Scaling-Up Techniques for the Nanofabrication of Cell Culture Substrates via Two-Photon Polymerization for Industrial-Scale Expansion of Stem Cells
Abstract
:1. Introduction
1.1. Rationale Underlying Industrial-Scale Expansion of Stem Cells and Limitation of Feeder-Cell Layers and Exogenous Conditioning
1.2. State of the Art in the Use of 3-D Substrates for the Expansion of Stem Cells
1.3. State of the Art in the Use of 2PP for the Nanofabrication of Substrates for Cell Culture
2. Materials and Methods
2.1. Description of Photosensitive Material and Photo-Initiator
2.2. The Laser Fabrication Set-Up
2.3. Description of the Up-Scaling Techniques
3. Results and Discussion
3.1. Parameter Optimization of 2PP-Engineered Elementary Nichoids
3.2. Increasing the Percentage of Glass Surface Covered by the Nichoids
3.3. Comparison with Results from the Literature
3.4. Future Prospectives
3.4.1. Further Reduction in the Machining Time
3.4.2. Decreasing Cell Adhesion in the Nichoid Surroundings
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Herberts, C.A.; Kwa, M.S.G.; Hermsen, H.P.H. Risk factors in the development of stem cell therapy. J. Transl. Med. 2011, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Ross, A.M.; Lahann, J.; Krebsbach, P.H. The evolution of human pluripotent stem cell culture: From feeder cells to synthetic coatings. Stem Cells 2013, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Miki, T.; Yasuda, S.Y.; Kahn, M. Wnt/β-catenin signaling in embryonic stem cell self-renewal and somatic cell reprogramming. Stem Cell Rev. Rep. 2011, 7, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, B. Reprogramming barriers and enhancers: Strategies to enhance the efficiency and kinetics of induced pluripotency. Cell Regen. 2015, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Homma, Y.; Lachaniette, C.H.F.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int. Orthop. 2013, 37, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Valamehr, B.; Abujarour, R.; Robinson, M.; Le, T.; Robbins, D.; Shoemaker, D.; Flynn, P. A novel platform to enable the high-throughput derivation and characterization of feeder-free human iPSCs. Sci. Rep. 2012, 2, 213. [Google Scholar] [CrossRef] [PubMed]
