An Effective Utilization of Solar Energy: Enhanced Photodegradation Efficiency of TiO2/Graphene-Based Composite
Abstract
:1. Introduction
2. TiO2/Graphene-Based Materials with Various Dimensional Morphologies
2.1. 0D TiO2 NP/2D Graphene/GO/rGO Sheet
2.1.1. Graphene/GO/rGO Sheet-Spherical TiO2 NPs
2.1.2. Spindle-Like TiO2 NP/Graphene/GO/rGO Sheet
2.2. 1D TiO2 Nanotube (Nanowire)/2D Graphene/GO/rGO Sheet
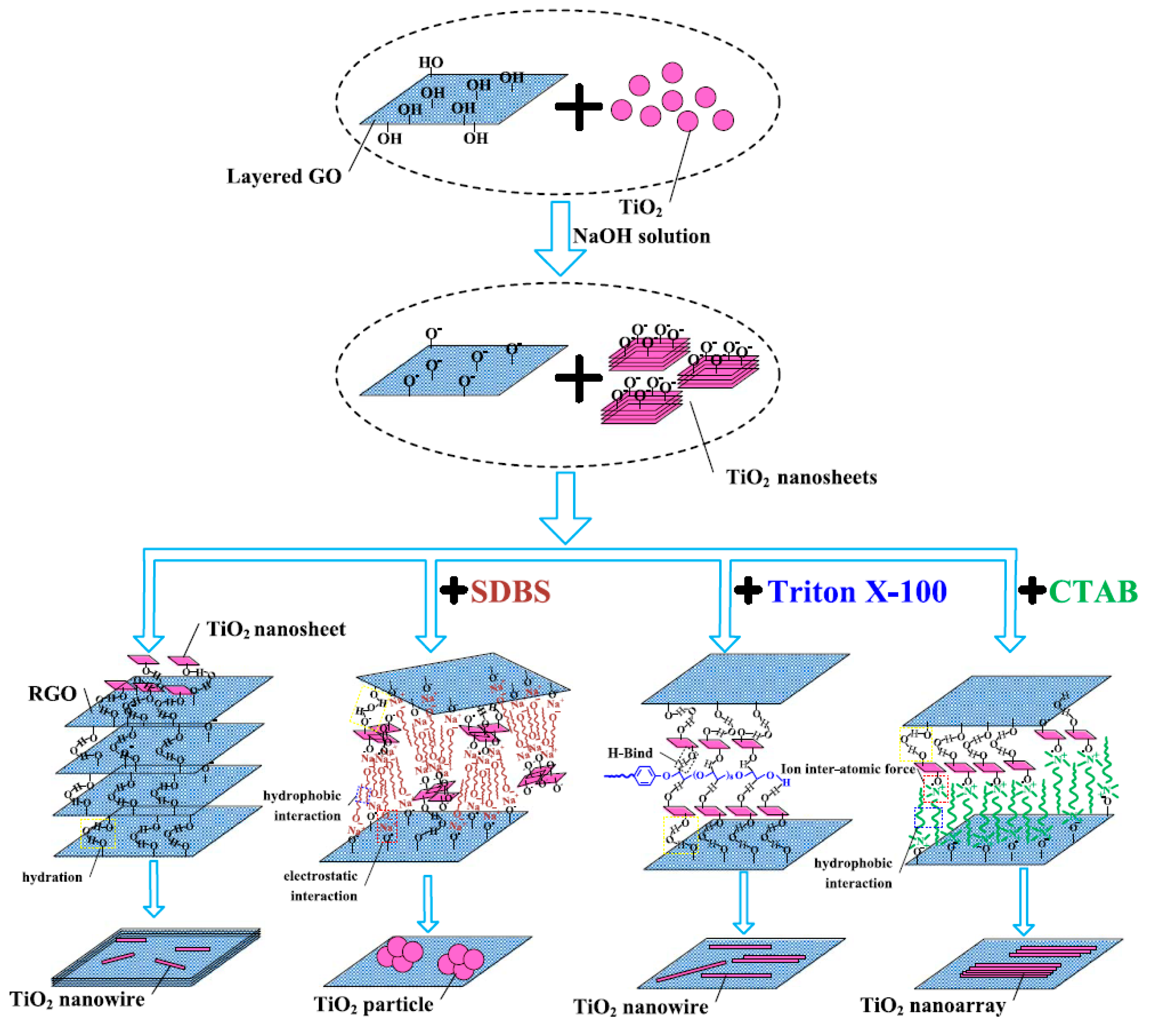
2.3. 2D TiO2 Nanosheet/2D Graphene/GO/rGO Sheet
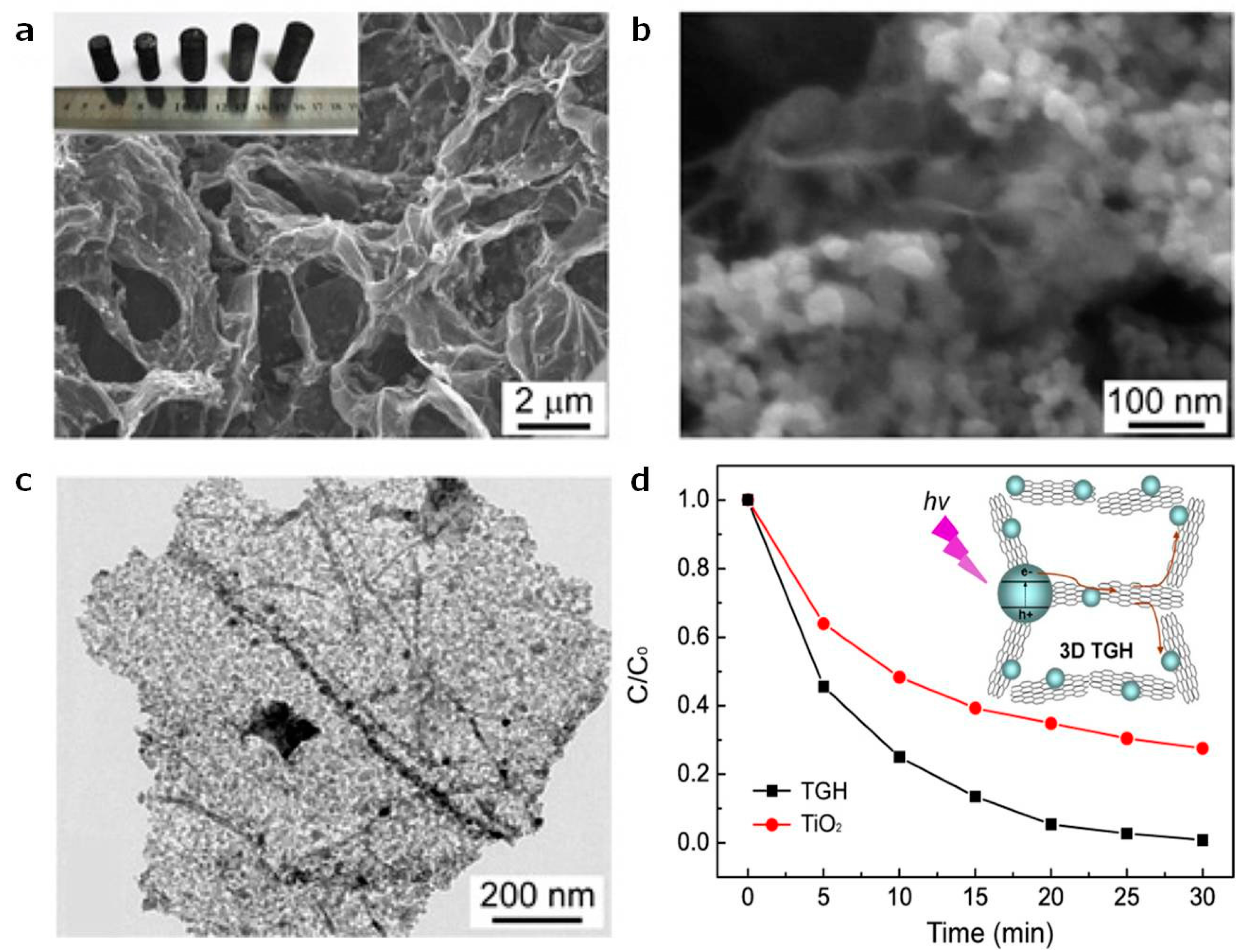
2.4. 3D TiO2 Hollow Nanospheres/2D Graphene/GO/rGO Sheets
3. TiO2/Graphene-Based Composite with Novel Construction or Additives
3.1. Novel Construction
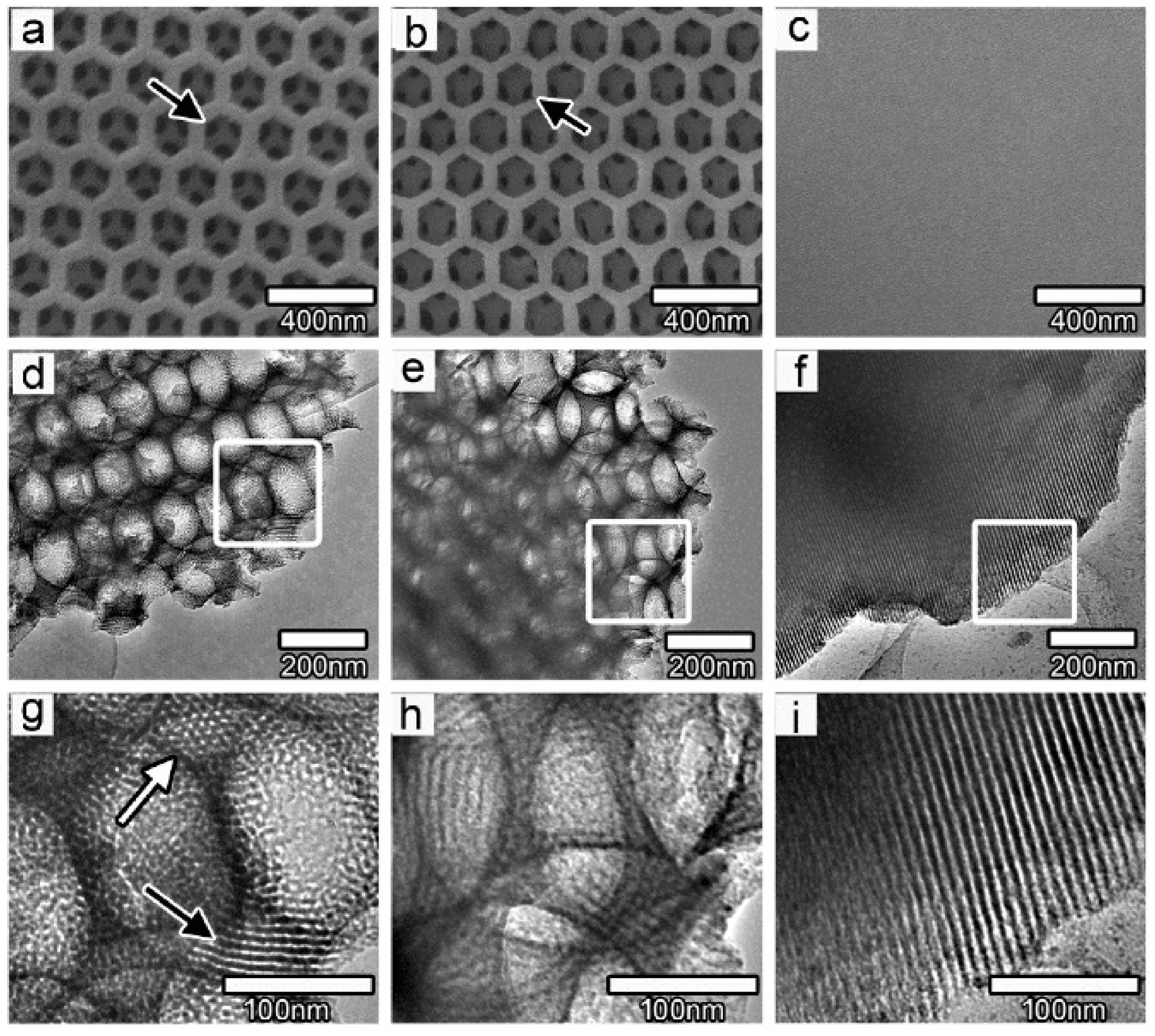
3.1.1. Hierarchically Ordered Macro-Mesoporous TiO2-Graphene Films
3.1.2. TiO2/Graphene Nanoflakes
3.1.3. TiO2 NP Surrounded by Mono or Bilayer Graphene
3.2. With Functional Additives
3.2.1. Graphene Coating of TiO2 NP Loaded on Mesoporous Silica
3.2.2. With Floating Autoclaved Cellular Concrete
3.2.3. Photocatalytic Reaction with Dye-Sensitization
3.2.4. Photocatalytic Reaction with a Cu(II) Graft
3.2.5. Phenylamine & Tourmaline Functionalization
3.2.6. Antibacterial Polyacylic Coating & Biopolymer
3.2.7. TiO2/Graphene Composite with Surfactant & Surface Fluorination
3.2.8. With Heteroatom Doping
4. Mechanism on Enhanced Photocatalytic Activity by Graphene-Based Material
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ohko, Y.; Luchi, K.; Niwa, C.; Tatsuma, T.; Nakashima, T.; Iguchi, T.; Kubota, Y.; Fujishima, A. 17β-estradiol degradation by TiO2 photocatalysis as a means of reducing estrogenic activity. Environ. Sci. Technol. 2002, 36, 4175–4181. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.L.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Choi, W. TiO2 photocatalytis for the redox conversion of aquatic pollutants. Aquat. Redox Chem. 2011, 10, 199–222. [Google Scholar] [CrossRef]
- Diesen, V.; Jonsson, M. Formation of H2O2 in TiO2 photocatalysis of oxygenated and deoxygenated aqueous system: A probe for photocatalytically produced hydroxyl radicals. J. Phys. Chem. C 2014, 118, 10083–10087. [Google Scholar] [CrossRef]
- Kuznetsov, V.N.; Serpone, N. On the origin of the spectral bands in the visible absorption spectra of visible-light-active TiO2 specimens analysis and assignments. J. Phys. Chem. C 2009, 113, 15110–15123. [Google Scholar] [CrossRef]
- Choi, J.; Park, H.; Hoffmann, M.R. Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J. Phys. Chem. C 2010, 114, 783–792. [Google Scholar] [CrossRef]
