Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature
Abstract
:1. Introduction
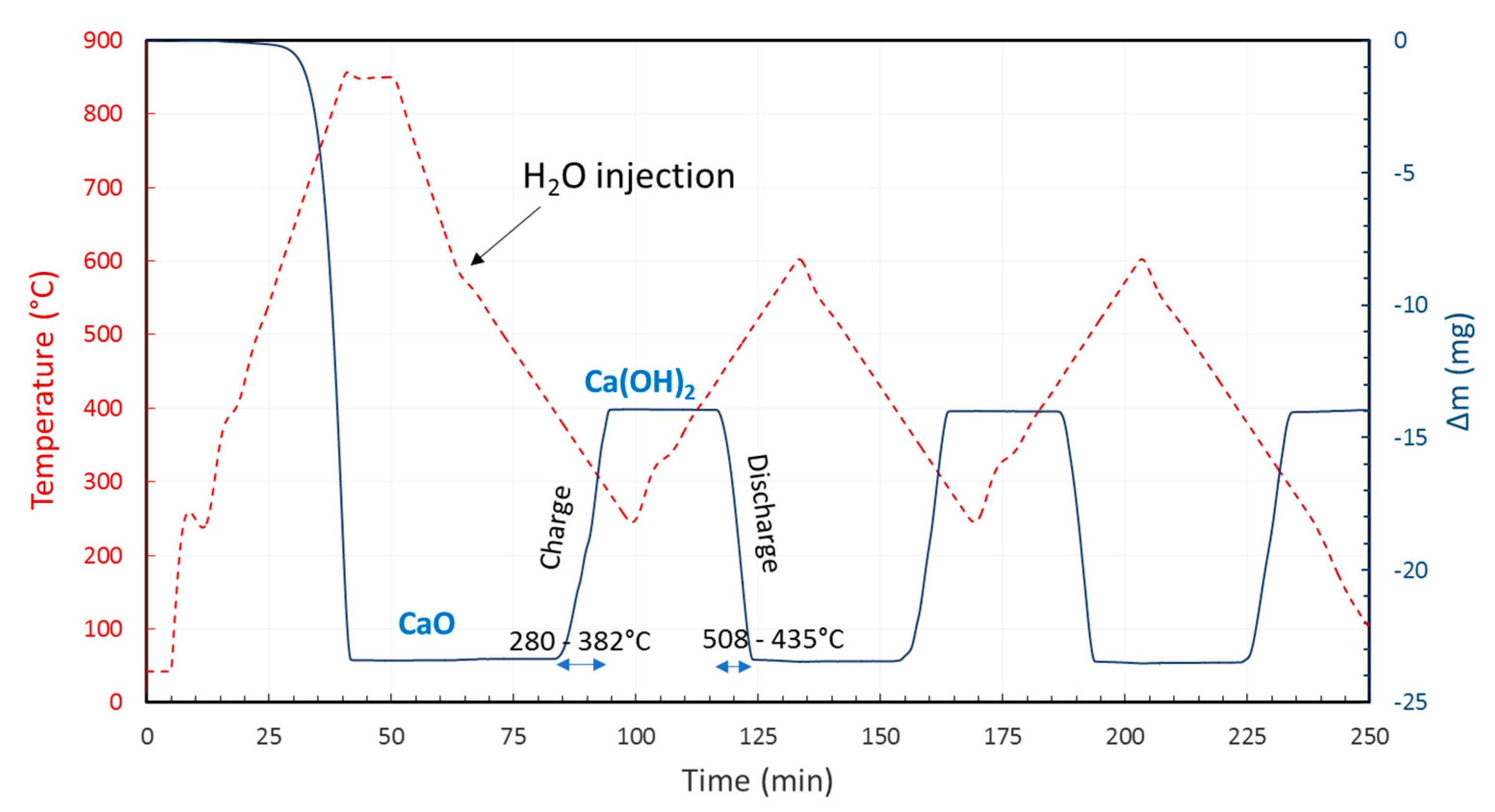
2. TCES Systems Based on Hydroxides
(ΔH° = −109.2 kJ/mol)
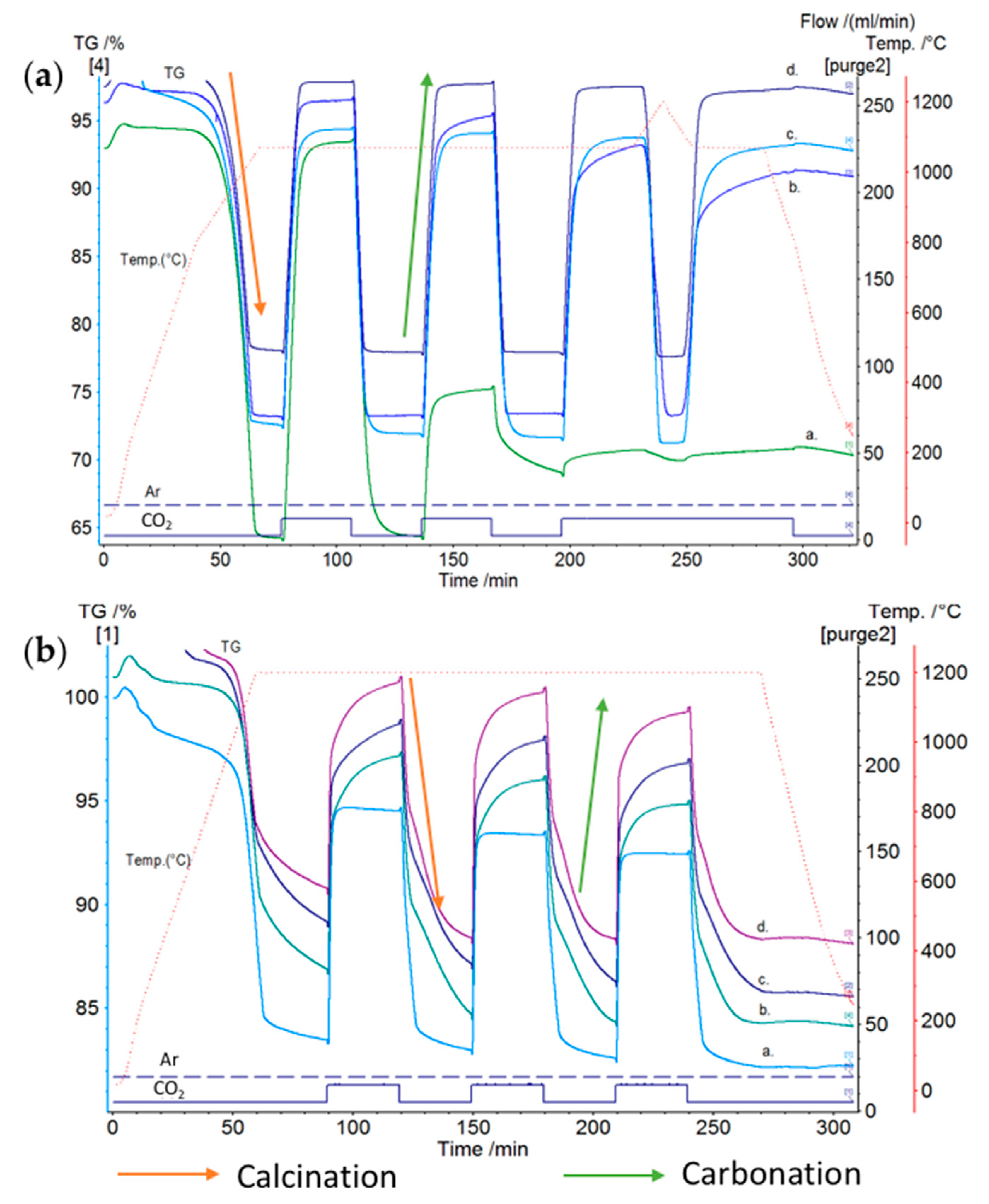
3. TCES Systems Based on Carbonates
(ΔH° = −178.2 kJ/mol)
(ΔH° = −241.5 kJ/mol)
(ΔH° = −272.5 kJ/mol)
(ΔH850 °C = 126.9 kJ/mol)
(ΔHr = −94 kJ/mol)
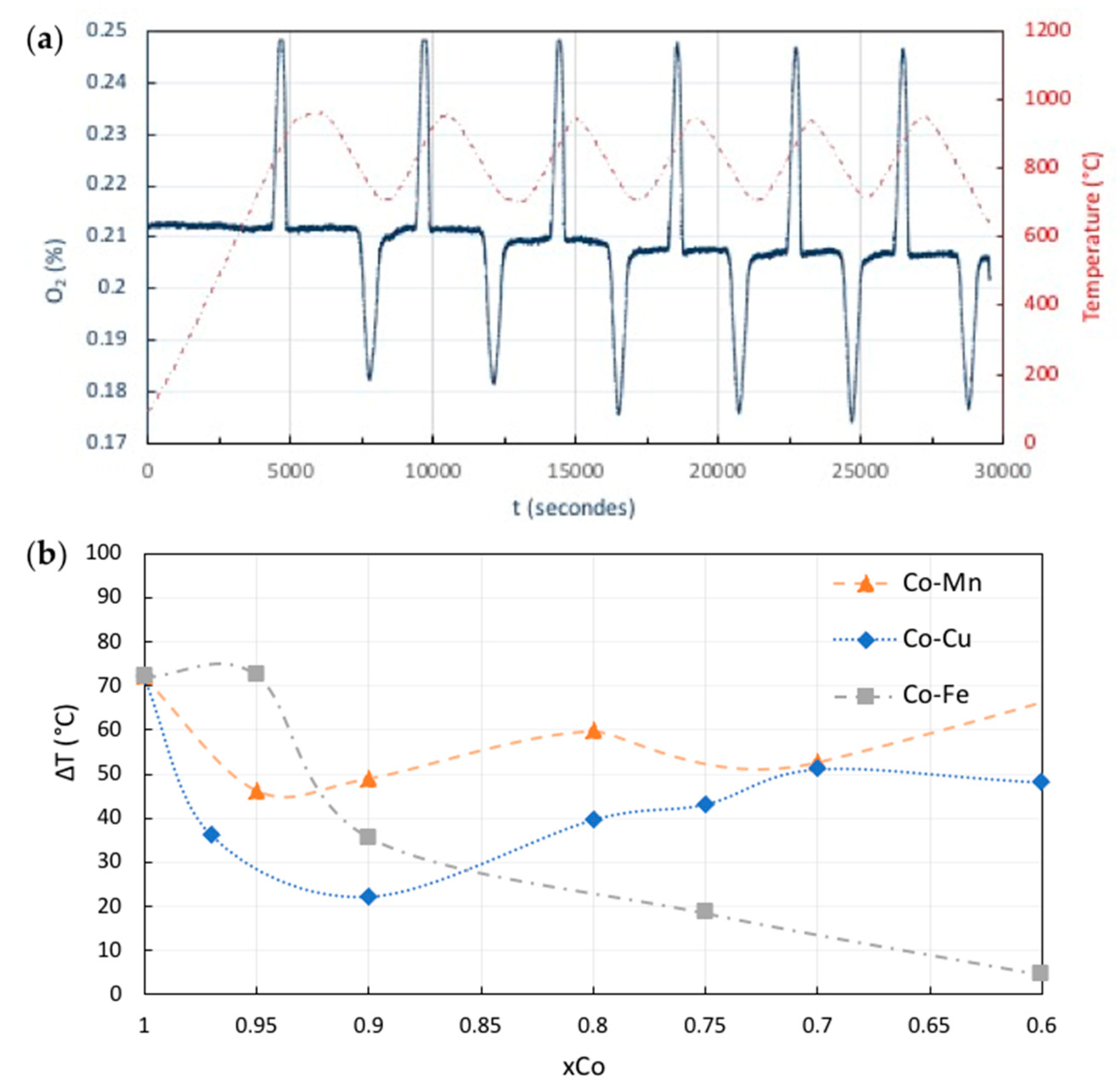
4. TCES systems Based on Metal Oxides
(ΔH° = −86.3 kJ/mol BaO)
5. TCES Systems Based on Perovskites
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yan, Y.; Wang, K.; Clough, P.T.; Anthony, E.J. Developments in calcium/chemical looping and metal oxide redox cycles for high-temperature thermochemical energy storage: A review. Fuel Process. Technol. 2020, 199, 106280. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Qi, C.; Ling, X.; Peng, H. State of the art on the high-temperature thermochemical energy storage systems. Energy Convers. Manag. 2018, 177, 792–815. [Google Scholar] [CrossRef]
- Bayon, A.; Bader, R.; Jafarian, M.; Fedunik-Hofman, L.; Sun, Y.; Hinkley, J.; Miller, S.; Lipiński, W. Techno-economic assessment of solid–gas thermochemical energy storage systems for solar thermal power applications. Energy 2018, 149, 473–484. [Google Scholar] [CrossRef]
- Pan, Z.H.; Zhao, C.Y. Gas–solid thermochemical heat storage reactors for high-temperature applications. Energy 2017, 130, 155–173. [Google Scholar] [CrossRef]
- Zsembinszki, G.; Solé, A.; Barreneche, C.; Prieto, C.; Fernández, A.; Cabeza, L. Review of Reactors with Potential Use in Thermochemical Energy Storage in Concentrated Solar Power Plants. Energies 2018, 11, 2358. [Google Scholar] [CrossRef] [Green Version]
- Pardo, P.; Deydier, A.; Anxionnaz-Minvielle, Z.; Rougé, S.; Cabassud, M.; Cognet, P. A review on high temperature thermochemical heat energy storage. Renew. Sustain. Energy Rev. 2014, 32, 591–610. [Google Scholar] [CrossRef] [Green Version]
- Sunku Prasad, J.; Muthukumar, P.; Desai, F.; Basu, D.N.; Rahman, M.M. A critical review of high-temperature reversible thermochemical energy storage systems. Appl. Energy 2019, 254, 113733. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Flamant, G. Screening of thermochemical systems based on solid-gas reversible reactions for high temperature solar thermal energy storage. Renew. Sustain. Energy Rev. 2016, 64, 703–715. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Investigation of metal oxides, mixed oxides, perovskites and alkaline earth carbonates/hydroxides as suitable candidate materials for high-temperature thermochemical energy storage using reversible solid-gas reactions. Mater. Today Energy 2018, 10, 48–61. [Google Scholar] [CrossRef]
- Schmidt, M.; Gutierrez, A.; Linder, M. Thermochemical energy storage with CaO/Ca(OH)2—Experimental investigation of the thermal capability at low vapor pressures in a lab scale reactor. Appl. Energy 2017, 188, 672–681. [Google Scholar] [CrossRef]
- Rougé, S.; Criado, Y.A.; Soriano, O.; Abanades, J.C. Continuous CaO/Ca(OH)2 Fluidized Bed Reactor for Energy Storage: First Experimental Results and Reactor Model Validation. Ind. Eng. Chem. Res. 2017, 56, 844–852. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Li, Y.; Zhao, J. Development on Thermochemical Energy Storage Based on CaO-Based Materials: A Review. Sustainability 2018, 10, 2660. [Google Scholar] [CrossRef] [Green Version]
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Performance of thermochemical energy storage of a packed bed of calcium hydroxide pellets. Energy Storage 2019, 1, e40. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Huai, X.; Cai, J. Agglomeration Behavior of Calcium Hydroxide/Calcium Oxide as Thermochemical Heat Storage Material: A Reactive Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 3025–3033. [Google Scholar] [CrossRef]
