Melting Behavior and Thermolysis of NaBH4−Mg(BH4)2 and NaBH4−Ca(BH4)2 Composites
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. In Situ Time-Resolved Synchrotron Radiation Powder X-ray Diffraction
2.3. Thermal Analysis
2.4. Temperature Programmed Photographic Analysis
3. Results
3.1. Differential Scanning Calorimetry
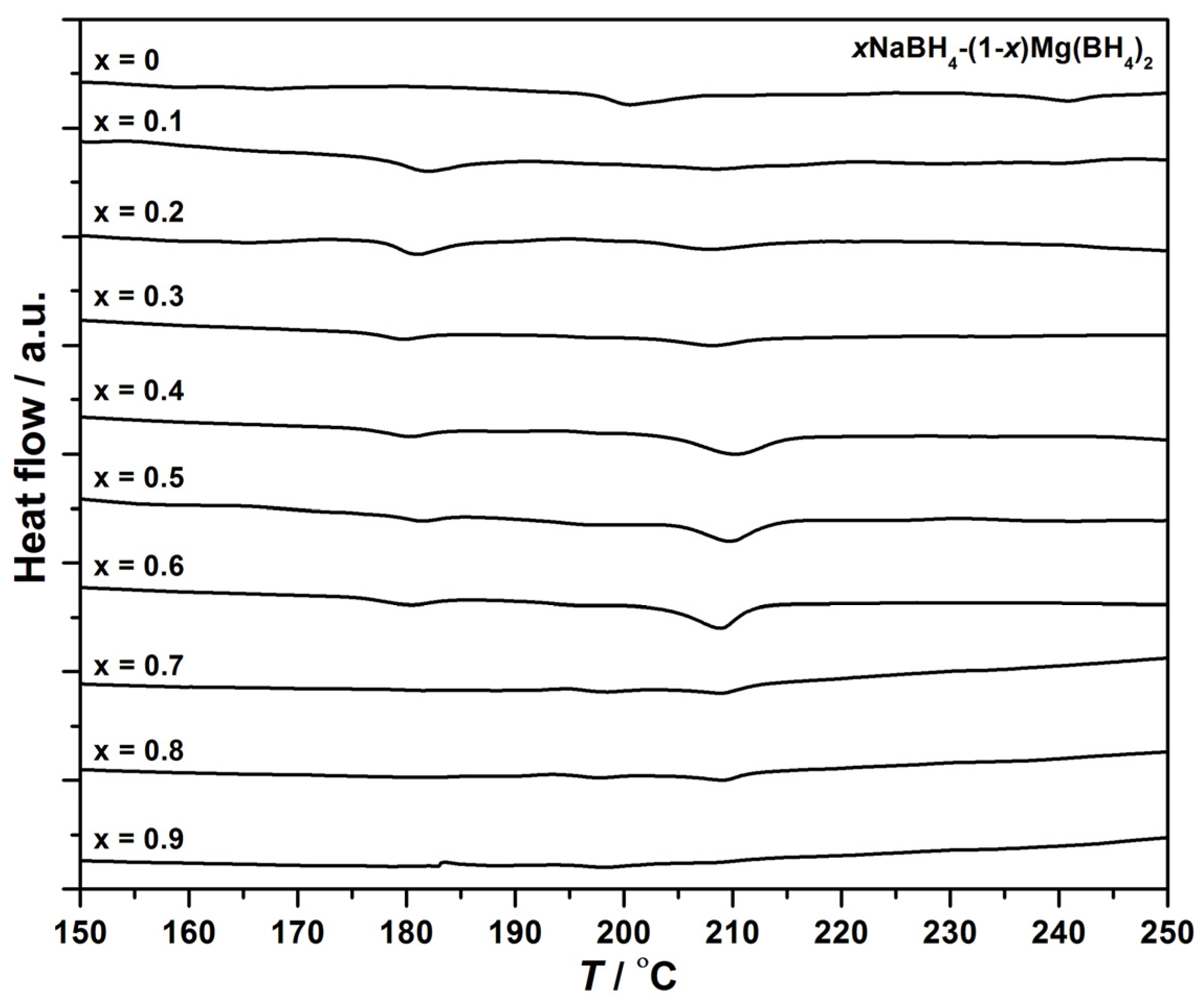
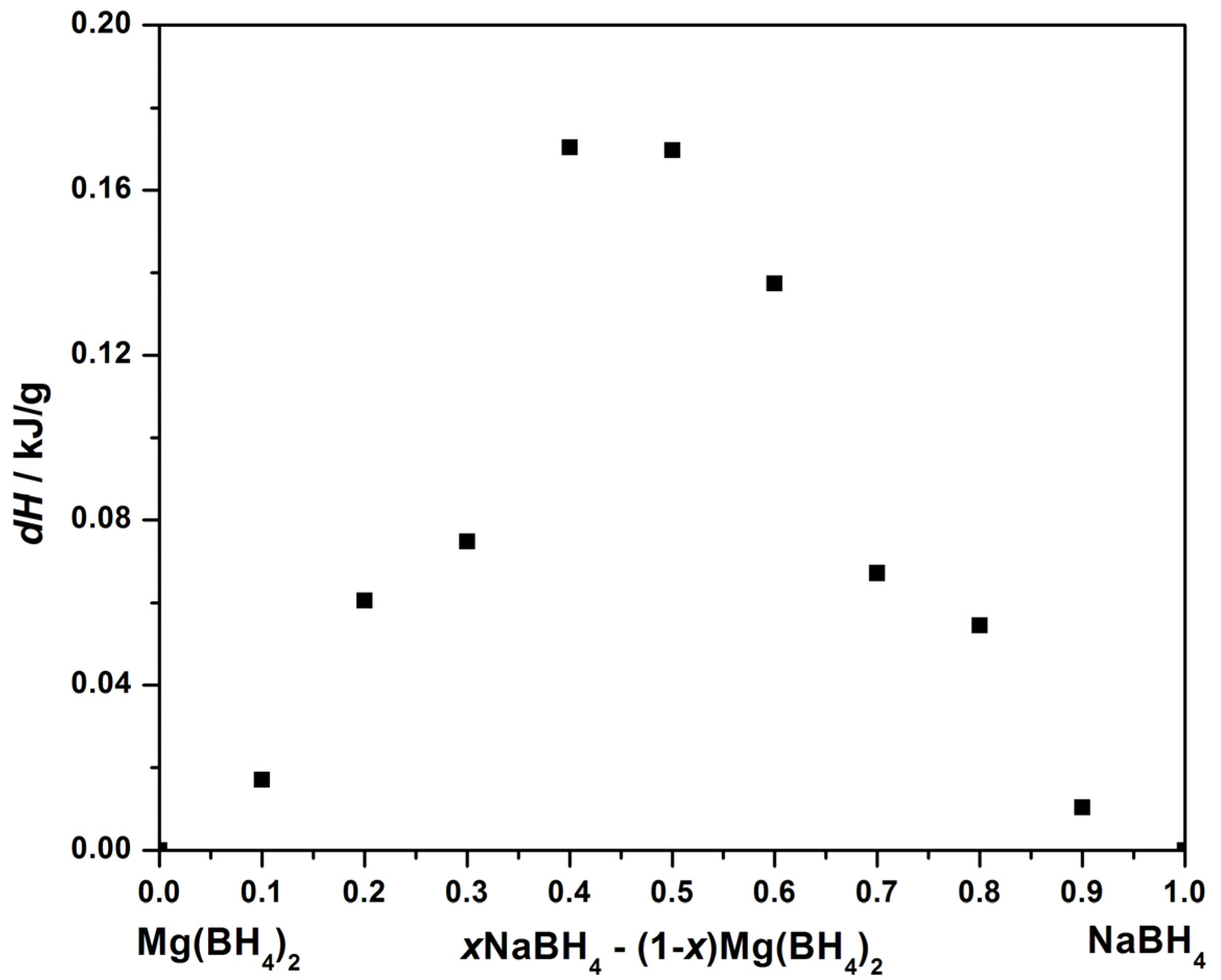
3.2. Temperature Programmed Photographic Analysis
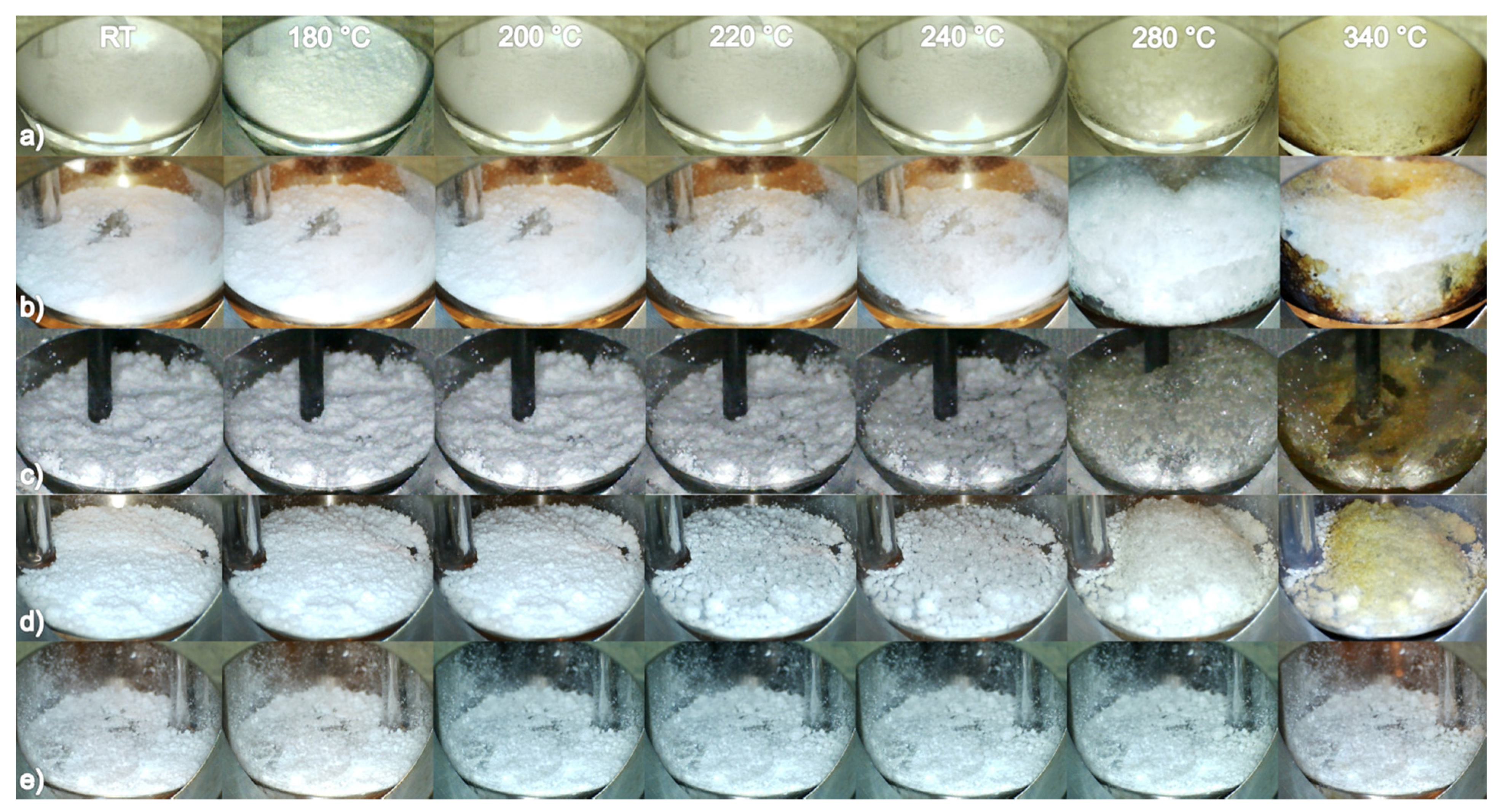
3.3. Thermogravimetric and Mass Spectrometry Analysis

3.4. Decomposition Mechanisms Observed by in Situ SR-PXD
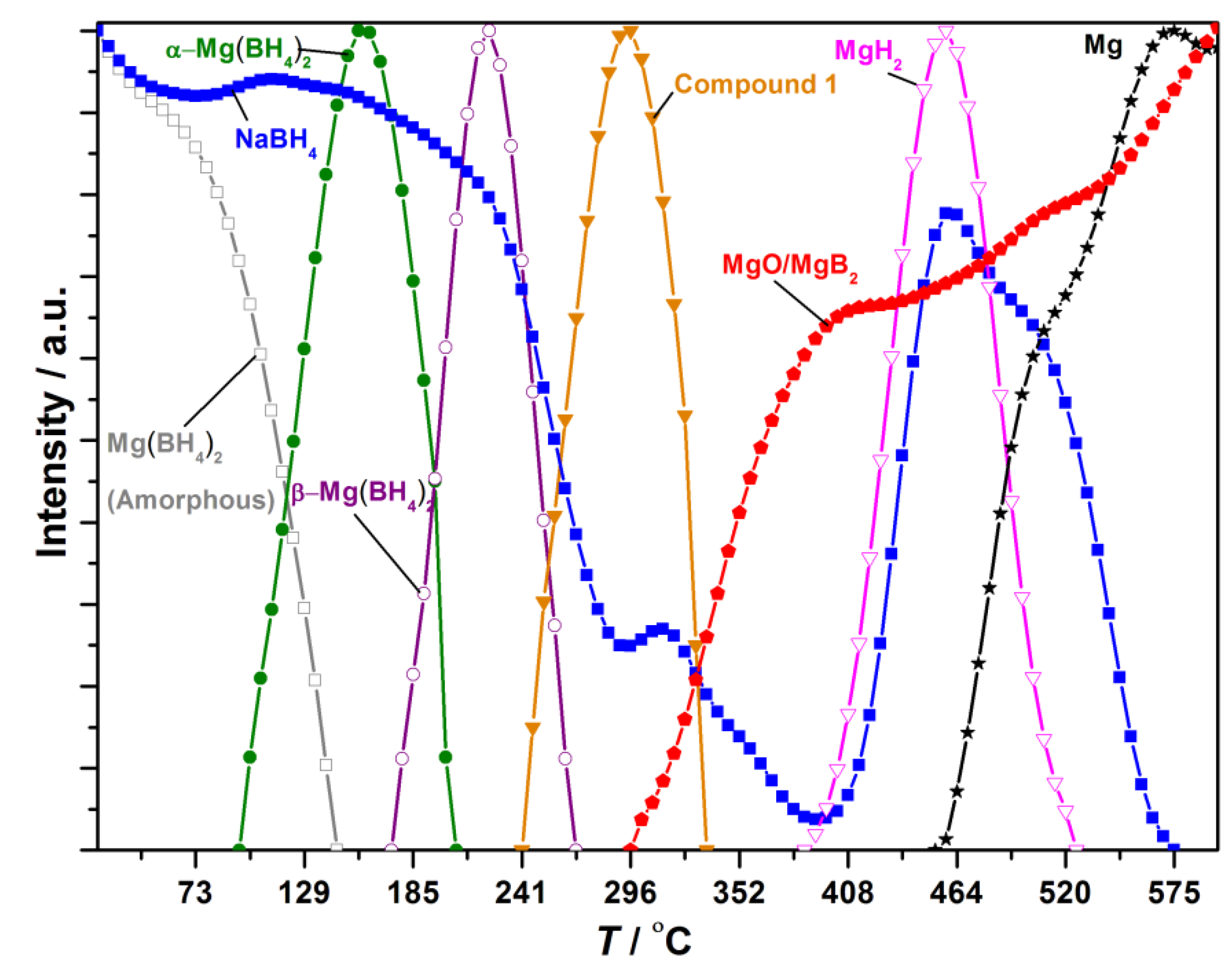
4. Discussion
4.1. Discussion of the xNaBH4‒(1 − x)Mg(BH4)2 Composite
4.2. Discussion of the xNaBH4‒(1 − x)Ca(BH4)2 Composite
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Bellosta von Colbe, J.M.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ley, M.B.; Lee, Y.-S.; Cho, Y.W.; Dornheim, M.; Jensen, J.O.; Filinchuk, Y.; Jørgensen, J.E.; Besenbacher, F.; Jensen, T.R. Boron-nitrogen based hydrides and reactive composites for hydrogen storage. Mater. Today 2014, 17, 129–135. [Google Scholar] [CrossRef]
- Fichtner, M. Conversion materials for hydrogen storage and electrochemical applications—Concepts and similarities. J. Alloys Compd. 2011, 509, 529–534. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2009, 22, 587–603. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Rude, L.H.; Nielsen, T.K.; Ravnsbæk, D.B.; Bösenberg, U.; Ley, M.B.; Richter, B.; Arnbjerg, L.M.; Dornheim, M.; Filinchuk, Y.; Besenbacher, F.; et al. Tailoring properties of borohydrides for hydrogen storage: A review. Phys. Status Solidi 2011, 208, 1754–1773. [Google Scholar] [CrossRef]