- Valamehr, B.; Robinson, M.; Abujarour, R.; Rezner, B.; Vranceanu, F.; Le, T.; Medcalf, A.; Lee, T.T.; Fitch, M.; Robbins, D.; et al. Platform for Induction and Maintenance of Transgene-free hiPSCs Resembling Ground State Pluripotent Stem Cells. Stem Cell Rep. 2014, 2, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Tamm, C.; Galito, S.P.; Annere, C.A. Comparative Study of Protocols for Mouse Embryonic Stem Cell Culturing. PLoS ONE 2013, 8, e81156. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Gaskell, T.; Moens, N.; Culley, O.J.; Hansen, D.; Gervasio, M.K.R.; Yeap, Y.J.; Danovi, D. Human pluripotent stem cells on artificial microenvironments: A high content perspective. Front. Pharmacol. 2014, 5, 150. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Lu, Y.; Li, J.; Liu, Y.; Liu, J.; Wang, W. Leukemia inhibitory factor promotes tumor growth and metastasis in human osteosarcoma via activating STAT3. APMIS 2015, 123, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Albrengues, J.; Bourget, I.; Pons, C.; Butet, V.; Hofman, P.; Tartare-Deckert, S.; Feral, C.C.; Meneguzzi, G.; Gaggioli, C. LIF Mediates Proinvasive Activation of Stromal Fibroblasts in Cancer. Cell Rep. 2014, 7, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Joddar, B.; Ito, Y. Artificial niche substrates for embryonic and induced pluripotent stem cell cultures. J. Biotechnol. 2013, 168, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Futaki, S.; Suemori, H.; Taniguchi, Y.; Yamada, M.; Kawasaki, M.; Hayashi, M.; Kumagai, H.; Nakatsuji, N.; Sekiguchi, K.; et al. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodin, S.; Domogatskaya, A.; Ström, S.; Hansson, E.M.; Chien, K.R.; Inzunza, J.; Hovatta, O.; Tryggvason, K. Long-term self-renewal of human pluripotent stem cells on human recombinant laminin-511. Nat. Biotechnol. 2010, 28, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Hongisto, H.; Vuoristo, S.; Mikhailova, A.; Suuronen, R.; Virtanen, I.; Otonkoski, T.; Skottman, H. Laminin-511 expression is associated with the functionality of feeder cells in human embryonic stem cell culture. Stem Cell Res. 2012, 8, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Liu, S.; Li, X.; Krawetz, R.; Rancourt, D.E. Extracellular matrix isolated from foreskin