- Pan, H.; Zhang, Y.W.; Shenoy, V.B.; Gao, H.J. Effects of H-, N-, and (H, N)-doping on the photocatalytic activity of TiO2. J. Phys. Chem. C 2011, 115, 12224–12231. [Google Scholar] [CrossRef]
- Valero, J.M.; Obregón, S.; Colón, G. Active site considerations on the photocatalytic H2 evolution performance of Cu-doped TiO2 obtained by different doping methods. ACS Catal. 2014, 4, 3320–3329. [Google Scholar] [CrossRef]
- Štengl, V.; Popelková, D.; Vlácil, P. TiO2-graphene nanocomposite as high performance photocatalysts. J. Phys. Chem. C 2011, 115, 25209–25218. [Google Scholar] [CrossRef]
- Zhu, P.N.; Nair, A.S.; Peng, S.J.; Yang, S.Y.; Ramakrishna, S. Facile fabrication of TiO2-graphene composite with ehhanced photovoltaic and photocatalytic properties by electrospinning. ACS Appl. Mater. Interfaces 2012, 4, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhou, X.F.; Wu, J.H.; Yao, X.Y.; Liu, Z.P. Scalable synthesis of TiO2/graphene nanostructured composite with high-rate performance for lithium ion batteries. ACS Nano 2012, 6, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Akhtar, M.S.; Yang, O.B.; Stadler, F.J. Novel preparation of anatase TiO2@reduced graphene oxide hybrids for high-performance dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 6635–6642. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.A.L.; Wang, J.Z.; Lim, T.K.; Bi, X.Z.; Lee, W.C.; Lin, Q.S.; Chang, Y.T.; Lim, C.T.; Loh, K.P. High-performance graphene-titania platform for detection of phosphopeptides in cancer cells. Anal. Chem. 2012, 84, 6693–6700. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Shiota, S.; Hirakawa, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Titanium dioxide/reduced graphene oxide hybrid photocatalysts for efficient and selective partial oxidation of cyclohexane. ACS Catal. 2017, 7, 293–300. [Google Scholar] [CrossRef]
- Li, W.; Wang, F.; Feng, S.S.; Wang, J.X.; Sun, Z.K.; Li, B.; Li, Y.H.; Yang, J.P.; Elzatahry, A.A.; Xia, Y.Y.; et al. Sol-gel design strategy for ultradispersed TiO2 nanoparticles on graphene for high-performance lithium ion batteries. J. Am. Chem. Soc. 2013, 135, 18300–18303. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family—A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tang, Z.R.; Fu, X.Z.; Xu, Y.J. TiO2–graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-graphene truly different from other TiO2-carbon composite materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.H.; Kim, D.H.; Kim, H.I.; Bokare, A.D.; Choi, W. Platinum-like behavior of reduced graphene oxide as a cocatalyst on TiO2 for the efficient photocatalytic oxidation of arsenite. Environ. Sci. Technol. Lett. 2014, 1, 185–190. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xiao, F.; Guo, Y.L.; Wang, S.; Liu, Y.Q. One-pot self-assembled three-dimensional TiO2-graphene hydrogel with improved adsorption capacities and photocatalytic and electrochemical activities. ACS Appl. Mater. Interfaces 2013, 5, 2227–2233. [Google Scholar] [CrossRef] [PubMed]
- Gan, Z.X.; Wu, X.L.; Meng, M.; Zhu, X.B.; Yang, L.; Chu, P.K. Photothermal contribution to enhanced photocatalytic performance of graphene-based nanocomposites. ACS Nano 2014, 8, 9304–9310. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cai, W.M.; Long, M.C.; Zhou, B.X.; Wu, Y.H.; Wu, D.Y.; Feng, Y.J. Synthesis of visible-light responsive graphene oxide/TiO2 composites with p/n heterojunction. ACS Nano 2010, 4, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Y.; Wang, H.L.; Casalongue, H.S.; Chen, Z.; Dai, H.J. TiO2 nanocrystals grown on graphene as advanced photocatalytic hybrid materials. Nano Res. 2010, 3, 701–705. [Google Scholar] [CrossRef]
- Zhu, C.Y.; Liu, G.G.; Han, K.; Ye, H.Q.; Wei, S.C.; Zhou, Y.H. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process. Process Intensif. 2017, 120, 20–26. [Google Scholar] [CrossRef]
- Pu, S.Y.; Zhu, R.X.; Ma, H.; Deng, D.L.; Pei, X.J.; Qi, F.; Chu, W. Facile in-situ design strategy to disperse TiO2 nanoparticles on graphene for the enhanced photocatalytic degradation of rhodamine 6G. Appl. Catal. B Environ. 2017, 218, 208–219. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Maiti, D.; Saha, A.; Devi, P.S. Shape transition of TiO2 nanocube to nanospindle embedded on reduced graphene oxide with enhanced photocatalytic activity. Cryst. Growth Des. 2016, 16, 6922–6932. [Google Scholar] [CrossRef]
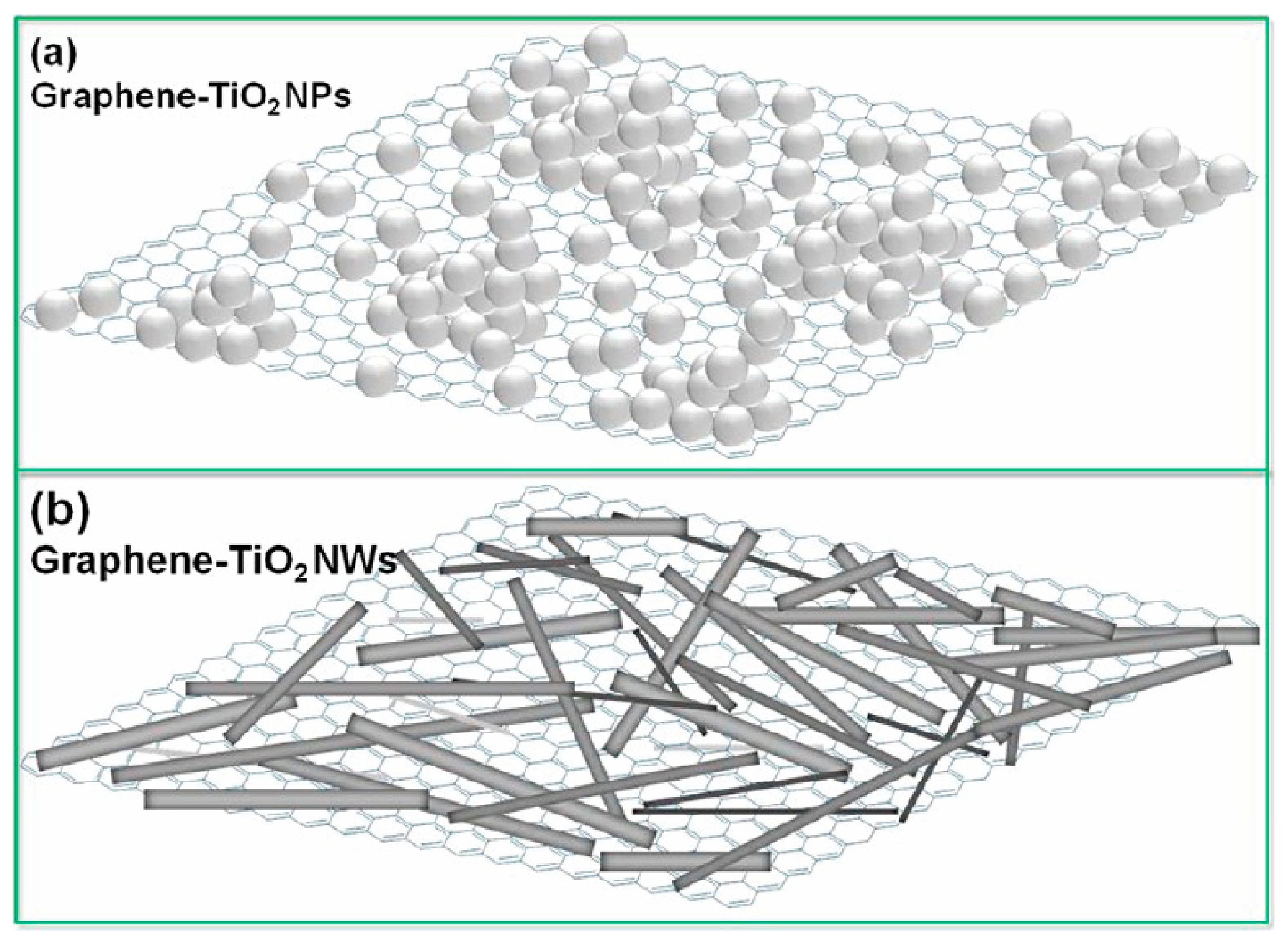
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z.Y. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, H.; Guo, L.H.; Zhao, L. Two-dimensional interface engineering of a titania-graphene nanosheet composite for improved photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 13035–13041. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, J.; Cheng, H.; Zhao, Y.; Liu, L.; Han, X. One-step preparation of graphene-supported anatase TiO2 with exposed {001} facets and mechanism of enhanced photocatalytic properties. ACS Appl. Mater. Interfaces 2013, 5, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Jiu, H.; Ni, C.; Zhang, X.; Xu, M. Graphene-based hollow TiO2 composites with enhanced photocatalytic activity for removal of pollutants. J. Alloys Compd. 2015, 86, 82–89. [Google Scholar] [CrossRef]