- Amieiro-Fonseca, A.; Ellis, S.R.; Nuttall, C.J.; Hayden, B.E.; Guerin, S.; Purdy, G.; Soulié, J.-P.; Callear, S.K.; Culligan, S.D.; David, W.I.F.; et al. A multidisciplinary combinatorial approach for tuning promising hydrogen storage materials towards automotive applications. Faraday Discuss. 2011, 151, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and dense magnesium borohydride frameworks: Synthesis, stability, and reversible absorption of guest species. Angew. Chem. 2011, 123, 11358–11362. [Google Scholar] [CrossRef]
- David, W.I.F.; Callear, S.K.; Jones, M.O.; Aeberhard, P.C.; Culligan, S.D.; Pohl, A.H.; Johnson, S.R.; Ryan, K.R.; Parker, J.E.; Edwards, P.P.; et al. The structure, thermal properties and phase transformations of the cubic polymorph of magnesium tetrahydroborate. Phys. Chem. Chem. Phys. 2012, 14, 11800–11807. [Google Scholar] [CrossRef] [PubMed]
- Her, J.H.; Stephens, P.W.; Gao, Y.; Soloveichik, G.L.; Rijssenbeek, J.; Andrus, M.; Zhao, J.C. Structure of unsolvated magnesium borohydride Mg(BH4)2. Acta Crystallogr. B 2007, 63, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Pitt, M.P.; Webb, C.J.; Sheppard, D.A.; Filsø, U.; Gray, E.M.; Buckley, C.E. In-Situ X-ray Diffraction Study of γ-Mg(BH4)2 Decomposition. J. Phys. Chem. C 2012, 116, 15231–15240. [Google Scholar] [CrossRef]