- Criado, Y.A.; Huille, A.; Rougé, S.; Abanades, J.C. Experimental investigation and model validation of a CaO/Ca(OH)2 fluidized bed reactor for thermochemical energy storage applications. Chem. Eng. J. 2017, 313, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Long, X.-F.; Lou, B.; Wu, J. Thermal cycling stability of thermochemical energy storage system Ca(OH)2/CaO. Appl. Therm. Eng. 2018, 133, 261–268. [Google Scholar] [CrossRef]
- Funayama, S.; Takasu, H.; Zamengo, M.; Kariya, J.; Kim, S.T.; Kato, Y. Composite material for high-temperature thermochemical energy storage using calcium hydroxide and ceramic foam. Energy Storage 2019, 1, e53. [Google Scholar] [CrossRef] [Green Version]
- Funayama, S.; Takasu, H.; Kim, S.T.; Kato, Y. Thermochemical storage performance of a packed bed of calcium hydroxide composite with a silicon-based ceramic honeycomb support. Energy 2020, 201, 117673. [Google Scholar] [CrossRef]
- Criado, Y.A.; Alonso, M.; Abanades, J.C. Enhancement of a CaO/Ca(OH)2 based material for thermochemical energy storage. Sol. Energy 2016, 135, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Gollsch, M.; Afflerbach, S.; Angadi, B.V.; Linder, M. Investigation of calcium hydroxide powder for thermochemical storage modified with nanostructured flow agents. Sol. Energy 2020, 201, 810–818. [Google Scholar] [CrossRef]
- Cosquillo Mejia, A.; Afflerbach, S.; Linder, M.; Schmidt, M. Experimental analysis of encapsulated CaO/Ca(OH)2 granules as thermochemical storage in a novel moving bed reactor. Appl. Therm. Eng. 2020, 169, 114961. [Google Scholar] [CrossRef]
- Xia, B.Q.; Zhao, C.Y.; Yan, J.; Khosa, A.A. Development of granular thermochemical heat storage composite based on calcium oxide. Renew. Energy 2020, 147, 969–978. [Google Scholar] [CrossRef]
- Shkatulov, A.; Aristov, Y. Calcium hydroxide doped by KNO3 as a promising candidate for thermochemical storage of solar heat. RSC Adv. 2017, 7, 42929–42939. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Zhao, C.Y.; Yan, J. Investigation on the Ca(OH)2/CaO thermochemical energy storage system with potassium nitrate addition. Sol. Energy Mater. Sol. Cells 2020, 215, 110646. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, C.Y. Experimental study of CaO/Ca(OH)2 in a fixed-bed reactor for thermochemical heat storage. Appl. Energy 2016, 175, 277–284. [Google Scholar] [CrossRef]
- Huang, C.; Xu, M.; Huai, X. Experimental investigation on thermodynamic and kinetic of calcium hydroxide dehydration with hexagonal boron nitride doping for thermochemical energy storage. Chem. Eng. Sci. 2019, 206, 518–526. [Google Scholar] [CrossRef]
- Huang, C.; Xu, M.; Huai, X. Synthesis and performances evaluation of the spindle-shaped calcium hydroxide nanomaterials for thermochemical energy storage. J. Nanopart. Res. 2019, 21, 262. [Google Scholar] [CrossRef]
- Flegkas, S.; Birkelbach, F.; Winter, F.; Groenewold, H.; Werner, A. Profitability Analysis and Capital Cost Estimation of a Thermochemical Energy Storage System Utilizing Fluidized Bed Reactors and the Reaction System MgO/Mg(OH)2. Energies 2019, 12, 4788. [Google Scholar] [CrossRef] [Green Version]
- Müller, D.; Knoll, C.; Gravogl, G.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Schreiner, M.; Harasek, M.; Hradil, K.; et al. Tuning the performance of MgO for thermochemical energy storage by dehydration—From fundamentals to phase impurities. Appl. Energy 2019, 253, 113562. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Tan, T.; Nie, J.; Zhang, H. Optimization of LiNO3–Mg(OH)2 composites as thermo-chemical energy storage materials. J. Environ. Manag. 2020, 262, 110258. [Google Scholar] [CrossRef]
- Shkatulov, A.I.; Aristov, Y. Thermochemical Energy Storage using LiNO3-Doped Mg(OH)2: A Dehydration Study. Energy Technol. 2018, 6, 1844–1851. [Google Scholar] [CrossRef]
