Piezoelectric Ceramics of the (1 − x)Bi0.50Na0.50TiO3–xBa0.90Ca0.10TiO3 Lead-Free Solid Solution: Chemical Shift of the Morphotropic Phase Boundary, a Case Study for x = 0.06
Abstract
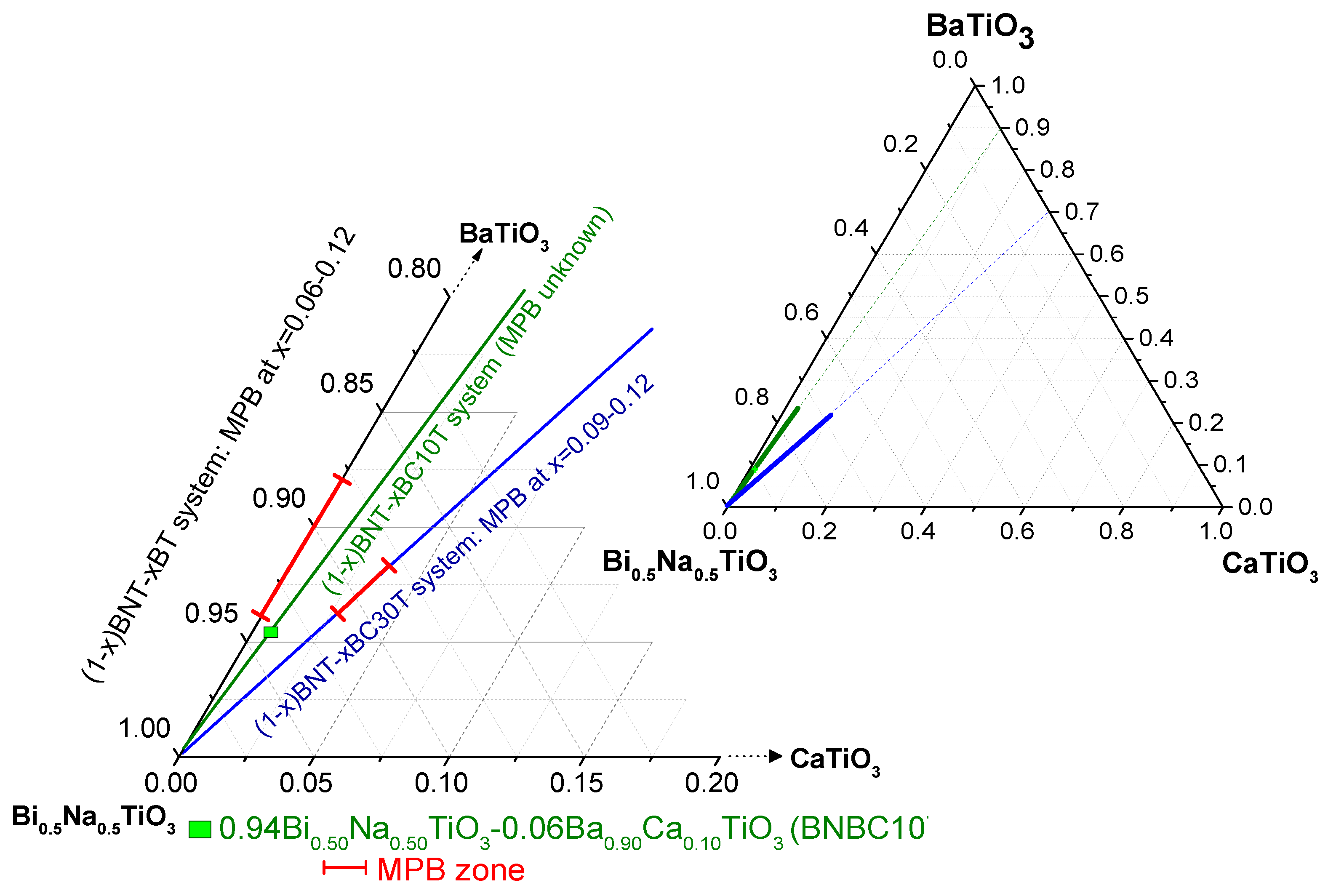
:1. Introduction
2. Results and Discussion
2.1. Structural and Electrical Characterization of Ceramics from the Solid State Route
2.2. Structural and Electrical Characterization of Ceramics from the Pechini Route
2.3. Comparative Structural Analysis of Ceramics from the Solid State and Pechini Routes: XRD
2.4. Comparative Structural Analysis of Ceramics from the Solid State and Pechini Routes: TEM
2.5. Comparative Dielectric Permittivity Curves of Ceramics from the Solid State and Pechini Routes
3. Experimental Method
3.1. Materials Preparation
3.2. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ringgarard, E.; Wurlitzer, T. Lead-free piezoceramics based on alkaliniobates. J. Eur. Ceram. Soc. 2005, 25, 2701–2706. [Google Scholar] [CrossRef]
- Priya, S.; Nahm, S. Lead-Free Piezoelectrics; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Rodel, J.; Kyle, G.; Dittmer, R.; Wook, J.; Kimura, M.; Damjanovic, D. Transferring lead-free piezoelectric ceramics into application. J. Eur. Ceram. Soc. 2015, 35, 1659–1681. [Google Scholar] [CrossRef]
- Villafuerte-Castrejon, M.E.; Morán, E.; Reyes-Montero, A.; Vivar-Ocampo, R.; Peña-Jiménez, J.A.; Rea-López, S.O.; Pardo, L. Towards lead-free piezoceramics: Facing a synthesis challenge. Materials 2016, 9, 21. [Google Scholar] [CrossRef]
- Jaffe, B.; Cook, W.R.; Haffe, H. Piezoelectric Ceramics; Academic Press: London, UK, 1971. [Google Scholar]
- Takenaka, T.; Nagata, H.; Hiruma, Y. Phase transition temperatures and piezoelectric properties of (Bi1/2Na1/2)TiO3 and (Bi1/2K1/2)TiO3 Based bismuth perovskite lead-free ferroelectric ceramics. Trans. UFFC 2009, 56, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Pardo, L.; García, A.; Brebol, K.; Mercadelli, E.; Galassi, C. Enhanced properties for ultrasonic transduction, phase transitions and thermal depoling in 0.96(Bi0.5Na0.5)TiO3–0.04BaTiO3 submicron structured ceramic. J. Phys. D 2011, 44, 335404. [Google Scholar] [CrossRef]
- Smolensky, G.A.; Isupov, V.A.; Agranovskaya, A.I.; Krainik, N.N. New Ferroelectrics of complex composition IV. Sov. Phys. Solid State 1961, 2, 2651–2654. [Google Scholar]
- Jones, G.O.; Thomas, P.A. Investigation of the structure and phase transitions in the novel A-site substituted disorted perovskite compound Na0.5Bi0.5TiO3. Acta Crystallogr. Sect. B 2002, 58, 168–178. [Google Scholar] [CrossRef]
- Jeong, I.K.; Sung, Y.S.; Song, T.K.; Kim, M.H.; Llobet, A. Structural evolution of bismuth sodium titanate induced by A-site non-stoichiometry: Neutron powder diffraction studies. J. Korean Phys. Soc. 2015, 67, 1583–1587. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Zhang, H.; Reaney, I.M.; Sinclair, D.C. Controlling mixed conductivity in Na1/2Bi1/2TiO3 using A-site non-stoichiometry and Nb-donor doping. J. Mater. Chem. C 2016, 4, 5779–5786. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, C.; Chen, C.; Luo, H.; Zhou, K.; Zhang, D. Morphology control and piezoelectric response of Na0.5Bi0.5TiO3 synthesized via hydrothermal method. CrysEngComm 2016, 18, 1302–1310. [Google Scholar] [CrossRef]