- Fitri, M.A.; Ota, M.; Hirota, Y.; Uchida, Y.; Hara, K.; Ino, D.; Nishiyama, N. Fabrication of TiO2-graphene photocatalyst by direct chemical vapor deposition and its anti-fouling property. Mater. Chem. Phys. 2017, 198, 42–48. [Google Scholar] [CrossRef]
- Fotiou, T.; Triantis, T.M.; Kaloudis, T.; Pastrana-Martínez, L.M.; Likodimos, V.; Falaras, P.; Silva, A.M.T.; Hiskia, A. Photocatalytic degradation of microcystin-LR and off-odor compounds in water under UV-A and solar light with a nanostructured photocatalyst based on reduced graphene oxide-TiO2 composite: Identification of intermediate products. Ind. Eng. Chem. Res. 2013, 52, 13991–14000. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Gan, Z.X.; Wu, X.L.; Zhou, G.X.; Shen, J.C.; Chu, P.K. Is there real upconversion photoluminescence from graphene quantum dots? Adv. Opt. Mater. 2013, 1, 554–558. [Google Scholar] [CrossRef]
- Jiang, B.J.; Tian, C.G.; Pan, Q.J.; Jiang, Z.; Wang, J.Q.; Yan, W.; Fu, H.G. Enhanced photocatalytic activity and electron transfer mechanisms of graphene/TiO2 with exposed {001} facets. J. Phys. Chem. C 2011, 115, 23718–23725. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Park, A.R.; Zhang, K.; Park, J.H.; Yoo, P.J. Green synthesis of biphasic TiO2-reduced graphene oxide nanocomposites with highly enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2012, 4, 3893–3901. [Google Scholar] [CrossRef] [PubMed]
- Li, H.N.; Zhu, M.Y.; Chen, W.; Xu, L.; Wang, K. Non-light-driven reduced graphene oxide anchored TiO2 nanocatalysts with enhanced catalytic oxidation performance. J. Colloid Interface Sci. 2017, 507, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.D.; Mariano, R.G.; Vu, K.; Nour, N.; Seitz, O.; Chabal, Y.; Balkus, K.J., Jr. Hydrothermal synthesis of graphene-TiO2 nanotube composites with enhanced photocatalytic activity. ACS Catal. 2012, 2, 949–956. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, L.; Liu, R.; Gao, Z.; Yang, X.; Tu, Z.; Yang, F.; Ye, Z.; Cui, L.; Xu, C.; et al. Hydrothermal synthesis of N-doped TiO2 nanowires and N-doped graphene heterostructures with enhanced photocatalytic properties. J. Alloys Compd. 2016, 656, 24–32. [Google Scholar] [CrossRef]