- Shkatulov, A.; Takasu, H.; Kato, Y.; Aristov, Y. Thermochemical energy storage by LiNO3-doped Mg(OH)2: Rehydration study. J. Energy Storage 2019, 22, 302–310. [Google Scholar] [CrossRef]
- André, L.; Abanades, S. Evaluation and performances comparison of calcium, strontium and barium carbonates during calcination/carbonation reactions for solar thermochemical energy storage. J. Energy Storage 2017, 13, 193–205. [Google Scholar] [CrossRef]
- Abanades, S.; André, L. Design and demonstration of a high temperature solar-heated rotary tube reactor for continuous particles calcination. Appl. Energy 2018, 212, 1310–1320. [Google Scholar] [CrossRef]
- Ortiz, C.; Romano, M.C.; Valverde, J.M.; Binotti, M.; Chacartegui, R. Process integration of Calcium-Looping thermochemical energy storage system in concentrating solar power plants. Energy 2018, 155, 535–551. [Google Scholar] [CrossRef]
- Ortiz, C.; Valverde, J.M.; Chacartegui, R.; Perez-Maqueda, L.A.; Giménez, P. The Calcium-Looping (CaCO3/CaO) process for thermochemical energy storage in Concentrating Solar Power plants. Renew. Sustain. Energy Rev. 2019, 113, 109252. [Google Scholar] [CrossRef]
- Astolfi, M.; De Lena, E.; Romano, M.C. Improved flexibility and economics of Calcium Looping power plants by thermochemical energy storage. Int. J. Greenh. Gas Control 2019, 83, 140–155. [Google Scholar] [CrossRef]
- Cannone, S.F.; Stendardo, S.; Lanzini, A. Solar-Powered Rankine Cycle Assisted by an Innovative Calcium Looping Process as an Energy Storage System. Ind. Eng. Chem. Res. 2020, 59, 6977–6993. [Google Scholar] [CrossRef]
- Durán-Martín, J.D.; Sánchez Jimenez, P.E.; Valverde, J.M.; Perejón, A.; Arcenegui-Troya, J.; García Triñanes, P.; Pérez Maqueda, L.A. Role of particle size on the multicycle calcium looping activity of limestone for thermochemical energy storage. J. Adv. Res. 2020, 22, 67–76. [Google Scholar] [CrossRef]
- Fedunik-Hofman, L.; Bayon, A.; Donne, S.W. Kinetics of Solid-Gas Reactions and Their Application to Carbonate Looping Systems. Energies 2019, 12, 2981. [Google Scholar] [CrossRef] [Green Version]
- Benitez-Guerrero, M.; Valverde, J.M.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Low-cost Ca-based composites synthesized by biotemplate method for thermochemical energy storage of concentrated solar power. Appl. Energy 2018, 210, 108–116. [Google Scholar] [CrossRef]
- Khosa, A.A.; Zhao, C.Y. Heat storage and release performance analysis of CaCO3/CaO thermal energy storage system after doping nano silica. Sol. Energy 2019, 188, 619–630. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Su, C.; Li, J.; Liu, Q.; Qin, Y. High-performance CaO-based composites synthesized using a space-confined chemical vapor deposition strategy for thermochemical energy storage. Sol. Energy Mater. Sol. Cells 2020, 206, 110346. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Sun, F.; Liu, Q.; Qin, Y. Development of dense Ca-based, Al-stabilized composites with high volumetric energy density for thermochemical energy storage of concentrated solar power. Energy Convers. Manag. 2020, 221, 113201. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Yan, X.; Zhao, J.; Wang, Z. Thermochemical energy storage performance of Al2O3/CeO2 co-doped CaO-based material under high carbonation pressure. Appl. Energy 2020, 263, 114650. [Google Scholar] [CrossRef]
- Møller, K.T.; Ibrahim, A.; Buckley, C.E.; Paskevicius, M. Inexpensive thermochemical energy storage utilising additive enhanced limestone. J. Mater. Chem. A 2020, 8, 9646–9653. [Google Scholar] [CrossRef]
- Chen, X.; Jin, X.; Liu, Z.; Ling, X.; Wang, Y. Experimental investigation on the CaO/CaCO3 thermochemical energy storage with SiO2 doping. Energy 2018, 155, 128–138. [Google Scholar] [CrossRef]