- Richter, B.; Ravnsbæk, D.B.; Tumanov, N.; Filinchuk, Y.; Jensen, T.R. Manganese borohydride; synthesis and characterization. Dalt. Trans. 2015, 44, 3988–3996. [Google Scholar] [CrossRef]
- Pistidda, C.; Garroni, S.; Dolci, F.; Bardají, E.G.; Khandelwal, A.; Nolis, P.; Dornheim, M.; Gosalawit, R.; Jensen, T.; Cerenius, Y.; et al. Synthesis of amorphous Mg(BH4)2 from MgB2 and H2 at room temperature. J. Alloys Compd. 2010, 508, 212–216. [Google Scholar] [CrossRef]
- Severa, G.; Rönnebro, E.; Jensen, C.M. Direct hydrogenation magnesium boride to magnesium borohydride: Demonstration of >11 weight percent reversible hydrogen storage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Ronnebro, E.; Chandra, D. Crystal structures and phase transformations in Ca(BH4)2. Acta Mater. 2009, 57, 732–738. [Google Scholar] [CrossRef]
- Aeberhard, P.C.; Refson, K.; Edwards, P.P.; David, W.I.F. High-pressure crystal structure prediction of calcium borohydride using density functional theory. Phys. Rev. B 2011, 83, 174102. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Chłopek, K.; Fichtner, M.; Hauback, B.C. The identification of a hitherto unknown intermediate phase CaB2Hx from decomposition of Ca(BH4)2. J. Mater. Chem. 2009, 19, 2754–2759. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Jensen, T.R.; Doppiu, S.; Bösenberg, U.; Borgschulte, A.; Gremaud, R.; Cerenius, Y.; Dornheim, M.; Klassen, T.; Bormann, R. Formation of Ca(BH4)2 from hydrogenation of CaH2+MgB2 composite. J. Phys. Chem. C 2008, 112, 2743–2749. [Google Scholar] [CrossRef]