- Wang, W.S.; Wang, D.H.; Qu, W.G.; Lu, L.Q.; Xu, A.W. Large ultrathin anatase TiO2 nanosheets with exposed {001} facets on graphene for enhanced visible light photocatalytic activity. J. Phys. Chem. C 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, J.; Dong, X.L.; Zhang, F.; Ma, L.; Fei, X.; Zhang, X.; Ma, H. Synthesis and catalytic performance of hierarchical TiO2 hollow sphere/reduced graphene oxide hybrid nanostructures. J. Alloys Compd. 2016, 656, 181–188. [Google Scholar] [CrossRef]
- Du, J.; Lai, X.Y.; Yang, N.L.; Zhai, J.; Kisailus, D.; Su, F.; Wang, D.; Jiang, L. Hierarchically ordered macro–mesoporous TiO2–graphene composite films: Improved mass transfer, reduced charge recombination, and their enhanced photocatalytic activities. ACS Nano 2011, 5, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Byeon, J.H.; Kim, Y.W. Gas-phase self-assembly of highly ordered titania@graphene nanoflakes for enhancement in photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 3959–3966. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Yamahana, D.; Yamashita, H. Graphene coating of TiO2 nanoparticles loaded on mesoporous silica for enhancement of photocatalytic activity. J. Phys. Chem. C 2010, 114, 15049–15053. [Google Scholar] [CrossRef]
- Suave, J.; Amorim, S.M.; Moreira, R.F.P.M. TiO2-graphene nanocomposite supported on floating autoclaved cellular concrete for photocatalytic removal of organic compounds. J. Environ. Chem. Eng. 2017, 5, 3215–3223. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Ming, T.S.; Wang, X.; Yu, H.G.; Yu, J.G.; Wang, Y.G.; Lei, M. Dye-sensitization-induced visible-light reduction of graphene oxide for the enhanced TiO2 photocatalytic performance. ACS Appl. Mater. Interfaces 2013, 5, 2924–2929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guo, L.H.; Wang, D.B.; Zhao, L.X.; Wan, B. Light-induced efficient molecular oxygen activation on a Cu(II)-grafted TiO2/graphene photocatalyst for phenol degradation. ACS Appl. Mater. Interfaces 2015, 7, 1816–1823. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.G.; Xiao, P.; Tian, J.; Wang, F.Z.; Yu, J.G. Phenylamine-functionalized rGO/TiO2 photocatalysts: Spatially separated adsorption sites and tunable photocatalytic selectivity. ACS Appl. Mater. Interfaces 2016, 8, 29470–29477. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.L.; Zhao, M.; Hu, L.H.; Ye, J.H.; Wang, D.F. Synthesis of graphene/tourmaline/TiO2 composites with enhanced activity for photocatalytic degradation of 2-propanol. Chin. J. Catal. 2017, 38, 1307–1314. [Google Scholar] [CrossRef]