- Yamatoh, T.; Fujimori, H.; Arimura, M. Polymerizable complex synthesis of lead-free ferroelectrics Na0.5Bi0.5TiO3 suppressing evaporation of sodium and bismuth. J. Ceram. Soc. Jpn. 2015, 123, 978–982. [Google Scholar] [CrossRef]
- Takenaka, T.; Maruyama, K.; Sakata, K. (Bi1/2Na1/2)TiO3-BaTiO3 system for lead-free piezoelectric ceramics. Jpn. J. Appl. Phys. 1991, 30, 2236–2239. [Google Scholar] [CrossRef]
- Jo, W.; Dittmer, R.; Acosta, M.; Zang, J.; Groh, C.; Sapper, E.; Wang, K.; Rödel, J. Giant electric-field-induced strains in lead-free ceramics for actuator applications status and perspective. J. Electroceram. 2012, 29, 71–93. [Google Scholar] [CrossRef]
- Li, T.; Lou, X.; Ke, X.; Cheng, S.; Mi, S.; Wang, X.; Shi, J.; Liu, X.; Dong, G.; Fan, H.; et al. Giant strain with low hysteresis in A-site-deficient (Bi0.5Na0.5)TiO3-based lead-free piezoceramics. Acta Mater. 2017, 128, 337–344. [Google Scholar] [CrossRef]
- Pardo, L.; Mercadelli, E.; García, A.; Brebol, K.; Galassi, C. Field-induced phase transition and relaxor character in submicron structured lead-free (Bi0.5Na0.5)0.94Ba0.06TiO3 piezoceramic at the morphotropic phase boundary. IEEE Trans. UFFC 2011, 58, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Mercadelli, E.; Galassi, C.; Costa, A.L.; Albonetti, S.; Sanson, A. Sol-gel combustion synthesis of BNBT powders. J. Sol Gel Sci. Technol. 2008, 46, 39–45. [Google Scholar] [CrossRef]
- Mitsui, T.; Westphal, W.B. Dielectric and X-ray studies of CaxBa1−xTiO3 and CaxSr1−xTiO3. Phys. Rev. 1961, 124, 1354–1359. [Google Scholar] [CrossRef]
- Panigrahi, M.R.; Panigrahi, S. Diffuse phase transition and dielectric study in Ba0.95Ca0.05TiO3 ceramic. Phys. B 2010, 405, 2556–2559. [Google Scholar] [CrossRef]
- Varatharajan, R.; Samanta, S.B.; Jayavel, R.; Subramanian, C.; Narlikar, A.V.; Ramasamy, P. Ferroelectric characterization studies on barium calcium titanate single crystals. Mater. Charact. 2000, 45, 89–93. [Google Scholar] [CrossRef]
- Jayanthi, S.; Kutty, T.R.N. Extended phase homogeneity and electrical properties of barium calcium titanate prepared by the wet chemical methods. Mater. Sci. Eng. B 2004, 110, 202–212. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, X.H.; Zhao, C.J.; Li, B.; Zhang, S.R. High-temperature capacitor based on Ca-doped Bi0.5Na0.5TiO3 ceramics. J. Electron. Mater. 2010, 39, 2471–2475. [Google Scholar] [CrossRef]
- Yang, O.J.; Liu, P.; Bian, X.; Jing, H.; Wang, Y.; Zhang, Y.; Wu, Y.; Song, W. Dielectric, ferroelectric and piezoelectric properties of Bi0.5Na0.5–(Ba0.7Ca0.3)TiO3 ceramics at morphotropic phase boundary composition. Mater. Sci. Eng. 2011, 176, 260–265. [Google Scholar] [CrossRef]
- Xu, K.; Li, J.; Lv, X.; Wu, J.; Zhang, X.; Xiao, D.; Zhu, J. Superior Piezoelectric properties in potassium–sodium niobate lead-free ceramics. Adv. Mater. 2016, 28, 8519–8523. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ren, X. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef] [PubMed]
- Montero-Cabrera, M.E.; Pardo, L.; García, A.; Fuentes-Montero, M.E.; Ballinas-Casarrubias, M.L.; Fuentes-Cobas, L.E. The global and local symetries of nanostructured ferroelectric relaxor 0.94(Bi0.5Na0.5)TiO3–0.06BaTiO3. Ferroelectrics 2014, 469, 50–60. [Google Scholar] [CrossRef]
- Chu, B.J.; Chen, D.R.; Li, G.R.; Yin, Q.R. Electrical properties of (Na1/2Bi1/2)TiO3-BaTiO3 ceramics. J. Eur. Ceram. Soc. 2002, 22, 2115–2121. [Google Scholar] [CrossRef]