- Schouwink, P.; D’Anna, V.; Ley, M.B.; Lawson Daku, L.M.; Richter, B.; Jensen, T.R.; Hagemann, H.; Černý, R. Bimetallic borohydrides in the system M(BH4)2—KBH4 (M = Mg, Mn): On the structural diversity. J. Phys. Chem. C 2012, 116, 10829–10840. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Jensen, T.R.; Smrčok, L.; Černý, R. Borohydrides: From sheet to framework topologies. Dalton Trans. 2014, 43, 7726–7733. [Google Scholar] [CrossRef] [PubMed]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the Decomposition Temperature in Complex Hydrides: Synthesis of a Mixed Alkalali Metal Borohydride. Angew. Chem. Int. Ed. 2008, 47, 2817–2819. [Google Scholar] [CrossRef]
- Ley, M.B.; Ravnsbæk, D.B.; Filinchuk, Y.; Lee, Y.-S.; Janot, R.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Ley, M.B.; Boulineau, S.; Janot, R.; Filinchuk, Y.; Jensen, T.R. New li ion conductors and solid state hydrogen storage materials: LiM(BH4)3Cl, M = La, Gd. J. Phys. Chem. C 2012, 116, 21267–21276. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrčok, L.; Černý, R. Structure and properties of complex hydride perovskite materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef] [PubMed]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Dornheim, M.; Doppiu, S.; Barkhordarian, G.; Bösenberg, U.; Klassen, T.; Gutfleisch, O.; Bormann, R. Hydrogen storage in magnesium-based hydrides and hydride composites. Scr. Mater. 2007, 56, 841–846. [Google Scholar] [CrossRef]