fibroblasts supports long-term xeno-free human embryonic stem cell culture. Stem Cells Dev. 2010, 19, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qiu, Y.; Zeng, X.; Ding, Y.; Zeng, J.; Lu, K.; Li, D. Effect of a feeder layer composed of mouse embryonic and human foreskin fibroblasts on the proliferation of human embryonic stem cells. Exp. Ther. Med. 2016, 11, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Totonchi, M.; Taei, A.; Seifinejad, A.; Tabebordbar, M.; Rassouli, H.; Farrokhi, A.; Gourabi, H.; Aghdami, N.; Hosseini-Salekdeh, G.; Baharvand, H. Feeder- and serum-free establishment and expansion of human induced pluripotent stem cells. Int. J. Dev. Biol. 2010, 54, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Pakzad, M.; Ashtiani, M.K.; Gargari, S.L.M.; Baharvand, H. Development of a simple, repeatable, and cost-effective extracellular matrix for long-term xeno-free and feeder-free self-renewal of human pluripotent stem cells. Histochem. Cell Biol. 2013, 140, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.; Wang, Y. Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Yang, K.; Kim, M.-J.; Jang, J.; Lee, M.; Kim, D.-W.; Lee, H.; Cho, S.-W. Bio-inspired oligovitronectin-grafted surface for enhanced self-renewal and long-term maintenance of human pluripotent stem cells under feeder-free conditions. Biomaterials 2015, 50, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Raimondi, M.T.; Pietrabissa, R. Controlling Self-Renewal and Differentiation of Stem Cells via Mechanical Cues. J. Biomed. Biotechnol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kress, S.; Neumann, A.; Weyand, B.; Kasper, C. Stem cell differentiation depending on different surfaces. Adv. Biochem. Eng. Biotechnol. 2012, 126, 263–283. [Google Scholar] [CrossRef] [PubMed]
- Peerani, R.; Zandstra, P.W. Enabling stem cell therapies through synthetic stem cell–niche engineering. J. Clin. Investig. 