- Han, R.; Gao, J.; Wei, S.; Su, Y.; Sun, F.; Zhao, G.; Qin, Y. Strongly coupled calcium carbonate/antioxidative graphite nanosheets composites with high cycling stability for thermochemical energy storage. Appl. Energy 2018, 231, 412–422. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, Y.; Ding, Y. Wettability of molten sodium sulfate salt on nanoscale calcium oxide surface in high-temperature thermochemical energy storage. Appl. Surf. Sci. 2020, 505, 144550. [Google Scholar] [CrossRef]
- Raganati, F.; Chirone, R.; Ammendola, P. Calcium-looping for thermochemical energy storage in concentrating solar power applications: Evaluation of the effect of acoustic perturbation on the fluidized bed carbonation. Chem. Eng. J. 2020, 392, 123658. [Google Scholar] [CrossRef]
- Da, Y.; Xuan, Y.; Teng, L.; Zhang, K.; Liu, X.; Ding, Y. Calcium-based composites for direct solar-thermal conversion and thermochemical energy storage. Chem. Eng. J. 2020, 382, 122815. [Google Scholar] [CrossRef]
- Zheng, H.; Song, C.; Bao, C.; Liu, X.; Xuan, Y.; Li, Y.; Ding, Y. Dark calcium carbonate particles for simultaneous full-spectrum solar thermal conversion and large-capacity thermochemical energy storage. Sol. Energy Mater. Sol. Cells 2020, 207, 110364. [Google Scholar] [CrossRef]
- Teng, L.; Xuan, Y.; Da, Y.; Liu, X.; Ding, Y. Modified Ca-Looping materials for directly capturing solar energy and high-temperature storage. Energy Storage Mater. 2020, 25, 836–845. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Z.; Huang, G. Fe- and Mn-Doped Ca-Based Materials for Thermochemical Energy Storage Systems. Energy Fuels 2020, 34, 11479–11488. [Google Scholar] [CrossRef]
- Tregambi, C.; Padula, S.; Galbusieri, M.; Coppola, G.; Montagnaro, F.; Salatino, P.; Troiano, M.; Solimene, R. Directly irradiated fluidized bed reactor for thermochemical energy storage and solar fuels production. Powder Technol. 2020, 366, 460–469. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Valverde, J.M.; Sanchez-Jimenez, P.E.; Perejon, A.; Perez-Maqueda, L.A. Multicycle activity of natural CaCO3 minerals for thermochemical energy storage in Concentrated Solar Power plants. Sol. Energy 2017, 153, 188–199. [Google Scholar] [CrossRef]
- Benitez-Guerrero, M.; Sarrion, B.; Perejon, A.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A.; Manuel Valverde, J. Large-scale high-temperature solar energy storage using natural minerals. Sol. Energy Mater. Sol. Cells 2017, 168, 14–21. [Google Scholar] [CrossRef]
- Wang, K.; Gu, F.; Clough, P.T.; Zhao, P.; Anthony, E.J. Porous MgO-stabilized CaO-based powders/pellets via a citric acid-based carbon template for thermochemical energy storage in concentrated solar power plants. Chem. Eng. J. 2020, 390, 124163. [Google Scholar] [CrossRef]
- Sánchez Jiménez, P.E.; Perejón, A.; Benítez Guerrero, M.; Valverde, J.M.; Ortiz, C.; Pérez Maqueda, L.A. High-performance and low-cost macroporous calcium oxide based materials for thermochemical energy storage in concentrated solar power plants. Appl. Energy 2019, 235, 543–552. [Google Scholar] [CrossRef]
- Humphries, T.D.; Møller, K.T.; Rickard, W.D.A.; Sofianos, M.V.; Liu, S.; Buckley, C.E.; Paskevicius, M. Dolomite: A low cost thermochemical energy storage material. J. Mater. Chem. A 2019, 7, 1206–1215. [Google Scholar] [CrossRef] [Green Version]
- Arcenegui-Troya, J.; Sánchez-Jiménez, P.E.; Perejón, A.; Valverde, J.M.; Chacartegui, R.; Pérez-Maqueda, L.A. Calcium-Looping Performance of Biomineralized CaCO3 for CO2 Capture and Thermochemical Energy Storage. Ind. Eng. Chem. Res. 2020, 59, 12924–12933. [Google Scholar] [CrossRef]
- Sun, H.; Li, Y.; Bian, Z.; Yan, X.; Wang, Z.; Liu, W. Thermochemical energy storage performances of Ca-based natural and waste materials under high pressure during CaO/CaCO3 cycles. Energy Convers. Manag. 2019, 197, 111885. [Google Scholar] [CrossRef]