- Roedern, E.; Jensen, T.R. Thermal decomposition of Mn(BH4)2−M(BH4)x and Mn(BH4)2−MHx composites with M = Li, Na, Mg, and Ca. J. Phys. Chem. C 2014, 118, 23567–23574. [Google Scholar] [CrossRef]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, K.N.; Chavgun, A.P.; Surov, V.N. Interaction of sodium tetrahydroborate with potassium and lithium tetrahydroborates. Russ. J. Inorg. Chem. 1971, 16, 271–273. [Google Scholar]
- Ley, M.B.; Roedern, E.; Jensen, T.R. Eutectic melting of LiBH4-KBH4. Phys. Chem. Chem. Phys. 2014, 16, 24194–24199. [Google Scholar] [CrossRef] [PubMed]
- Bardaji, E.G.; Zhao-Karger, Z.; Boucharat, N.; Nale, A.; van Setten, M.J.; Lohstroh, W.; Rohm, E.; Catti, M.; Fichtner, M. LiBH4-Mg(BH4)2: A physical mixture of metal borohydrides as hydrogen storage material. J. Phys. Chem. C 2011, 115, 6095–6101. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ravnsbæk, D.; Lee, Y.-S.; Kim, Y.; Cerenius, Y.; Shim, J.-H.; Jensen, T.R.; Hur, N.H.; Cho, Y.W. Decomposition reactions and reversibility of the LiBH4-Ca(BH4)2 composite. J. Phys. Chem. C 2009, 113, 15080–15086. [Google Scholar] [CrossRef]
- Lee, H.-S.; Lee, Y.-S.; Suh, J.-Y.; Kim, M.; Yu, J.-S.; Cho, Y.W. Enhanced Desorption and Absorption properties of eutectic LiBH4-Ca(BH4)2 infiltrated into mesoporous carbon. J. Phys. Chem. C 2011, 115, 20027–20035. [Google Scholar] [CrossRef]