2010, 120, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Liu, F.; Lan, S.; Li, P.; Wang, L.; Kou, J.; Qi, X.; Fan, R.; Hao, D.; Wu, C.; et al. Large-scale expansion of Wharton’s jelly-derived mesenchymal stem cells on gelatin microbeads, with retention of self-renewal and multipotency characteristics and the capacity for enhancing skin wound healing. Stem Cell Res. Ther. 2015, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Leisten, I.; Kramann, R.; Ferreira, M.S.V.; Bovi, M.; Neuss, S.; Ziegler, P.; Wagner, W.; Knüchel, R.; Schneider, R.K. 3D co-culture of hematopoietic stem and progenitor cells and mesenchymal stem cells in collagen scaffolds as a model of the hematopoietic niche. Biomaterials 2012, 33, 1736–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rowe, R.G.; Hiraoka, N.; George, J.P.; Wirtz, D.; Mosher, D.F.; Virtanen, I.; Chernousov, M.A.; Weiss, S.J. Fibronectin fibrillogenesis regulates three-dimensional neovessel formation. Genes Dev. 2008, 22, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Dong, Y.; Wang, W. Encapsulation and 3D culture of human adipose-derived stem cells in an in-situ crosslinked hybrid hydrogel composed of PEG-based hyperbranched copolymer and hyaluronic acid. Stem Cell Res. Ther. 2013, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Alamein, M.A.; Stephens, S.; Liu, Q.; Skabo, S.; Warnke, P.H. Mass Production of Nanofibrous Extracellular Matrix with Controlled 3D Morphology for Large-Scale Soft Tissue Regeneration. Tissue Eng. Part C Methods 2013, 19, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Alamein, M.A.; Wolvetang, E.J.; Ovchinnikov, D.A.; Stephens, S.; Sanders, K.; Warnke, P.H. Polymeric nanofibrous substrates stimulate pluripotent stem cells to form three-dimensional multilayered patty-like spheroids in feeder-free culture and maintain their pluripotency. J. Tissue Eng. Regen. Med. 2015, 9, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.B.S.; Burdick, J.A. Influence of 3D Hyaluronic Acid Microenvironments on Mesenchymal Stem Cell Chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.J.; Miller, L.M.; Blaschke, A.J.; Doss, E.L.; Bonham, A.J.; Hikita, S.T.; Johnson, L.V.; Clegg, D.O. Roles of integrins in human induced pluripotent stem cell growth on Matrigel and vitronectin. Stem Cells Dev. 2010, 19, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Schaffer, D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc. Natl. Acad. Sci. USA 2013, 110, 5039–5048. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Jeong, D.; Xiao, J.; Schaffer, D.V. Developing defined and scalable 3d culture systems for culturing human pluripotent stem cells at high densities. Cell Mol. Bioeng. 2014, 7, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Liao, Y.-W.; Liud, D.-M.; Lin, H.-L.; Chen, S.-J.; Chen, H.-L.; Peng, C.-H.; Lian, C.-M.; Mou, C.-Y.; Chiou, S.-H. Corneal repair by human corneal keratocyte-reprogrammed iPSCs and amphiphatic carboxymethyl-hexanoyl chitosan hydrogel. Biomaterials 2012, 33, 8003–8016. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, O.A.M.; Darwish, S.M.H. Review of Rapid Prototyping Techniques for Tissue Engineering Scaffolds Fabrication. In Characterization and Development of Biosystems and Biomaterials; Springer: Berlin/Heidelberg, Germany, 2013; pp. 33–54. [Google Scholar] [CrossRef]
- Maruo, S.; Fourkas, J.T. Recent progress in multiphoton microfabrication. Laser Photon. Rev. 2008, 2, 100–111. [Google Scholar] [CrossRef]
- Hofmeister, L.H.; Costa, L.; Balikov, D.A.; Crowder, S.W.; Terekhov, A.; Sung, H.J.; Hofmeister, W.H. Patterned polymer matrix promotes stemness and cell-cell interaction of adult stem cells. J. Biol. Eng. 2015, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Kawata, S.; Sun, H.-B.; Tanaka, T.; Takada, K. Finer features for functional microdevices. Nature 2001, 412, 697–698. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.J.; Doraiswamy, A.; Chrisey, D.B.; Chichkov, B.N. Medical prototyping using two photon polymerization. Materialstoday 2010, 13, 42–48. [Google Scholar] [CrossRef]
- Malinauskas, M.; Danilevičius, P.; Baltriukienė, D.; Rutkauskas, M.; Žukauskas, A.; Kairytė, Ž.; Bičkauskaitė, G.; Purlys, V.; Paipulas, D.; Bukelskienė, V.; et al. 3D artificial polymeric scaffolds for stem cell growth fabricated by femtosecond laser. Lith. J. Phys. 2010, 50, 75–82. [Google Scholar] [CrossRef]
- Danilevičius, P.; Rekštytė, S.; Balčiūnas, E.; Kraniauskas, A.; Širmenis, R.; Baltriukienė, D.; Bukelskienė, V.; Gadonas, R.; Sirvydis, V.; Piskarskas, A.; et al. Laser 3D micro/nanofabrication of polymers for tissue engineering applications. Optlastec 2013, 45, 518–524. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Malinauskas, M.; Schlie, S.; Chichkov, B.; Gittard, S.; Narayan, R.; Löbler, M.; Sternberg, K.; Schmitz, K.P.; Haverich, A. Three-dimensional laser micro- and nano-structuring of acrylated poly(ethylene glycol) materials and evaluation of their cytoxicity for tissue engineering applications. Acta Biomater. 2011, 7, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Melissinaki, V.; Gill, A.A.; Ortega, I.; Vamvakaki, M.; Ranella, A.; Haycock, J.W.; Fotakis, C.; Farsari, M.; Claeyssens, F. Direct laser writing of 3D scaffolds for neural tissue engineering applications. Biofabrication 2011, 3, 045005. [Google Scholar] [CrossRef] [PubMed]
- Felfel, R.M.; Poocza, L.; Gimeno-Fabra, M.; Milde, T.; Hildebrand, G.; Ahmed, I.; Scotchford, C.; Sottile, V.; Grant, D.M.; Liefeith, K. In vitro degradation and mechanical properties of PLA-PCL copolymer unit cell scaffolds generated by two-photon polymerization. Biomed. Mater. 2016, 11, 015011. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Eaton, S.M.; Laganà, M.; Aprile, V.; Nava, M.M.; Cerullo, G.; Osellame, R. Three-dimensional structural niches engineered via two-photon laser polymerization promote stem cell homing. Acta Biomater. 2013, 9, 4579–4584. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Nava, M.M.; Eaton, S.M.; Bernasconi, A.; Vishnubhatla, K.C.; Cerullo, G.; Osellame, R. Optimization of Femtosecond Laser Polymerized Structural Niches to Control Mesenchymal Stromal Cell Fate in Culture. Micromachines 2014, 5, 341–358. [Google Scholar] [CrossRef]
- Nava, M.M.; Raimondi, M.T.; Credi, C.; de Marco, C.; Turri, S.; Cerullo, G.; Osellame, R. Interactions between structural and chemical biomimetism in synthetic stem cell niches. Biomed. Mater. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Di Maggio, N.; Zandrini, T.; Cerullo, G.; Osellame, R.; Martin, I.; Raimondi, M.T. Synthetic niche substrates engineered via two-photon laser polymerization for the expansion of human mesenchymal stromal cells. J. Tissue Eng. Regen. Med. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovsianikov, A.; Mironov, V.; Stampf, J.; Liska, R. Engineering 3D cell-culture matrices: Multiphoton processing technologies for biological and tissue engineering applications. Expert Rev. Med. Devices 2012, 9, 613–633. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-S.; Yang, D.-Y.; Park, S.H.; Kim, R.H. Recent developments in the use of two-photon polymerization in precise 2D and 3D microfabrications. Polym. Adv. Technol. 2006, 17, 72–82. [Google Scholar] [CrossRef]