- Ma, C.; Guo, H.; Beckman, S.P.; Tan, X. Creation and destruction of morphotropic phase boundaries through electrical poling. A case study of lead-free (Bi1/2Na1/2)TiO3-BaTiO3 piezoelectrics. Phys. Rev. Lett. 2012, 109, 107602. [Google Scholar] [CrossRef] [PubMed]
- Simons, H.; Daniels, J.; Jo, W.; Dittmer, R.; Studer, A.; Avdeev, M.; Rödel, J.; Hoffman, M. Electric-field-induced strain mechanisms in lead-ree 94%Bi1/2Na1/2TiO3–6%BaTiO3. Appl. Phys. Lett. 2011, 98, 082901. [Google Scholar] [CrossRef]
- Fuentes-Cobas, L.E.; Montero-Cabrera, M.E.; Pardo, L.; Fuentes-Montero, L. Ferroelectrics under the synchrotron light: A review. Materials 2016, 9, 14. [Google Scholar] [CrossRef]
- Gonzalez, A.M.; Pardo, L.; Montero-Cabrera, M.E.; Fuentes-Cobas, L.E. Analysis of the rombohedral and tetragonal symmetries coexistence in lead-free 0.94(Bi0.5Na0.5)TiO3–0.06BaTiO3 ceramics from nanopowders. Adv. Appl. Ceram. Struct. Funct. Bioceram. 2016, 115, 96–105. [Google Scholar] [CrossRef]
- Anthoniappen, J.; Tu, C.S.; Chen, P.Y.; Chen, C.S.; Chiu, S.J.; Lee, H.Y.; Ting, Y.; Wang, S.F.; Chai, C.K. Structural phase stability and electric field induced relaxor-ferroelectric phase transition in (1 − x)(Bi0.5Na0.5)TiO3–xBaTiO3 ceramics. J. Alloys Compd. 2015, 618, 120–126. [Google Scholar] [CrossRef]
- Fuentes-Cobas, L.E.; Pardo, L.; Montero-Cabrera, M.E.; Plaisier, J.R.; Garcia, A.; Brebol, K.; Mercadelli, E.; Galassi, C. The 0.96(Bi0.5Na0.5)TiO3–0.04BaTiO3 crystal structure: A high-Q, high counting statistics synchrotron diffraction analysis. Cryst. Res. Technol. 2014, 49, 190–194. [Google Scholar] [CrossRef]
- Bruker, A.X.S. DIFFRACplus EVA Aplication, version 6.0.0.1; SOCABIM SAS: Karlsruhe, Germany, 2000. [Google Scholar]
- Ranjan, R.; Dviwedi, A. Structure and dielectric properties of (Na0.50Bi0.50)1 – xBaxTiO3. Sol. State Commun. 2005, 135, 394–399. [Google Scholar] [CrossRef]
- Jo, W.; Daniels, J.E.; Jones, J.L.; Tan, X.; Thomas, P.; Damjanovic, D.; Rödel, J. Evolving morphotropic phase boundary in lead-free (Bi1/2Na1/2)TiO3-BaTiO3 piezoceramics. Appl. Phys. 2011, 109, 014110. [Google Scholar] [CrossRef]
- Tellier, J.; Malic, B.; Dkhil, B.; Jenko, D.; Cilensek, J.; Kosec, M. Crystal structure and phase transitions of sodium potassium niobate perovskites. Solid State Sci. 2009, 11, 320–324. [Google Scholar] [CrossRef]
- Moure, A.; Hungria, T.; Castro, A.; Pardo, L. Microstructural effects on the phase transitions and the thermal evolution of elastic and piezoelectric properties in highly dense, submicron structured NaNbO3 ceramics. J. Mater. Sci. 2010, 45, 1211–1219. [Google Scholar] [CrossRef]
- Koruza, J.; Groszewicz, P.; Breitzke, H.; Buntkowsky, G.; Rojac, T.; Malic, B. Grain-size-induced ferroelectricity in NaNbO3. Acta Mater. 2017, 126, 77–85. [Google Scholar] [CrossRef]
- Craciun, F.; Galassi, C.; Birjega, R. Electric-field-induced and spontaneous relaxor-ferroelectric phase transitions in (Na1/2Bi1/2)1 − xBaxTiO3. J. Appl. Phys. 2012, 112, 124106. [Google Scholar] [CrossRef]
- Vögler, M.; Novak, N.; Shader, F.H.; Rödel, J. Temperature-dependent volume fraction ofpolar nanoregions in lead-free (1 − x)(Bi0.5Na0.5)TiO3–xBaTiO3 ceramics. Phys. Rev. B 2017, 95, 024104. [Google Scholar] [CrossRef]
- Cohelo, A. TOPAS-Academic, version 4.1; Cohelo Software: Brisbane, Australia, 2007. [Google Scholar]
- Digital Micrograph. 1.71.38; Gatan Inc.: Pleasanton, CA, USA, 2010.