- Cerenius, Y.; Stahl, K.; Svensson, L.A.; Ursby, T.; Oskarsson, A.; Albertsson, J.; Liljas, A. The crystallography beamline I711 at MAX II. J. Synchrotron Radiat. 2000, 7, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.R.; Nielsen, T.K.; Filinchuk, Y.; Jørgensen, J.E.; Cerenius, Y.; Gray, E.M.; Webb, C.J. Versatile in situ powder X-ray diffraction cells for solid–gas investigations. J. Appl. Crystallogr. 2010, 43, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Hammersley, A.P.; Svensson, S.O.; Hanfland, M.; Fitch, A.N.; Hausermann, D. Two-dimensional detector software: From real detector to idealised image or two-theta scan. High Press. Res. 1996, 14, 235–248. [Google Scholar] [CrossRef]
- Martelli, P.; Caputo, R.; Remhof, A.; Mauron, P.; Borgschulte, A.; Züttel, A. Stability and decomposition of NaBH4. J. Phys. Chem. C 2010, 114, 7173–7177. [Google Scholar] [CrossRef]
- Ban, V.; Soloninin, A.V.; Skripov, A.V.; Hadermann, J.; Abakumov, A.; Filinchuk, Y. Pressure-collapsed amorphous Mg(BH4)2: An Ultradense complex hydride showing a reversible transition to the porous framework. J. Phys. Chem. C 2014, 118, 23402–23408. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Ravnsbæk, D.B.; Filinchuk, Y.; Vang, R.T.; Cerenius, Y.; Besenbacher, F.; Jørgensen, J.-E.; Jakobsen, H.J.; Jensen, T.R. Structure and dynamics for LiBH4—LiCl solid solutions. Chem. Mater. 2009, 21, 5772–5782. [Google Scholar] [CrossRef]
- Riktor, M.D.; Filinchuk, Y.; Vajeeston, P.; Bardají, E.G.; Fichtner, M.; Fjellvåg, H.; Sørby, M.H.; Hauback, B.C. The crystal structure of the first borohydride borate, Ca3(BD4)3(BO3). J. Mater. Chem. 2011, 21, 7188–7193. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Filinchuk, Y.; Lee, H.-S.; Suh, J.-Y.; Kim, J.W.; Yu, J.-S.; Cho, Y.W. On the formation and the structure of the first bimetallic borohydride borate, LiCa3(BH4)(BO3)2. J. Phys. Chem. C 2011, 115, 10298–10304. [Google Scholar] [CrossRef]
