Phys. Chem. Chem. Phys. 2013, 15, 5782–5787. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Rizzi, P.; Battezzati, L. De-alloying kinetics of an Au-based amorphous alloys. J. Alloys Compd. 2012, 536S, S60–S64. [Google Scholar] [CrossRef]
- Alonso, J.A.; de Tendler, R.H. Mechanism of amorphisation in Cu–Ru, a binary alloy with a positive heat of mixing. Phys. Chem. Liq. 2008, 46, 669–675. [Google Scholar] [CrossRef]
- Lee, D.J.; Yim, S.S.; Kim, K.S.; Kim, S.H.; Kim, K.B. Formation of Ru Nanotubes by Atomic Layer Deposition onto an Anodized Aluminum Oxide Template. Electrochem. Solid-State Lett. 2008, 11, K61–K63. [Google Scholar] [CrossRef]

| Catalysts | Dealloying Time (min) | Ru (wt %) | Cu (wt %) | Ratio (Ru/Cu) |
|---|---|---|---|---|
| Bare Alloy | Without Dealloying | 7.12 | 92.88 | 0.08 |
| Dealloyed | 30 | 40.75 | 59.25 | 0.69 |
| 60 | 83.84 | 16.16 | 5.19 | |
| 75 | 92.90 | 7.10 | 13.08 | |
| 90 | 93.84 | 6.16 | 15.23 | |
| 105 | 94.14 | 5.86 | 16.06 | |
| 120 | 96.35 | 3.65 | 26.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serin, R.B.; Abdullayeva, N.; Sankir, M. Dealloyed Ruthenium Film Catalysts for Hydrogen Generation from Chemical Hydrides. Materials 2017, 10, 738. https://doi.org/10.3390/ma10070738
Serin RB, Abdullayeva N, Sankir M. Dealloyed Ruthenium Film Catalysts for Hydrogen Generation from Chemical Hydrides. Materials. 2017; 10(7):738. https://doi.org/10.3390/ma10070738
Chicago/Turabian StyleSerin, Ramis B., Nazrin Abdullayeva, and Mehmet Sankir. 2017. "Dealloyed Ruthenium Film Catalysts for Hydrogen Generation from Chemical Hydrides" Materials 10, no. 7: 738. https://doi.org/10.3390/ma10070738