- Nosrati, R.; Olad, A.; Shakoori, S. Preparation of an antibacterial, hydrophilic and photocatalytically active polyacrylic coating using TiO2 nanoparticles sensitized by graphene oxide. Mater. Sci. Eng. C 2017, 80, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Natarajan, T.S.; Sheikh, M.U.D.; Bano, M.; Khan, F. Self-organized graphene oxide and TiO2 nanoparticles incorporated alginate/carboxymethyl cellulose nanocomposites with efficient photocatalytic activity under direct sunlight. J. Photochem. Photobiol. A Chem. 2017, 346, 113–125. [Google Scholar] [CrossRef]
- Martín-García, B.; Velázquez, M.M.; Rossella, F.; Bellani, V.; Diez, E.; Fierro, J.L.G.; Pérez-Hernández, J.A.; Hernández-Toro, J.; Claramunt, S.; Cirera, A. Functionalization of reduced graphite oxide sheets with a zwitterionic surfactant. ChemPhysChem 2012, 13, 3682–3690. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, D.H.; Li, X.L.; Xu, W.; Wang, D.Y.; Graff, G.L.; Bennett, W.D.; Nie, Z.M.; Saraf, L.V.; Aksay, I.A.; et al. Hierarchically porous graphene as a lithium-air battery electrode. Nano Lett. 2011, 11, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.S.; Muhammad, S.; Wu, Q.; Zhao, Y.; Jiao, Q.Z. Surfactant-assisted hydrothermal synthesis of TiO2/reduced graphene oxide nanocomposites and their photocatalytic performances. J. Solid State Chem. 2017, 253, 113–120. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, L.J.; Xiao, M.; Li, H.; Pan, X.J.; Jiang, F.Z. One-step hydrothermal synthesis of surface fluorinated TiO2/reduced graphene oxide nanocomposites for photocatalytic degradation of estrogens. Mater. Sci. Semicond. Process. 2015, 40, 183–193. [Google Scholar] [CrossRef]
- Xu, Y.; Mo, Y.P.; Tian, J.; Wang, P.; Yu, H.G.; Yu, J.G. The synergistic effect of graphitic N and pyrrolic N for the enhanced photocatalytic performance of nitrogen-doped graphene/TiO2 nanocomposites. Appl. Catal. B Environ. 2016, 181, 810–817. [Google Scholar] [CrossRef]
- Chen, Z.P.; Ma, J.F.; Yang, K.; Feng, S.; Tan, W.S.; Tao, Y.X.; Mao, H.H.; Kong, Y. Preparation of S-doped TiO2-three dimensional graphene aerogels as a highly efficient photocatalyst. Synth. Met. 2017, 231, 51–57. [Google Scholar] [CrossRef]
- Gu, Y.J.; Xing, M.Y.; Zhang, J.L. Synthesis and photocatalytic activity of graphene based doped TiO2 nanocomposites. Appl. Surf. Sci. 2014, 319, 8–15. [Google Scholar] [CrossRef]
- Silva, C.G.; Faria, J.L. Photocatalytic oxidation of benzene derivatives in aqueous suspensions: Synergic effect induced by the introduction of carbon nanotubes in a TiO2 matrix. Appl. Catal. B Environ. 2010, 101, 81–89. [Google Scholar] [CrossRef]
- Caridad, J.M.; Rossella, F.; Bellani, V.; Maicas, M.; Patrini, M.; Díez, E. Effects of particle contamination and substrate interaction on the Raman response of unintentionally doped graphene. J. Appl. Phys. 2010, 108, 085321. [Google Scholar] [CrossRef]