- Maaten, B.; Konist, A.; Siirde, A. Potential of solid residues from power plants as thermochemical energy storage materials. J. Anal. Calorim. 2020. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Jordan, C.; Azam, M.; Werner, A.; Harasek, M.; Winter, F. Fly Ash from Municipal Solid Waste Incineration as a Potential Thermochemical Energy Storage Material. Energy Fuels 2019, 33, 5810–5819. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Azam, M.; Huber, F.; Jordan, C.; Wesenauer, F.; Huber, C.; Naghdi, S.; Schwendtner, K.; Neuwirth, E.; Laminger, T.; et al. Comparing Fly Ash Samples from Different Types of Incinerators for Their Potential as Storage Materials for Thermochemical Energy and CO2. Materials 2019, 12, 3358. [Google Scholar] [CrossRef] [Green Version]
- Valverde, J.M.; Miranda-Pizarro, J.; Perejón, A.; Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A. Calcium-Looping performance of steel and blast furnace slags for thermochemical energy storage in concentrated solar power plants. J. CO2 Util. 2017, 22, 143–154. [Google Scholar] [CrossRef]
- Bagherisereshki, E.; Tran, J.; Lei, F.; AuYeung, N. Investigation into SrO/SrCO3 for high temperature thermochemical energy storage. Sol. Energy 2018, 160, 85–93. [Google Scholar] [CrossRef]
- Meroueh, L.; Yenduru, K.; Dasgupta, A.; Jiang, D.; AuYeung, N. Energy storage based on SrCO3 and Sorbents—A probabilistic analysis towards realizing solar thermochemical power plants. Renew. Energy 2019, 133, 770–786. [Google Scholar] [CrossRef]
- Zare Ghorbaei, S.; Ale Ebrahim, H. Carbonation reaction of strontium oxide for thermochemical energy storage and CO2 removal applications: Kinetic study and reactor performance prediction. Appl. Energy 2020, 277, 115604. [Google Scholar] [CrossRef]
- Ammendola, P.; Raganati, F.; Miccio, F.; Murri, A.N.; Landi, E. Insights into utilization of strontium carbonate for thermochemical energy storage. Renew. Energy 2020, 157, 769–781. [Google Scholar] [CrossRef]
- Gigantino, M.; Kiwic, D.; Steinfeld, A. Thermochemical energy storage via isothermal carbonation-calcination cycles of MgO-stabilized SrO in the range of 1000–1100 °C. Sol. Energy 2019, 188, 720–729. [Google Scholar] [CrossRef]
- Møller, K.T.; Williamson, K.; Buckley, C.E.; Paskevicius, M. Thermochemical energy storage properties of a barium based reactive carbonate composite. J. Mater. Chem. A 2020, 8, 10935–10942. [Google Scholar] [CrossRef]
- Takasu, H.; Ryu, J.; Kato, Y. Application of lithium orthosilicate for high-temperature thermochemical energy storage. Appl. Energy 2017, 193, 74–83. [Google Scholar] [CrossRef]
- Gravogl, G.; Knoll, C.; Artner, W.; Welch, J.M.; Eitenberger, E.; Friedbacher, G.; Harasek, M.; Hradil, K.; Werner, A.; Weinberger, P.; et al. Pressure effects on the carbonation of MeO (Me = Co, Mn, Pb, Zn) for thermochemical energy storage. Appl. Energy 2019, 252, 113451. [Google Scholar] [CrossRef]
- Silakhori, M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Thermogravimetric analysis of Cu, Mn, Co, and Pb oxides for thermochemical energy storage. J. Energy Storage 2019, 23, 138–147. [Google Scholar] [CrossRef]
- Setoodeh Jahromy, S.; Birkelbach, F.; Jordan, C.; Huber, C.; Harasek, M.; Werner, A.; Winter, F. Impact of Partial Pressure, Conversion, and Temperature on the Oxidation Reaction Kinetics of Cu2O to CuO in Thermochemical Energy Storage. Energies 2019, 12, 508. [Google Scholar] [CrossRef] [Green Version]
- Silakhori, M.; Jafarian, M.; Arjomandi, M.; Nathan, G.J. Comparing the thermodynamic potential of alternative liquid metal oxides for the storage of solar thermal energy. Sol. Energy 2017, 157, 251–258. [Google Scholar] [CrossRef]