- Sun, H.-B.; Takada, K.; Kim, M.-S.; Lee, K.-S.; Kawata, S. Scaling laws of voxels in two-photon photopolymerization nanofabrication. Appl. Phys. Lett. 2003, 83, 1104. [Google Scholar] [CrossRef]
- Nava, M.M.; Piuma, A.; Figliuzzi, M.; Cattaneo, I.; Bonandrini, B.; Zandrini, T.; Cerullo, G.; Osellame, R.; Remuzzi, A.; Raimondi, M.T. Two-photon polymerized “nichoid” substrates maintain function of pluripotent stem cells when expanded under feeder-free conditions. Stem Cell Res. Ther. 2016, 7, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uppal, N. A Mathematical Model Development and Sensitivity Analysis of Two Photon Polymerization for 3d Micro/Nano Fabrication. Ph.D. Thesis, University of Texas, Arlington, TX, USA, August 2008. [Google Scholar]
- LaFratta, C.N.; Li, L.; Fourkas, J.T. Soft-lithographic replication of 3D microstructures with closed loops. Proc. Natl. Acad. Sci. USA 2006, 103, 8589–8594. [Google Scholar] [CrossRef] [PubMed]
- Formanek, F.; Takeyasu, N.; Tanaka, T.; Chiyoda, K.; Ishikawa, A.; Kawata, S. Three-dimensional fabrication of metallic nanostructures over large areas by two-photon polymerization. Opt. Express 2006, 14, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; El-Tamer, A.; Hinze, U.; Li, J.; Hu, Y.; Huang, W.; Chu, J.; Chichkov, B. Parallel direct laser writing of micro-optical and photonic structures using spatial light modulator. Opt. Lasers Eng. 2015, 7, 26–32. [Google Scholar] [CrossRef]
- Li, Z.; Pucher, N.; Cicha, K.; Torgersen, J.; Ligon, S.C.; Ajami, A.; Husinsky, W.; Rosspeintner, A.; Vauthey, E.; Naumov, S.; et al. A Straightforward Synthesis and Structure—Activity Relationship of Highly Efficient Initiators for Two-Photon Polymerization. Macromolecules 2013, 46, 352–361. [Google Scholar] [CrossRef]
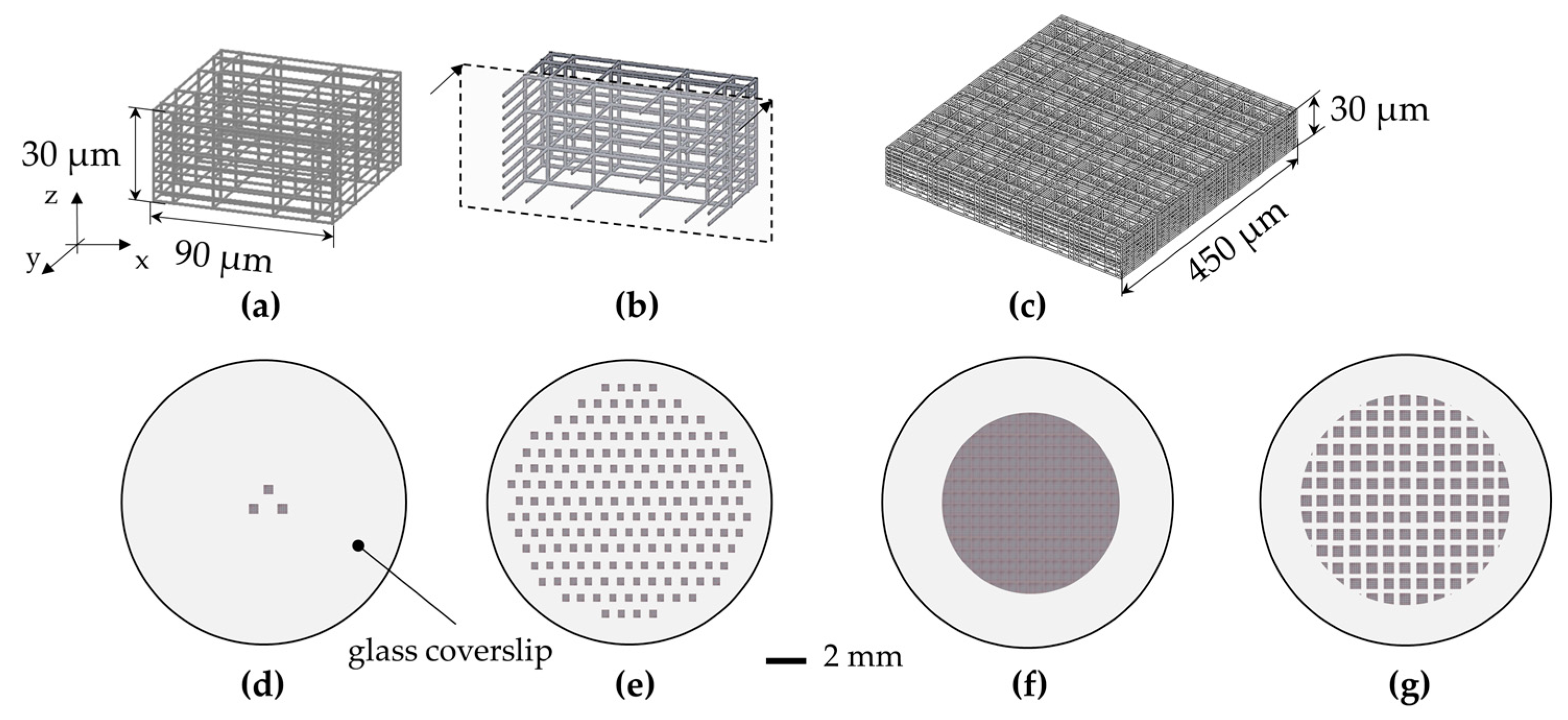
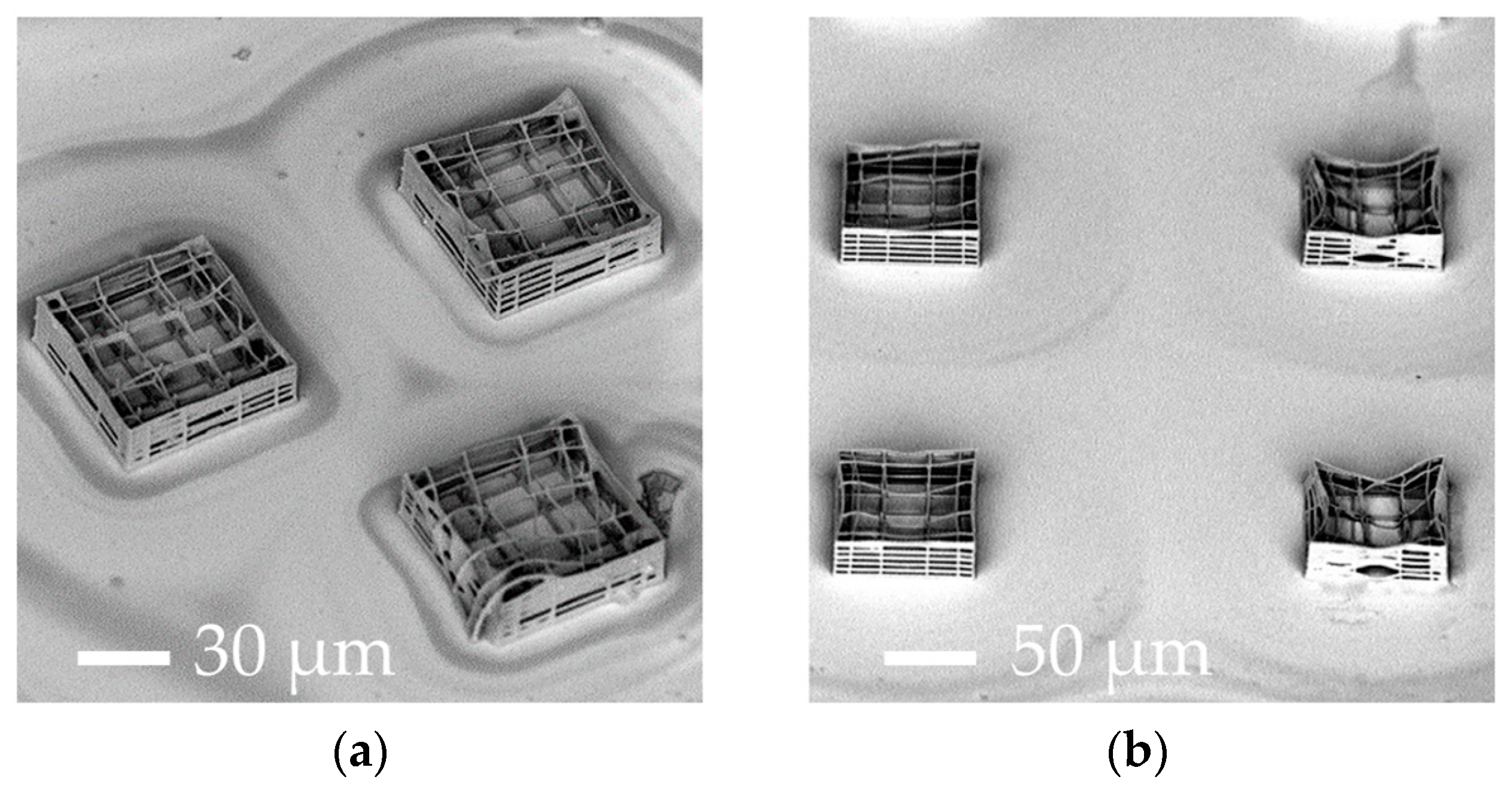
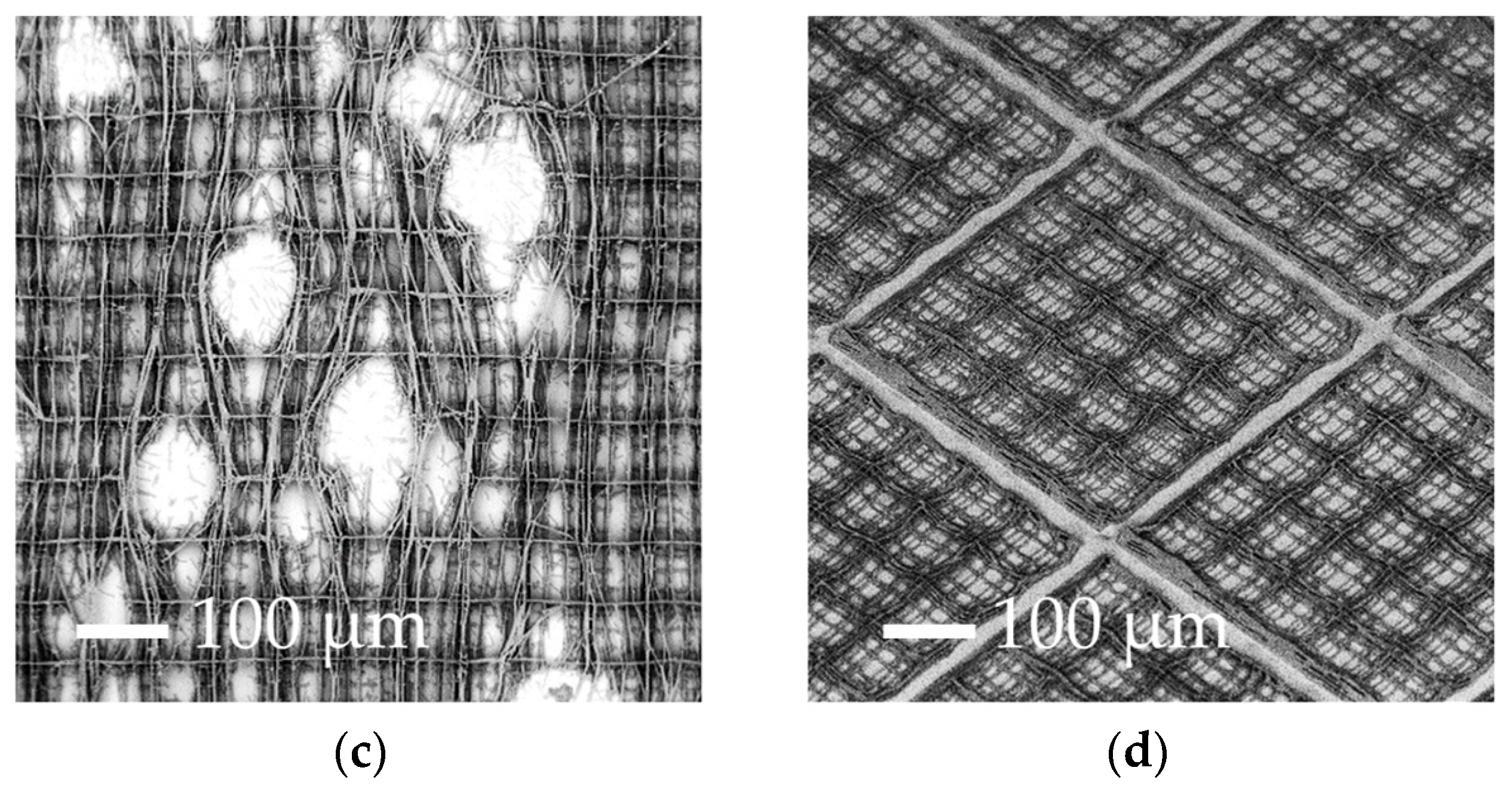
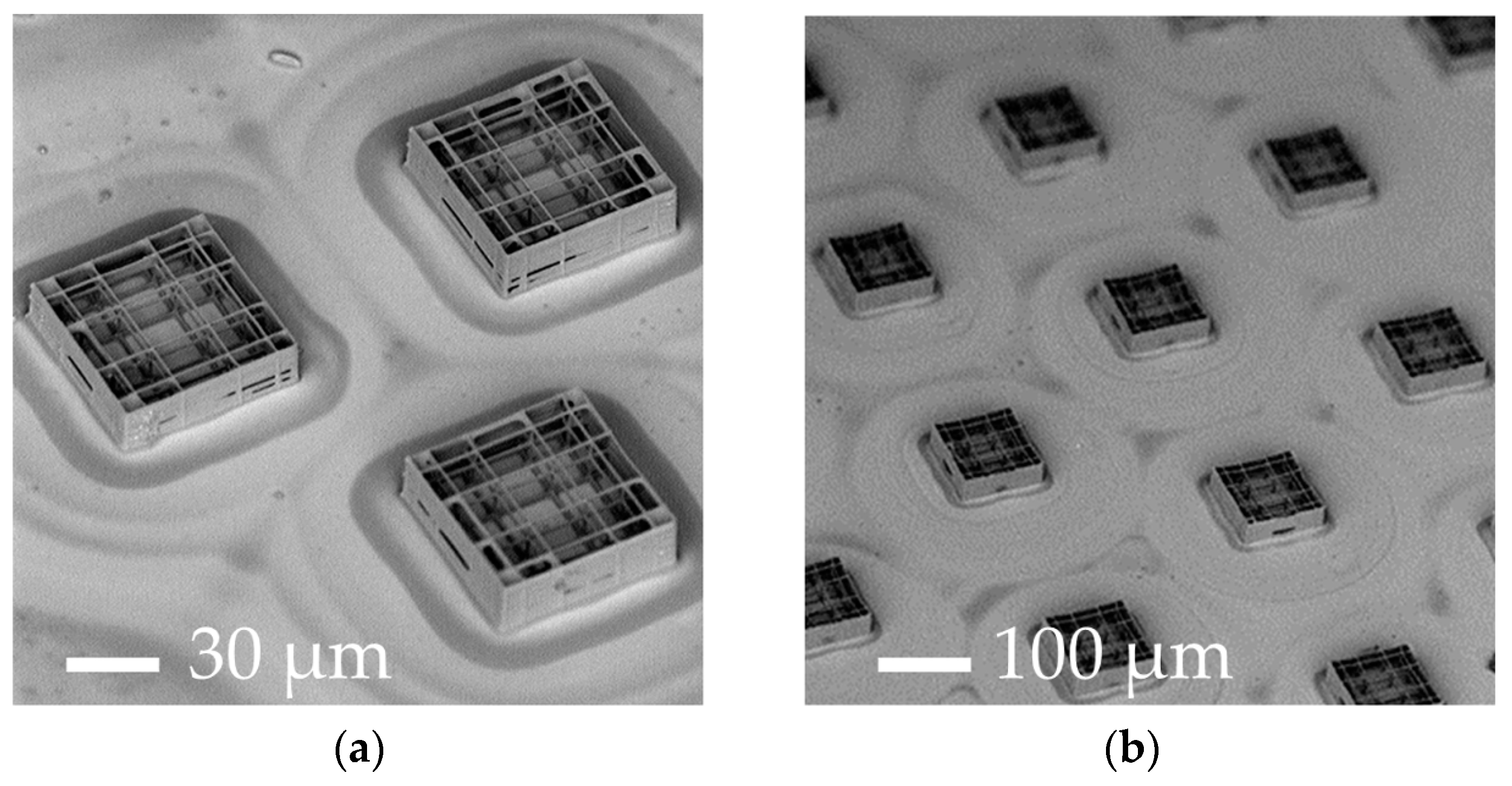
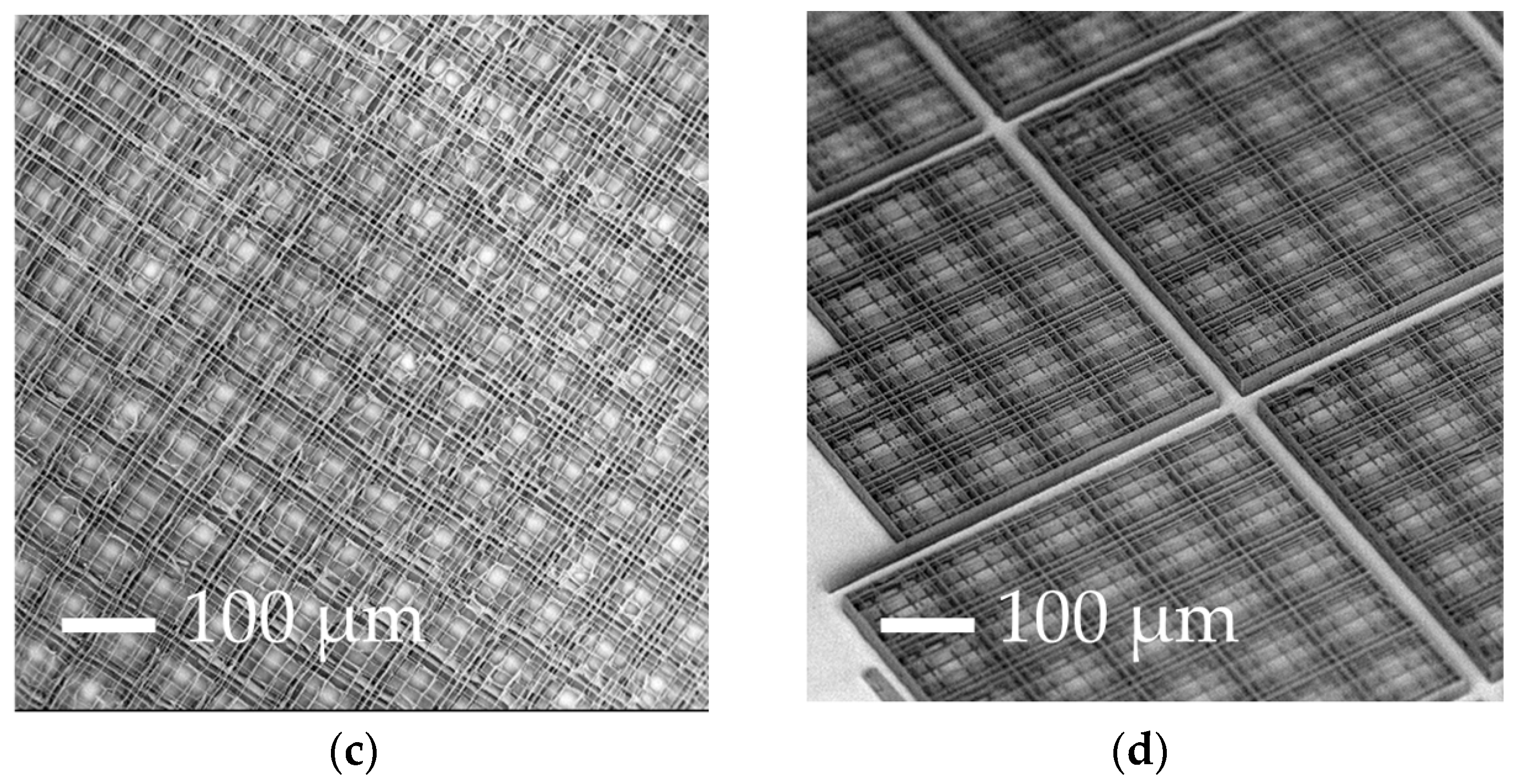
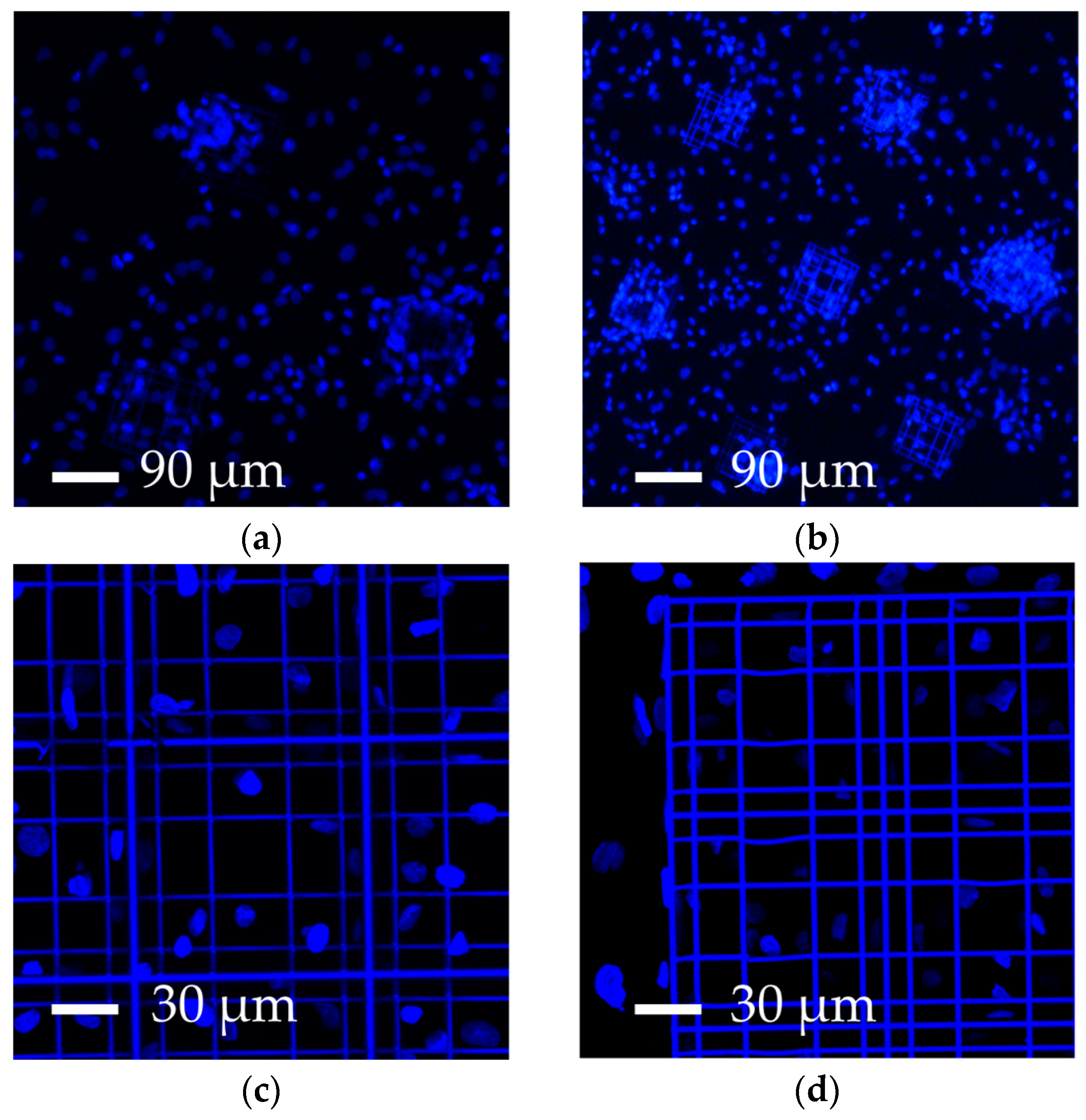
| Parameters | A | B | C | D |
|---|---|---|---|---|