- Caridad, J.M.; Rossella, F.; Bellani, V.; Grandi, M.S.; Díez, E. Automated detection and characterization of graphene and few-layer graphite via Raman spectroscopy. J. Raman Spectrosc. 2011, 42, 286–293. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Mayer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 2, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chen, G.; Joshi, P.; Tadigadapa, S.; Eklund, P.C. Raman scattering from high-frequency phonons in supported n-graphene layer films. Nano Lett. 2006, 6, 2667–2673. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, C.; Hartschuh, A.; Lidorikis, E.; Qian, H.; Harutyunyan, H.; Gokus, T.; Novoselov, K.S.; Ferrari, A.C. Rayleigh imaging of graphene and graphene layers. Nano Lett. 2007, 7, 2711–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sojoudi, H.; Baltazar, J.; Henderson, C.; Graham, S. Impact of post-growth thermal annealing and environmental exposure on the unintentional doping of CVD graphene films. J. Vac. Sci. Technol. B 2012, 30, 041213. [Google Scholar] [CrossRef]
- Yang, R.; Huang, Q.S.; Chen, X.L.; Zhang, G.Y.; Gao, H.J. Substrate doping effects on Raman spectrum of epitaxial graphene on SiC. J. Appl. Phys. 2010, 107, 034305. [Google Scholar] [CrossRef]
- Lin, S.S.; Chen, B.G.; Pan, C.T.; Hu, S.; Tian, P.; Tong, L.M. Unintentional doping induced splitting of G peak in bilayer graphene. Appl. Phys. Lett. 2011, 99, 233110. [Google Scholar] [CrossRef]
- Ni, Z.H.; Yu, T.; Luo, Z.Q.; Wang, Y.Y.; Liu, L.; Wong, C.P.; Miao, J.M.; Huang, W.; Shen, Z.X. Probing charged impurities in suspended graphene using Raman spectroscopy. ACS Nano 2009, 3, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Berciaud, S.; Ryu, S.; Brus, L.E.; Heinz, T.F. Probing the intrinsic properties of exfoliated graphene: Raman spectroscopy of free-standing monolayers. Nano Lett. 2009, 9, 346–352. [Google Scholar] [CrossRef] [PubMed]
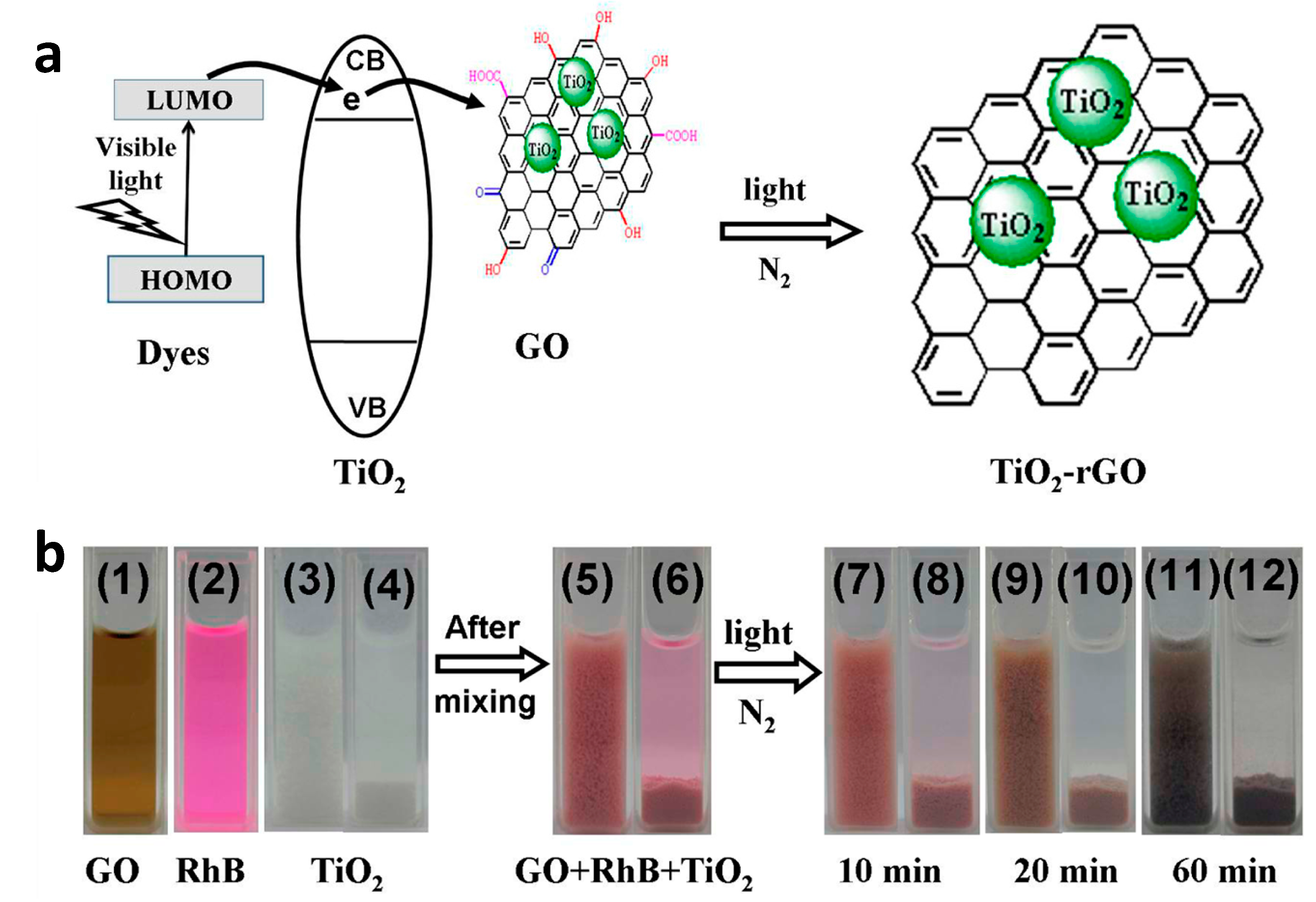

| [Ref.] Catalyst Abbreviation | Chemical to Be Degraded | Photocatalytic Activity | Surface Area (m2/g) | Light Source | Band Gap (eV) | Active Radical |
|---|---|---|---|---|---|---|
| [17] TiO2/Graphene | Benzene & MB | much higher than that of bare TiO2 | UV& visible light(with a UV cutoff filter, λ > 400 nm) | 2.83 | hydroxyl radical & superoxide redical | |
| [18] TiO2/rGO | Arsenite | much the same as Pt/TiO2 | 51.9 | λ ≥ 320 nm | superoxide & OH radicals | |