- Alemany, C.; Gonzalez, A.M.; Pardo, L.; Jimenez, B.; Carmona, F.; Mendiola, J. Automatic determination of complex constants piezoelectric lossy materials in the radial model. Physica D 1995, 28, 945–956. [Google Scholar] [CrossRef]

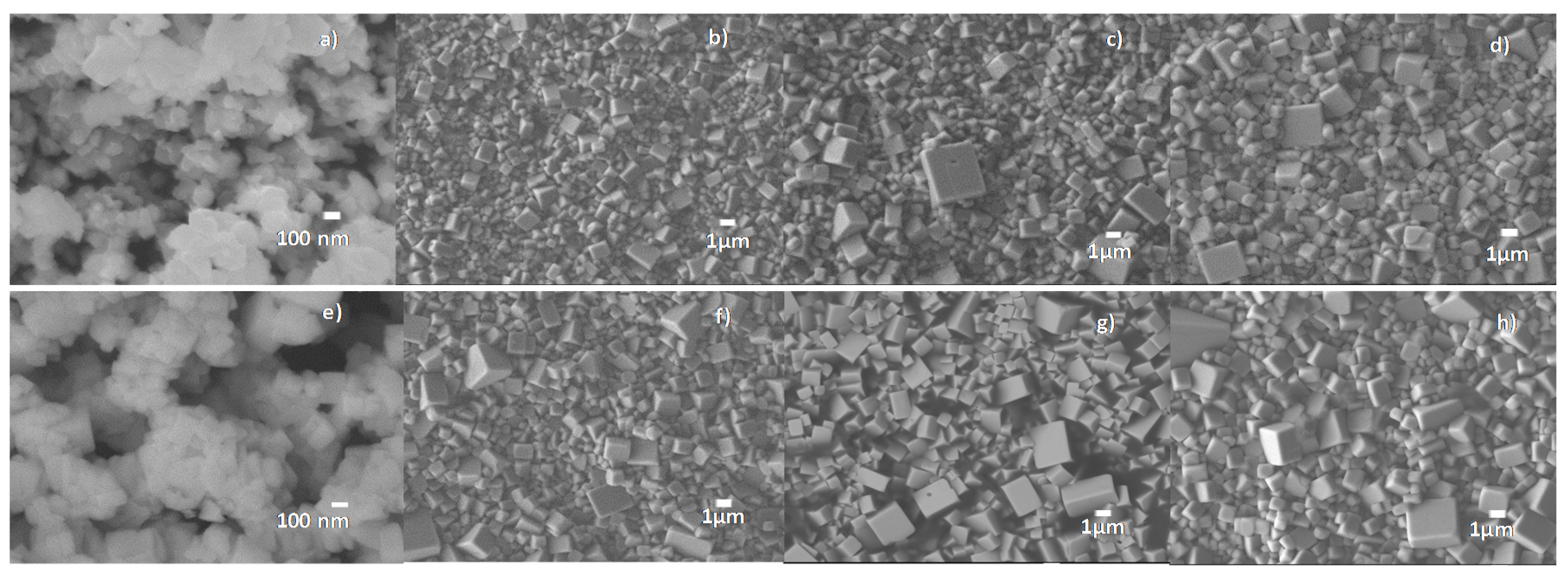

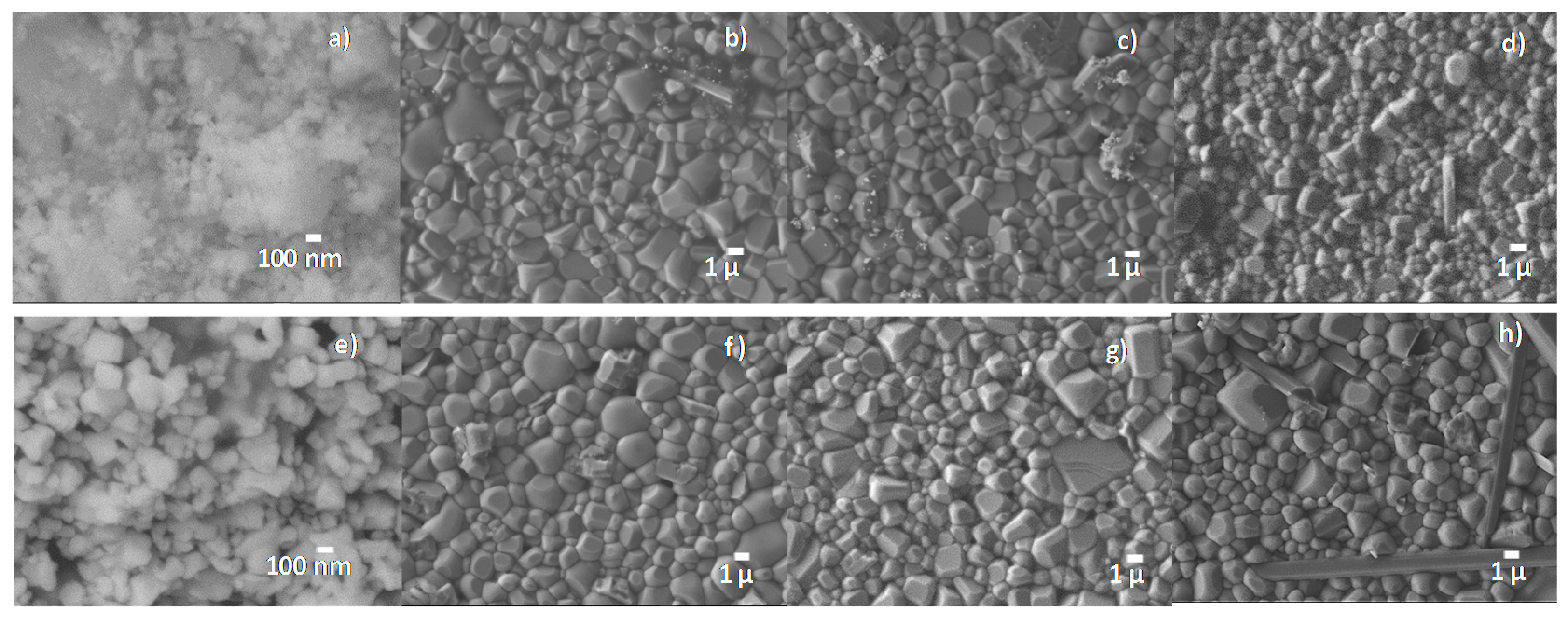
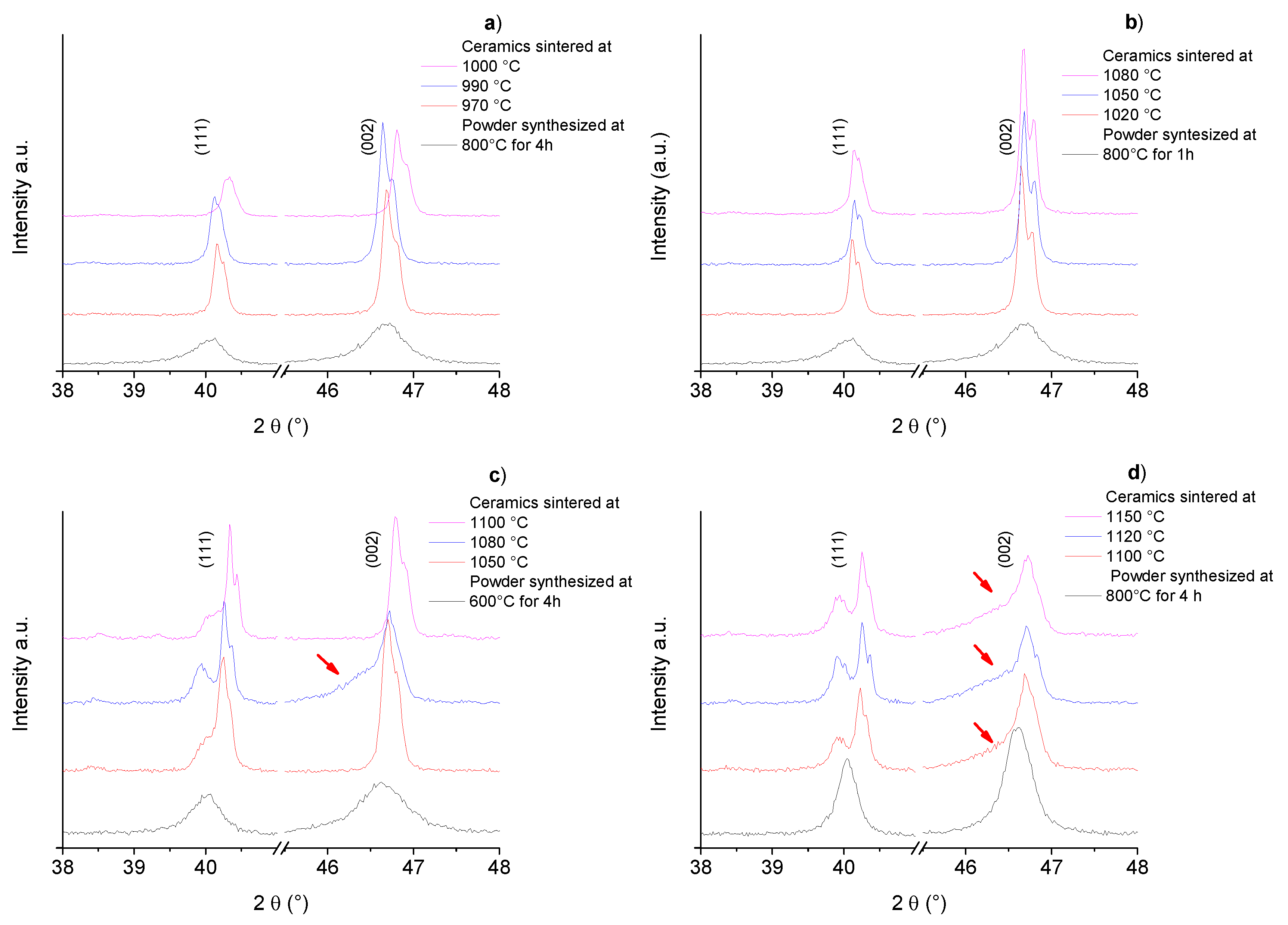
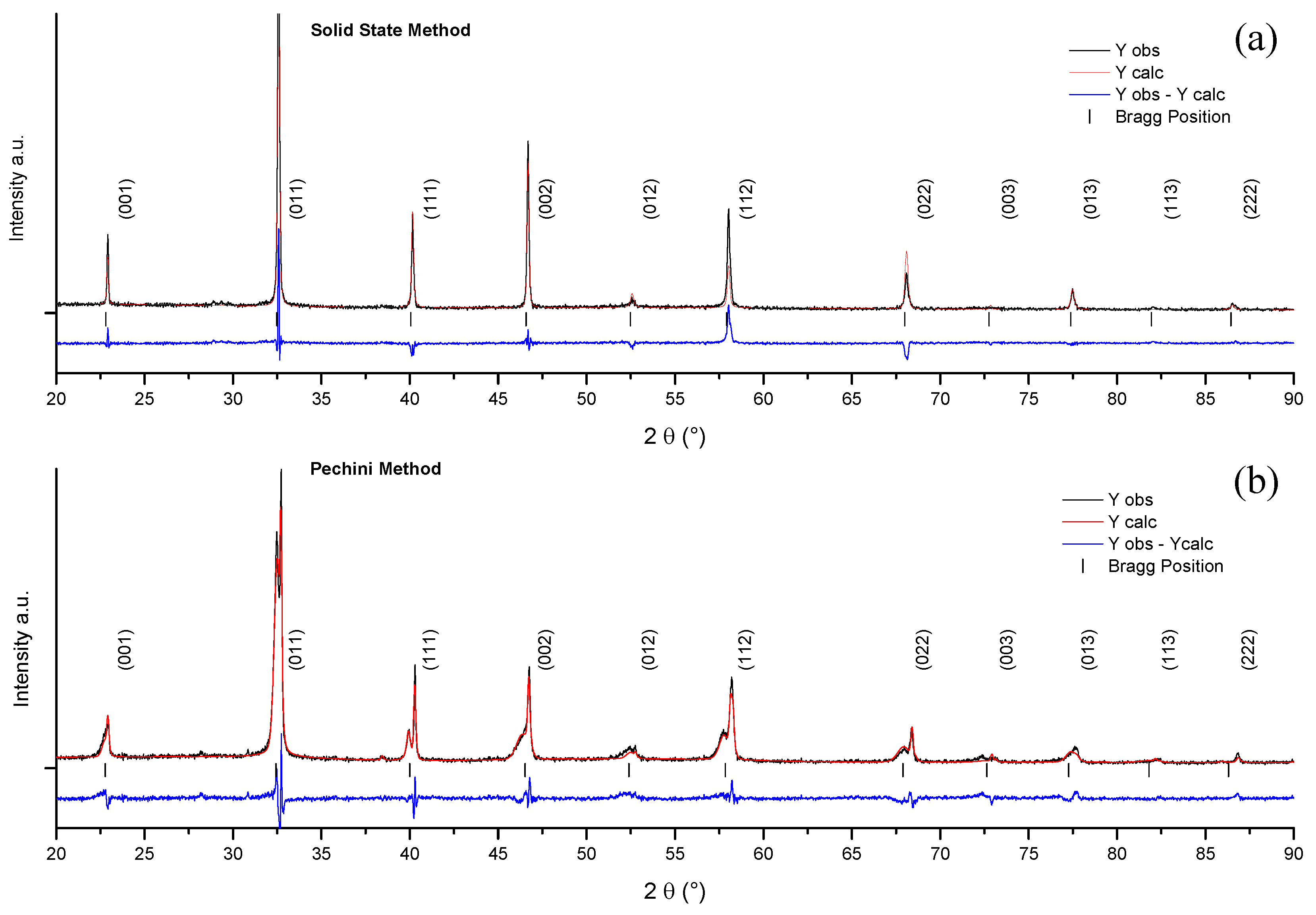
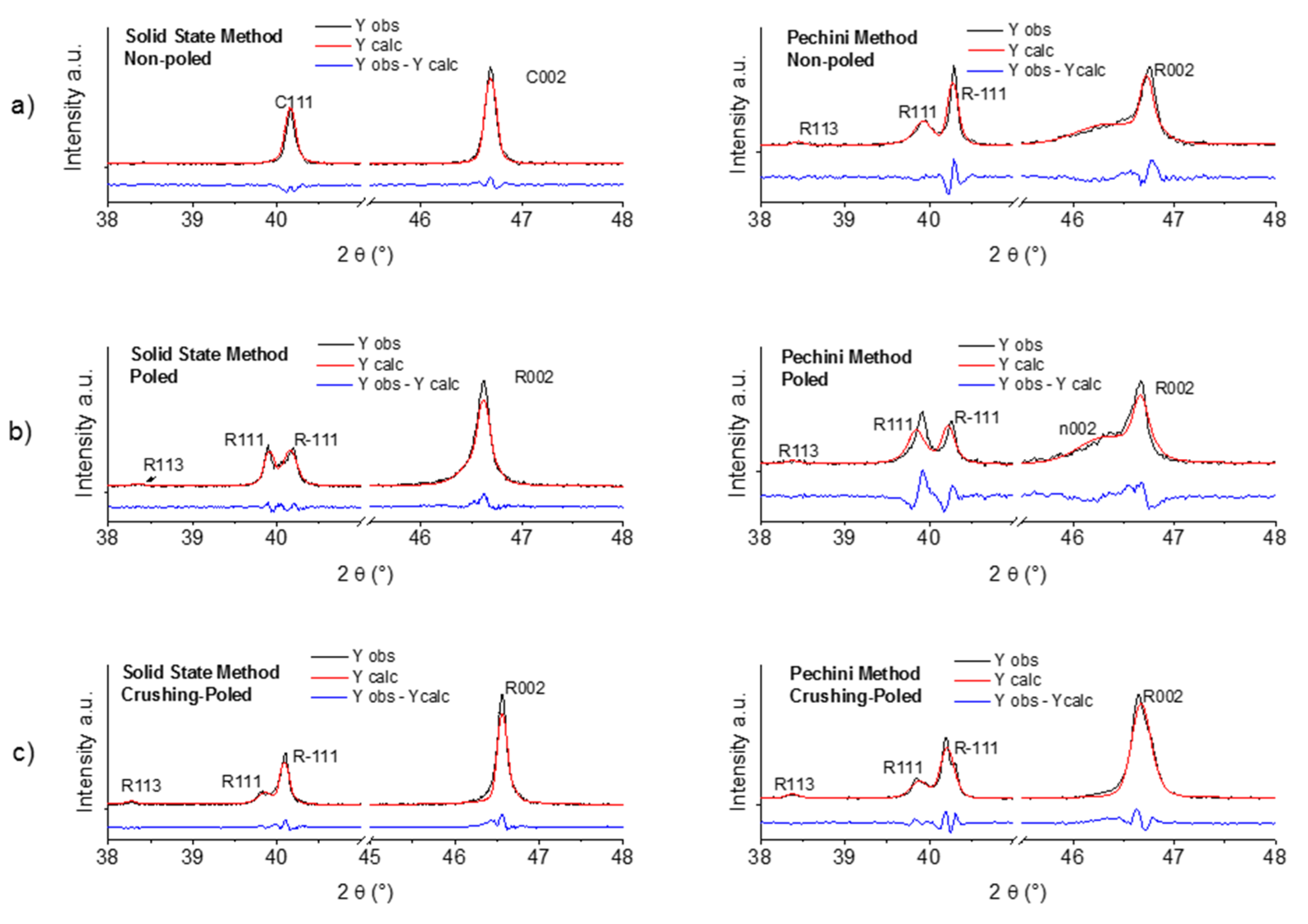

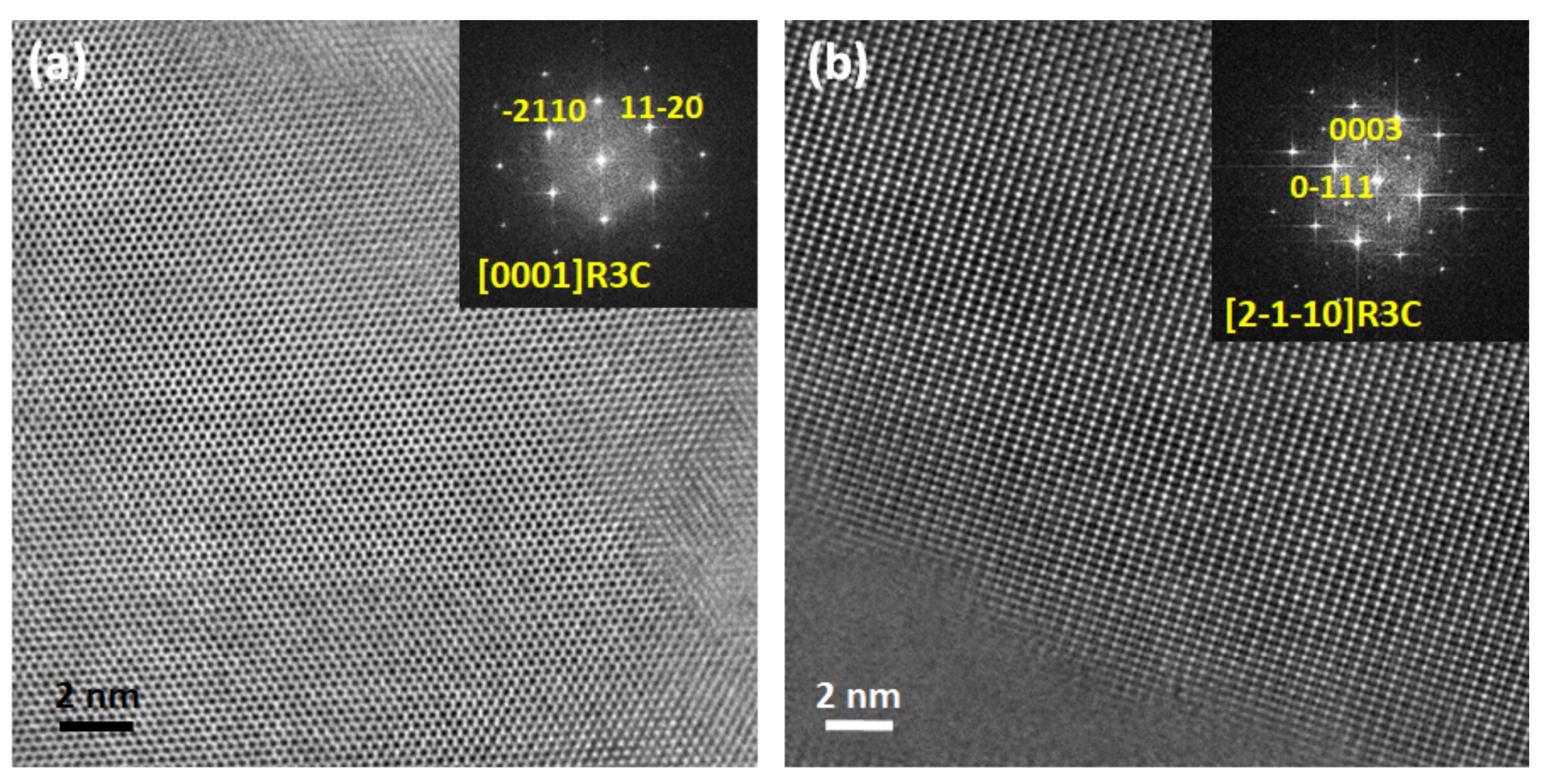
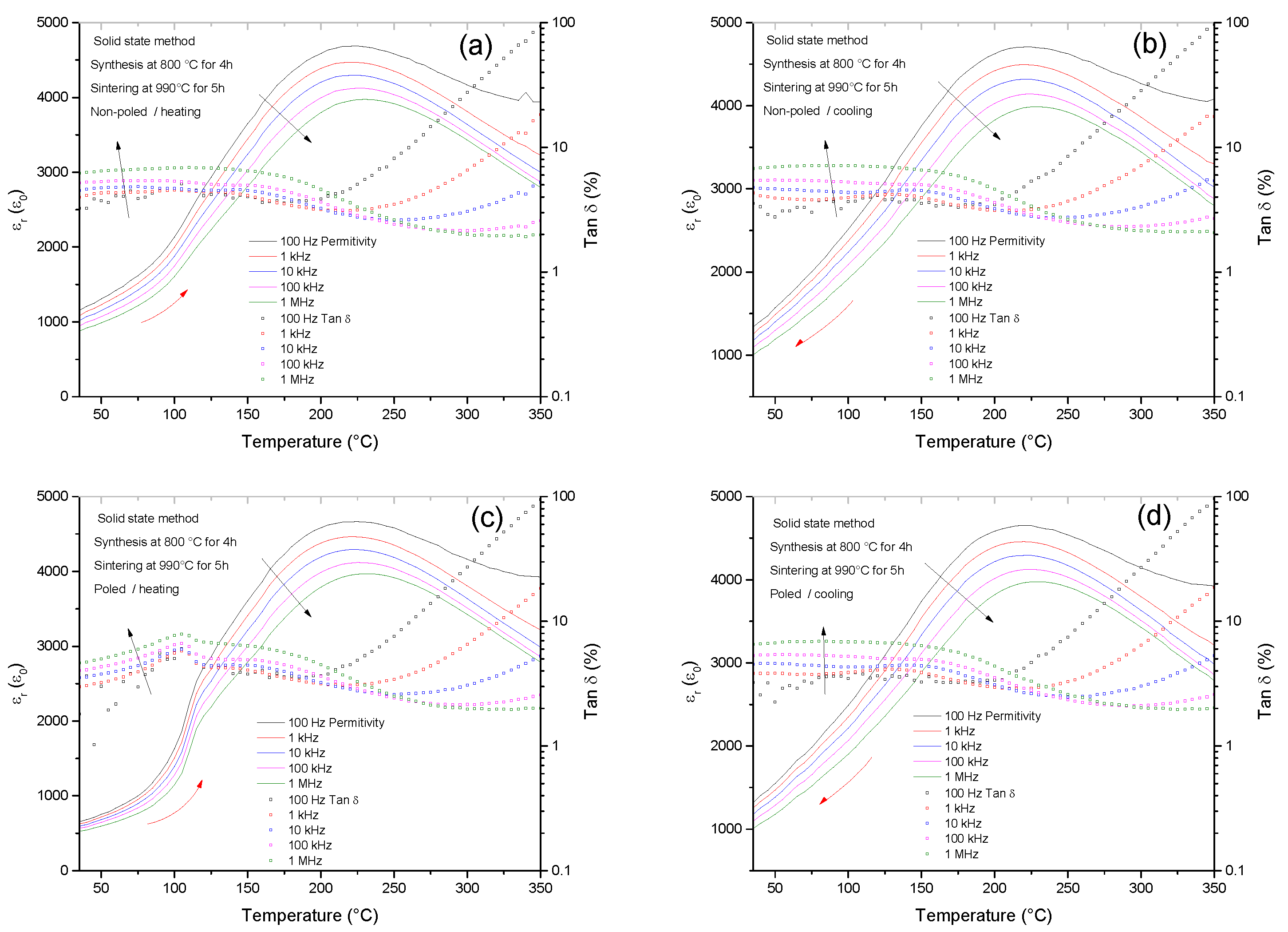

| Temp/Time | Temp/Time | Density | d33 | d31 | Kp | R.T. | R.T. |
|---|---|---|---|---|---|---|---|
| Synthesis (°C/h) | Sintering (°C/h) | (g/cm3) | (pC/N) | (pC/N) | (%) | εT33 | tan δ (%) |