| Total machining time | 1.5 h | 3 h | 17 h | 12 h |
| Power–scan speed (mW–mm∙s−1) | 15 1–0.01 | 12 2–1.5 | 12 2–1 | 13 2–3 |
| Elementary nichoid writing time | 30 min | 30 s | 18 s | 7 s |
| Surface of cell culture (mm2) | 0.24 | 28.27 | 28.27 | 50.26 |
| % of surface covered by the nichoids | 10% | 10% | 100% | 88% |
| Number of nichoids | 3 | 367 | 3500 | 5450 |
| Estimated nichoid-cultured cells/sample | 60 | 8000 | 7 × 104 | 10.9 × 104 |
| Scan Speed (mm∙s−1) | Power (mW) | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|
| 1 | STABLE | DAMAGED | DAMAGED | DAMAGED | |
| 2 | STABLE | DAMAGED | DAMAGED | DAMAGED | |
| 3 | UNSTABLE | STABLE | STABLE | DAMAGED | |
| 4 | UNSTABLE | STABLE | STABLE | STABLE | |
| 5 | UNSTABLE | STABLE | STABLE | STABLE | |
| 6 | UNSTABLE | UNSTABLE | STABLE | STABLE | |
| 7 | Ø | UNSTABLE | STABLE | STABLE | |
| 8 | Ø | UNSTABLE | UNSTABLE | UNSTABLE | |
| 9 | Ø | Ø | UNSTABLE | UNSTABLE | |
| 10 | Ø | Ø | UNSTABLE | UNSTABLE |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricci, D.; Nava, M.M.; Zandrini, T.; Cerullo, G.; Raimondi, M.T.; Osellame, R. Scaling-Up Techniques for the Nanofabrication of Cell Culture Substrates via Two-Photon Polymerization for Industrial-Scale Expansion of Stem Cells. Materials 2017, 10, 66. https://doi.org/10.3390/ma10010066
Ricci D, Nava MM, Zandrini T, Cerullo G, Raimondi MT, Osellame R. Scaling-Up Techniques for the Nanofabrication of Cell Culture Substrates via Two-Photon Polymerization for Industrial-Scale Expansion of Stem Cells. Materials. 2017; 10(1):66. https://doi.org/10.3390/ma10010066
Chicago/Turabian StyleRicci, Davide, Michele M. Nava, Tommaso Zandrini, Giulio Cerullo, Manuela T. Raimondi, and Roberto Osellame. 2017. "Scaling-Up Techniques for the Nanofabrication of Cell Culture Substrates via Two-Photon Polymerization for Industrial-Scale Expansion of Stem Cells" Materials 10, no. 1: 66. https://doi.org/10.3390/ma10010066
APA StyleRicci, D., Nava, M. M., Zandrini, T., Cerullo, G., Raimondi, M. T., & Osellame, R. (2017). Scaling-Up Techniques for the Nanofabrication of Cell Culture Substrates via Two-Photon Polymerization for Industrial-Scale Expansion of Stem Cells. Materials, 10(1), 66. https://doi.org/10.3390/ma10010066