- Deutsch, M.; Horvath, F.; Knoll, C.; Lager, D.; Gierl-Mayer, C.; Weinberger, P.; Winter, F. High-Temperature Energy Storage: Kinetic Investigations of the CuO/Cu2O Reaction Cycle. Energy Fuels 2017, 31, 2324–2334. [Google Scholar] [CrossRef]
- Bush, H.E.; Loutzenhiser, P.G. Solar electricity via an Air Brayton cycle with an integrated two-step thermochemical cycle for heat storage based on Fe2O3/Fe3O4 redox reactions: Thermodynamic and kinetic analyses. Sol. Energy 2018, 174, 617–627. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Sastre, D.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Revisiting the BaO2/BaO redox cycle for solar thermochemical energy storage. Phys. Chem. Chem. Phys. 2016, 18, 8039–8048. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Experimental Investigation of Co–Cu, Mn–Co, and Mn–Cu Redox Materials Applied to Solar Thermochemical Energy Storage. ACS Appl. Energy Mater. 2018, 1, 3385–3395. [Google Scholar] [CrossRef]
- Block, T.; Schmücker, M. Metal oxides for thermochemical energy storage: A comparison of several metal oxide systems. Sol. Energy 2016, 126, 195–207. [Google Scholar] [CrossRef]
- Carrillo, A.J.; Serrano, D.P.; Pizarro, P.; Coronado, J.M. Manganese oxide-based thermochemical energy storage: Modulating temperatures of redox cycles by Fe–Cu co-doping. J. Energy Storage 2016, 5, 169–176. [Google Scholar] [CrossRef]
- André, L.; Abanades, S.; Cassayre, L. Mixed Metal Oxide Systems Applied to Thermochemical Storage of Solar Energy: Benefits of Secondary Metal Addition in Co and Mn Oxides and Contribution of Thermodynamics. Appl. Sci. 2018, 8, 2618. [Google Scholar] [CrossRef] [Green Version]
- Dizaji, H.B.; Hosseini, H. A review of material screening in pure and mixed-metal oxide thermochemical energy storage (TCES) systems for concentrated solar power (CSP) applications. Renew. Sustain. Energy Rev. 2018, 98, 9–26. [Google Scholar] [CrossRef]
- Wu, S.; Zhou, C.; Doroodchi, E.; Nellore, R.; Moghtaderi, B. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle. Energy Convers. Manag. 2018, 168, 421–453. [Google Scholar] [CrossRef]
- Liu, J.; Baeyens, J.; Deng, Y.; Wang, X.; Zhang, H. High temperature Mn2O3/Mn3O4 and Co3O4/CoO systems for thermo-chemical energy storage. J. Environ. Manag. 2020, 267, 110582. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.; Knoll, C.; Artner, W.; Harasek, M.; Gierl-Mayer, C.; Welch, J.M.; Werner, A.; Weinberger, P. Combining in-situ X-ray diffraction with thermogravimetry and differential scanning calorimetry—An investigation of Co3O4, MnO2 and PbO2 for thermochemical energy storage. Sol. Energy 2017, 153, 11–24. [Google Scholar] [CrossRef]
- Yilmaz, D.; Darwish, E.; Leion, H. Investigation of the combined Mn-Si oxide system for thermochemical energy storage applications. J. Energy Storage 2020, 28, 101180. [Google Scholar] [CrossRef]
- Bielsa, D.; Zaki, A.; Arias, P.L.; Faik, A. Improving the redox performance of Mn2O3/Mn3O4 pair by Si doping to be used as thermochemical energy storage for concentrated solar power plants. Sol. Energy 2020, 204, 144–154. [Google Scholar] [CrossRef]
- King, K.; Randhir, K.; Klausner, J. Calorimetric method for determining the thermochemical energy storage capacities of redox metal oxides. Thermochim. Acta 2019, 673, 105–118. [Google Scholar] [CrossRef]
- Randhir, K.; King, K.; Rhodes, N.; Li, L.; Hahn, D.; Mei, R.; AuYeung, N.; Klausner, J. Magnesium-manganese oxides for high temperature thermochemical energy storage. J. Energy Storage 2019, 21, 599–610. [Google Scholar] [CrossRef]
- King, K.; Randhir, K.; Petrasch, J.; Klausner, J. Enhancing thermochemical energy storage density of magnesium-manganese oxides. Energy Storage 2019, 1, e83. [Google Scholar] [CrossRef] [Green Version]
- Preisner, N.C.; Block, T.; Linder, M.; Leion, H. Stabilizing Particles of Manganese-Iron Oxide with Additives for Thermochemical Energy Storage. Energy Technol. 2018, 6, 2154–2165. [Google Scholar] [CrossRef]