| 800/4 | 970/5 | 5.66 | 98 | 21 | 13 | 597 | 6 |
| 800/4 | 990/5 | 5.67 | 95 | 21 | 16 | 595 | 4 |
| 800/4 | 1000/5 | 5.79 | 20 | 5 | 4 | 591 | 40 |
| 800/1 | 1020/1 | 5.72 | 52 | 8 | 5 | 706 | 42 |
| 800/1 | 1050/1 | 5.65 | 68 | 18 | 13 | 642 | 5 |
| 800/1 | 1080/1 | 5.68 | 82 | 20 | 16 | 689 | 14 |
| Temp/Time | Temp/Time | Density | d33 | d31 | Kp | R.T. | R.T. |
|---|---|---|---|---|---|---|---|
| Synthesis (°C/h) | Sintering(°C/h) | (g/cm3) | (pC/N) | (pC/N) | (%) | εT33 | tan δ (%) |
| 600/4 | 1050/2.5 | 5.64 | 81 | 19 | 20 | 319 | 2 |
| 600/4 | 1080/2.5 | 5.67 | 83 | 19 | 20 | 357 | 2 |
| 600/4 | 1100/2.5 | 5.75 | 74 | 19 | 20 | 359 | 2 |
| 800/4 | 1100/1 | 5.44 | 59 | 14 | 14 | 379 | 5 |
| 800/4 | 1120/1 | 5.64 | 77 | 15 | 12 | 538 | 2 |
| 800/4 | 1150/1 | 5.64 | 61 | 15 | 15 | 330 | 5 |
| Sample | Solid State | Pechini | ||
|---|---|---|---|---|
| Phase Symmetry | R3c | Pm-3m | R3c | Pm-3m |
| As-sintered | ||||
| a (Å) | - | 3.8964 | 3.8928 | 3.9229 |
| α (°) | - | - | 89.63 | - |
| Crystal size (nm) | - | 128 | 158 | 7 |
| Content (%) | - | 100 | 48.9 | 51.1 |
| rwp | - | 21.8 | 16.0 | |
| Poled | ||||
| a (Å) | 3.9006 | - | 3.8930 | 3.9221 |
| α (°) | 89.76 | - | 89.61 | - |
| Crystal size (nm) | 169 | - | 185 | 6 |
| Content (%) | 100 | - | 49.6 | 50.4 |
| rwp | 16.0 | - | 17.3 | |