| [19] TGH | MB | complete degradation within 30 min | 267.98 | UV, centered at 365nm | ||
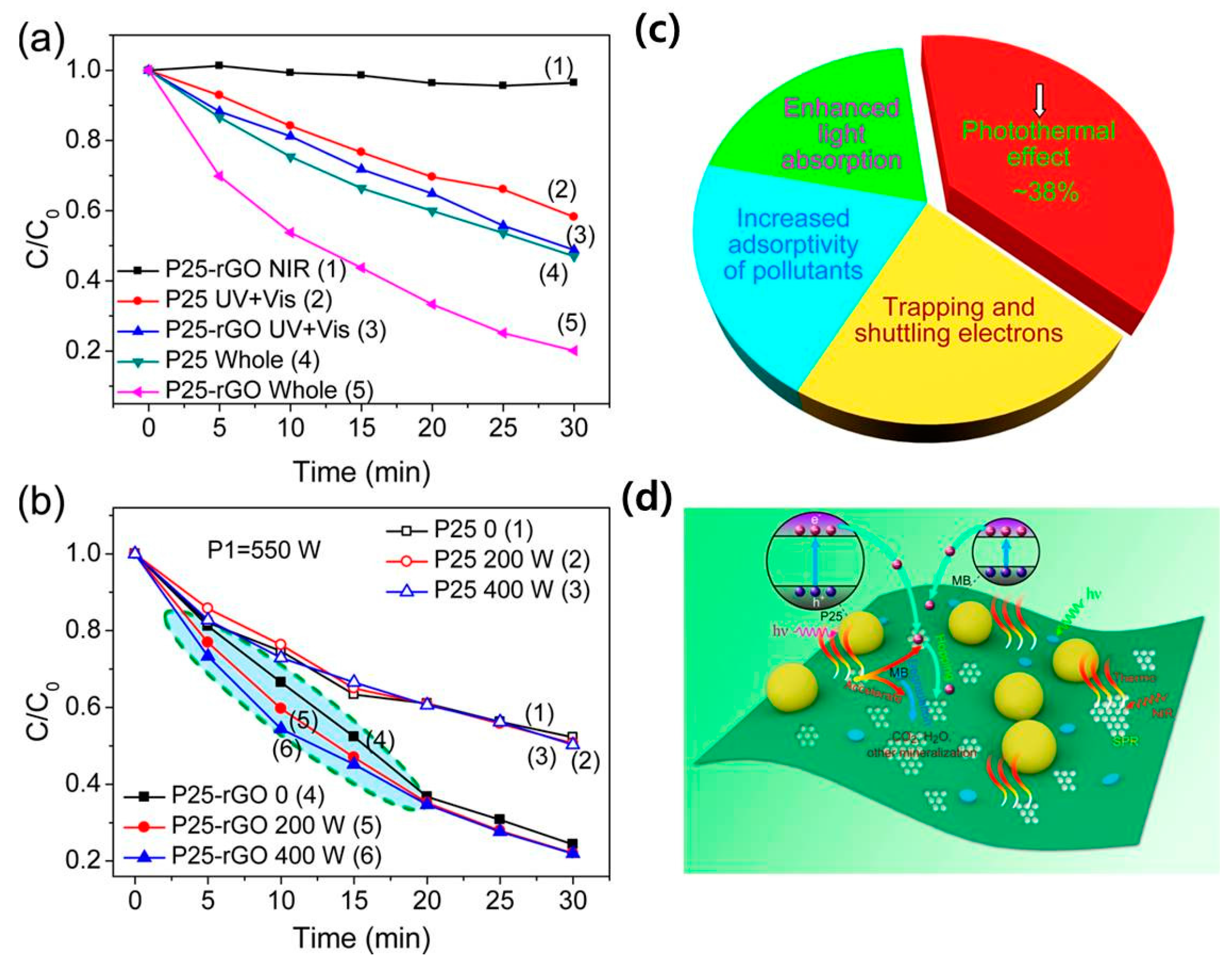
| [20] TiO2/rGO | MB | photothermal effect contributes ∼38% degradation | xenon lamp with a 720 nm short-path filter and a 720 nm long pass filter | |||
| [21] TiO2/GO | MO | improved performance than P25 | λ > 400 nm | 2.43 | ||
| [22] TiO2/GO | RhD B | a three-fold photocatalytic enhancement over P25 | 190 | mercury lamp | ||
| [23] TiO2/GO | MB | 92% MB degraded after 110 min irradiation | mercury lamp | |||
| [24] TiO2/rGO | RhD 6G | more than triple higher photodegradation rate than P25 | mercury lamp λ > 400 nm | 2.71 | •OH radicals | |
| [25] TiO2 spindle/rGO | MG | a 6 fold increase in efficiency over the native TiO2 cube | 89.34 | mercury lamp λ ≥ 420 nm | 2.91 | |
| [26] TiO2 NW/GO | MB | much higher than TiO2 NP/GO and pure TiO2 NWs or NPs | solar light | |||
| [27] TiO2nanosheet/Graphene | RhD B & 2,4-dichlorophenol | 95% dyes degraded within 60 min | mercury lamp centered at 365 nm | •OH & O2•− | ||
| [28] TiO2 nanosheet/rGO | MB | higher than that of TiO2 | mercury lamp | •OH radicals & holes | ||
| [29] TiO2 hollow sphere/GO | RhD B | 95% degraded within 60 min | mercury lamp | 2.51 | hydroxyl radicals | |
| [30] TiO2/Graphene | MB & estradiol | 2 fold higher photocatalytic activity than TiO2 | xenon lamp, centered at 350 nm | 2.95 |
| Material | Abbreviation | Formulation |
|---|---|---|
| graphene | two-dimensional sheet of graphite arrayed hexagonally with monolayer thickness | |
| graphite oxide | oxidized graphite with oxygen functional groups on the basal planes and increased interlayer spacing | |
| graphene oxide | GO | exfoliated form of graphite oxide |
| reduced graphene oxide | rGO | reduced form of graphene oxide via a chemical, thermal, solvothermal etc. process |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, P.; Zhao, P.; Wang, Y.; Liu, B.; Dong, M. An Effective Utilization of Solar Energy: Enhanced Photodegradation Efficiency of TiO2/Graphene-Based Composite. Energies 2018, 11, 630. https://doi.org/10.3390/en11030630
Huo P, Zhao P, Wang Y, Liu B, Dong M. An Effective Utilization of Solar Energy: Enhanced Photodegradation Efficiency of TiO2/Graphene-Based Composite. Energies. 2018; 11(3):630. https://doi.org/10.3390/en11030630
Chicago/Turabian StyleHuo, Peipei, Peng Zhao, Yin Wang, Bo Liu, and Mingdong Dong. 2018. "An Effective Utilization of Solar Energy: Enhanced Photodegradation Efficiency of TiO2/Graphene-Based Composite" Energies 11, no. 3: 630. https://doi.org/10.3390/en11030630