- Zhao-Karger, Z.; Witter, R.; Bardají, E.G.; Wang, D.; Cossement, D.; Fichtner, M. Altered reaction pathways of eutectic LiBH4-Mg(BH4)2 by nanoconfinement. J. Mater. Chem. A 2013, 1, 3379. [Google Scholar] [CrossRef]
- Javadian, P.; Jensen, T.R. Enhanced hydrogen reversibility of nanoconfined LiBH4–Mg(BH4)2. Int. J. Hydrog. Energy 2014, 39, 9871–9876. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4–Ca(BH4)2. Nano Energy 2015, 11, 96–103. [Google Scholar] [CrossRef]
- Zavorotynska, O.; Saldan, I.; Hino, S.; Humphries, T.D.; Deledda, S.; Hauback, B.C. Hydrogen cycling in γ-Mg(BH4)2 with cobalt-based additives. J. Mater. Chem. A 2015, 3, 6592–6602. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ley, M.B.; Roedern, E.; Thygesen, P.M.M.; Jensen, T.R. Melting Behavior and Thermolysis of NaBH4−Mg(BH4)2 and NaBH4−Ca(BH4)2 Composites. Energies 2015, 8, 2701-2713. https://doi.org/10.3390/en8042701
Ley MB, Roedern E, Thygesen PMM, Jensen TR. Melting Behavior and Thermolysis of NaBH4−Mg(BH4)2 and NaBH4−Ca(BH4)2 Composites. Energies. 2015; 8(4):2701-2713. https://doi.org/10.3390/en8042701
Chicago/Turabian StyleLey, Morten B., Elsa Roedern, Peter M. M. Thygesen, and Torben R. Jensen. 2015. "Melting Behavior and Thermolysis of NaBH4−Mg(BH4)2 and NaBH4−Ca(BH4)2 Composites" Energies 8, no. 4: 2701-2713. https://doi.org/10.3390/en8042701