| Poled and powdered | ||||
| a (Å) | 3.8962 | - | 3.8940 | 4.07 |
| α (°) | 89.76 | - | 89.62 | |
| Crystal size (nm) | 382 | - | 190 | 6 |
| Content (%) | 100 | - | 85.5 | 14.5 |
| rwp | 15.2 | - | 11.3 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vivar-Ocampo, R.; Pardo, L.; Ávila, D.; Morán, E.; González, A.M.; Bucio, L.; Villafuerte-Castrejón, M.-E. Piezoelectric Ceramics of the (1 − x)Bi0.50Na0.50TiO3–xBa0.90Ca0.10TiO3 Lead-Free Solid Solution: Chemical Shift of the Morphotropic Phase Boundary, a Case Study for x = 0.06. Materials 2017, 10, 736. https://doi.org/10.3390/ma10070736
Vivar-Ocampo R, Pardo L, Ávila D, Morán E, González AM, Bucio L, Villafuerte-Castrejón M-E. Piezoelectric Ceramics of the (1 − x)Bi0.50Na0.50TiO3–xBa0.90Ca0.10TiO3 Lead-Free Solid Solution: Chemical Shift of the Morphotropic Phase Boundary, a Case Study for x = 0.06. Materials. 2017; 10(7):736. https://doi.org/10.3390/ma10070736
Chicago/Turabian StyleVivar-Ocampo, Rodrigo, Lorena Pardo, David Ávila, Emilio Morán, Amador M. González, Lauro Bucio, and María-Elena Villafuerte-Castrejón. 2017. "Piezoelectric Ceramics of the (1 − x)Bi0.50Na0.50TiO3–xBa0.90Ca0.10TiO3 Lead-Free Solid Solution: Chemical Shift of the Morphotropic Phase Boundary, a Case Study for x = 0.06" Materials 10, no. 7: 736. https://doi.org/10.3390/ma10070736