- Al-Shankiti, I.A.; Ehrhart, B.D.; Ward, B.J.; Bayon, A.; Wallace, M.A.; Bader, R.; Kreider, P.; Weimer, A.W. Particle design and oxidation kinetics of iron-manganese oxide redox materials for thermochemical energy storage. Sol. Energy 2019, 183, 17–29. [Google Scholar] [CrossRef]
- Hamidi, M.; Wheeler, V.M.; Gao, X.; Pye, J.; Catchpole, K.; Weimer, A.W. Reduction of iron–manganese oxide particles in a lab-scale packed-bed reactor for thermochemical energy storage. Chem. Eng. Sci. 2020, 221, 115700. [Google Scholar] [CrossRef]
- Wokon, M.; Kohzer, A.; Linder, M. Investigations on thermochemical energy storage based on technical grade manganese-iron oxide in a lab-scale packed bed reactor. Sol. Energy 2017, 153, 200–214. [Google Scholar] [CrossRef]
- Wokon, M.; Block, T.; Nicolai, S.; Linder, M.; Schmücker, M. Thermodynamic and kinetic investigation of a technical grade manganese-iron binary oxide for thermochemical energy storage. Sol. Energy 2017, 153, 471–485. [Google Scholar] [CrossRef]
- Tescari, S.; Singh, A.; Agrafiotis, C.; de Oliveira, L.; Breuer, S.; Schlögl-Knothe, B.; Roeb, M.; Sattler, C. Experimental evaluation of a pilot-scale thermochemical storage system for a concentrated solar power plant. Appl. Energy 2017, 189, 66–75. [Google Scholar] [CrossRef]
- Zhou, X.; Mahmood, M.; Chen, J.; Yang, T.; Xiao, G.; Ferrari, M.L. Validated model of thermochemical energy storage based on cobalt oxides. Appl. Therm. Eng. 2019, 159, 113965. [Google Scholar] [CrossRef]
- Zaki, A.; Bielsa, D.; Faik, A. Development of a continuous solid solution with extended Red-Ox temperature range and unexpected high reaction enthalpies for thermochemical energy storage. AIP Conf. Proc. 2019, 2126, 210010. [Google Scholar] [CrossRef]
- Zaki, A.; Carrasco, J.; Bielsa, D.; Faik, A. Tunable Redox Temperature of a Co3−xMnxO4 (0 ≤ x ≤ 3) Continuous Solid Solution for Thermochemical Energy Storage. ACS Appl. Mater. Interfaces 2020, 12, 7010–7020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Andre, L.; Abanades, S. Experimental assessment of oxygen exchange capacity and thermochemical redox cycle behavior of Ba and Sr series perovskites for solar energy storage. Sol. Energy 2016, 134, 494–502. [Google Scholar] [CrossRef]
- Gokon, N.; Yawata, T.; Bellan, S.; Kodama, T.; Cho, H.-S. Thermochemical behavior of perovskite oxides based on LaxSr1-x(Mn, Fe, Co)O3-δ and BaySr1-yCoO3-δ redox system for thermochemical energy storage at high temperatures. Energy 2019, 171, 971–980. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Coker, E.N.; Miller, J.E.; Ambrosini, A. Investigation of LaxSr1−xCoyM1−yO3−δ (M = Mn, Fe) perovskite materials as thermochemical energy storage media. Sol. Energy 2015, 118, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Babiniec, S.M.; Coker, E.N.; Ambrosini, A.; Miller, J.E. ABO3 (A = La, Ba, Sr, K; B = Co, Mn, Fe) perovskites for thermochemical energy storage. AIP Conf. Proc. 2016, 1734, 050006. [Google Scholar] [CrossRef] [Green Version]
- Bork, A.H.; Carrillo, A.J.; Hood, Z.D.; Yildiz, B.; Rupp, J.L.M. Oxygen Exchange in Dual-Phase La0.65Sr0.35MnO3–CeO2 Composites for Solar Thermochemical Fuel Production. ACS Appl. Mater. Interfaces 2020, 12, 32622–32632. [Google Scholar] [CrossRef]
- Xu, M.; Ermanoski, I.; Stechel, E.B.; Deng, S. Oxygen pumping characteristics of YBaCo4O7+δ for solar thermochemical cycles. Chem. Eng. J. 2020, 389, 124026. [Google Scholar] [CrossRef]
- Babiniec, S.M.; Coker, E.N.; Miller, J.E.; Ambrosini, A. Doped calcium manganites for advanced high-temperature thermochemical energy storage. Int. J. Energy Res. 2016, 40, 280–284. [Google Scholar] [CrossRef]
- Albrecht, K.J.; Jackson, G.S.; Braun, R.J. Thermodynamically consistent modeling of redox-stable perovskite oxides for thermochemical energy conversion and storage. Appl. Energy 2016, 165, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, K.J.; Jackson, G.S.; Braun, R.J. Evaluating thermodynamic performance limits of thermochemical energy storage subsystems using reactive perovskite oxide particles for concentrating solar power. Sol. Energy 2018, 167, 179–193. [Google Scholar] [CrossRef]
- Imponenti, L.; Albrecht, K.J.; Wands, J.W.; Sanders, M.D.; Jackson, G.S. Thermochemical energy storage in strontium-doped calcium manganites for concentrating solar power applications. Sol. Energy 2017, 151, 1–13. [Google Scholar] [CrossRef]
- Imponenti, L.; Albrecht, K.J.; Kharait, R.; Sanders, M.D.; Jackson, G.S. Redox cycles with doped calcium manganites for thermochemical energy storage to 1000 °C. Appl. Energy 2018, 230, 1–18. [Google Scholar] [CrossRef]
- Lucio, B.; Romero, M.; González-Aguilar, J. Analysis of solid-state reaction in the performance of doped calcium manganites for thermal storage. Solid State Ion. 2019, 338, 47–57. [Google Scholar] [CrossRef]
- Pein, M.; Agrafiotis, C.; Vieten, J.; Giasafaki, D.; Brendelberger, S.; Roeb, M.; Sattler, C. Redox thermochemistry of Ca-Mn-based perovskites for oxygen atmosphere control in solar-thermochemical processes. Sol. Energy 2020, 198, 612–622. [Google Scholar] [CrossRef]
- Schrader, A.J.; Schieber, G.L.; Ambrosini, A.; Loutzenhiser, P.G. Experimental demonstration of a 5 kWth granular-flow reactor for solar thermochemical energy storage with aluminum-doped calcium manganite particles. Appl. Therm. Eng. 2020, 173, 115257. [Google Scholar] [CrossRef]
- Schrader, A.J.; Bush, H.E.; Ranjan, D.; Loutzenhiser, P.G. Aluminum-doped calcium manganite particles for solar thermochemical energy storage: Reactor design, particle characterization, and heat and mass transfer modeling. Int. J. Heat Mass Transf. 2020, 152, 119461. [Google Scholar] [CrossRef]
- Vieten, J.; Bulfin, B.; Starr, D.E.; Hariki, A.; de Groot, F.M.F.; Azarpira, A.; Zachäus, C.; Hävecker, M.; Skorupska, K.; Knoblauch, N.; et al. Redox Behavior of Solid Solutions in the SrFe1-xCuxO3-δ System for Application in Thermochemical Oxygen Storage and Air Separation. Energy Technol. 2019, 7, 131–139. [Google Scholar] [CrossRef] [Green Version]
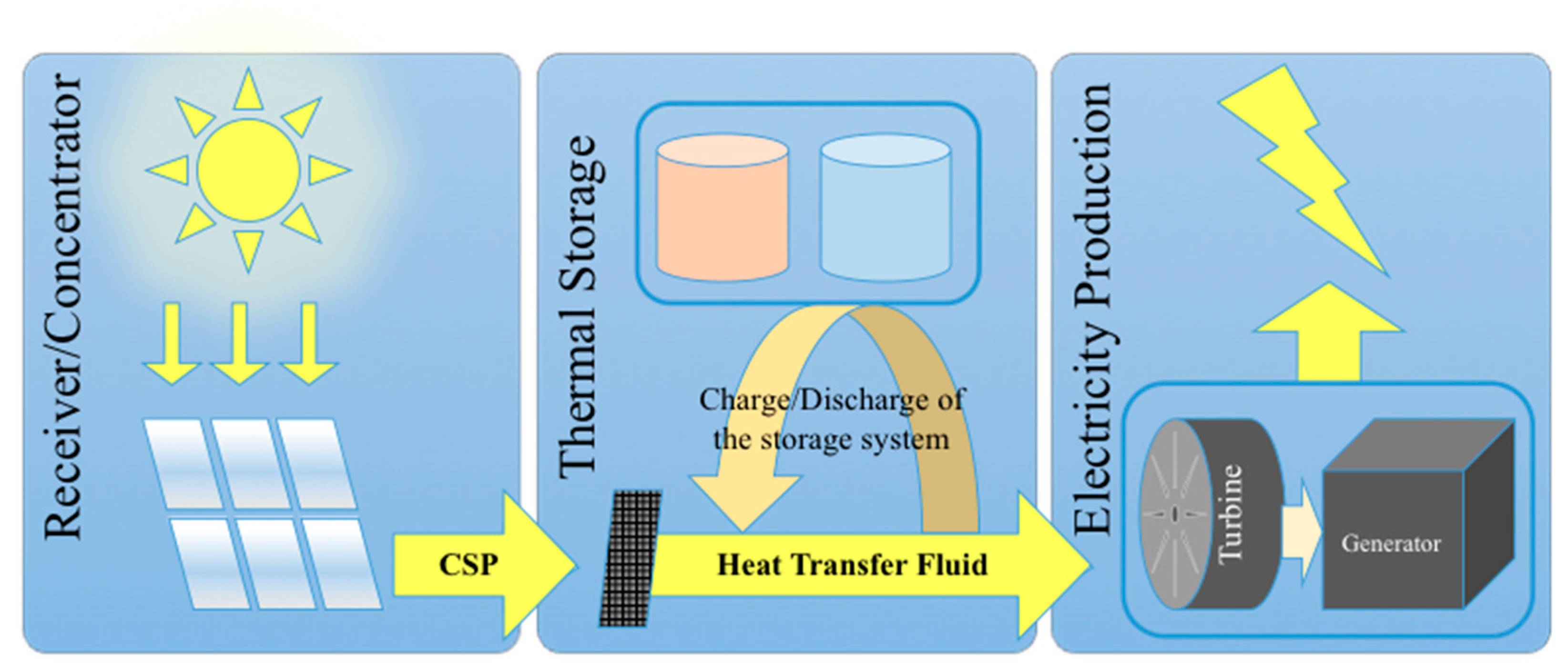




| Storage Type | Sensible Heat Storage (SHS) | Latent Heat Storage (LHT) | Thermochemical Energy Storage (TCES) |
|---|---|---|---|
| Gravimetric energy density | ~0.02–0.03 kWh/kg | ~0.05–0.1 kWh/kg | ~0.5–1 kWh/kg |
| Volumetric energy density | ~50 kWh/m3 | ~100 kWh/m3 | ~500 kWh/m3 |
| Storage temperature | Charging step temperature | Charging step temperature | Room temperature |
| Technology development | Industrial scale | Pilot scale | Laboratory and pilot scale |
| Energy storage period | Limited (Thermal loss) | Limited (Thermal loss) | Theoretically unlimited |
| Theoretical energy transport | Very short distance | Very short distance | Very long distance (>100 km) |
| Technology complexity | Simple | Medium | Complex |
| Drawbacks | Important thermal losses over time Large quantity of storage material required | Important thermal losses over time Corrosive materials Low heat conductivity | Expensive investment cost Complex technique |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, L.; Abanades, S. Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature. Energies 2020, 13, 5859. https://doi.org/10.3390/en13225859
André L, Abanades S. Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature. Energies. 2020; 13(22):5859. https://doi.org/10.3390/en13225859
Chicago/Turabian StyleAndré, Laurie, and Stéphane Abanades. 2020. "Recent Advances in Thermochemical Energy Storage via Solid–Gas Reversible Reactions at High Temperature" Energies 13, no. 22: 5859. https://doi.org/10.3390/en13225859





