Effect of Temperature and Mineral Matter on the Formation of NOx Precursors during Fast Pyrolysis of 2,5-Diketopiperazine
Abstract
:1. Introduction
2. Experimental
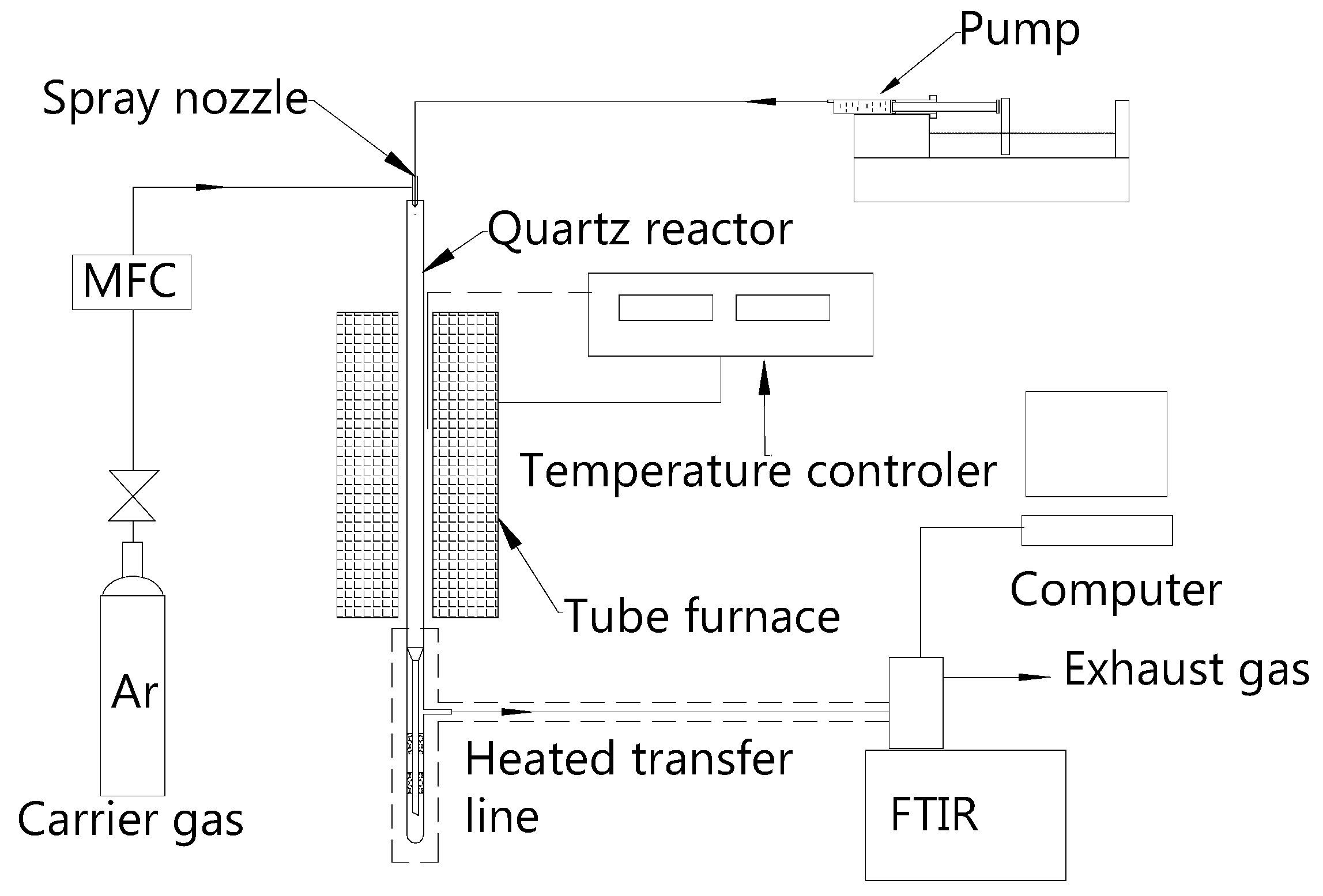
2.1. Apparatus
2.2. Materials
2.3. Pyrolysis Experiments
3. Results and Discussion
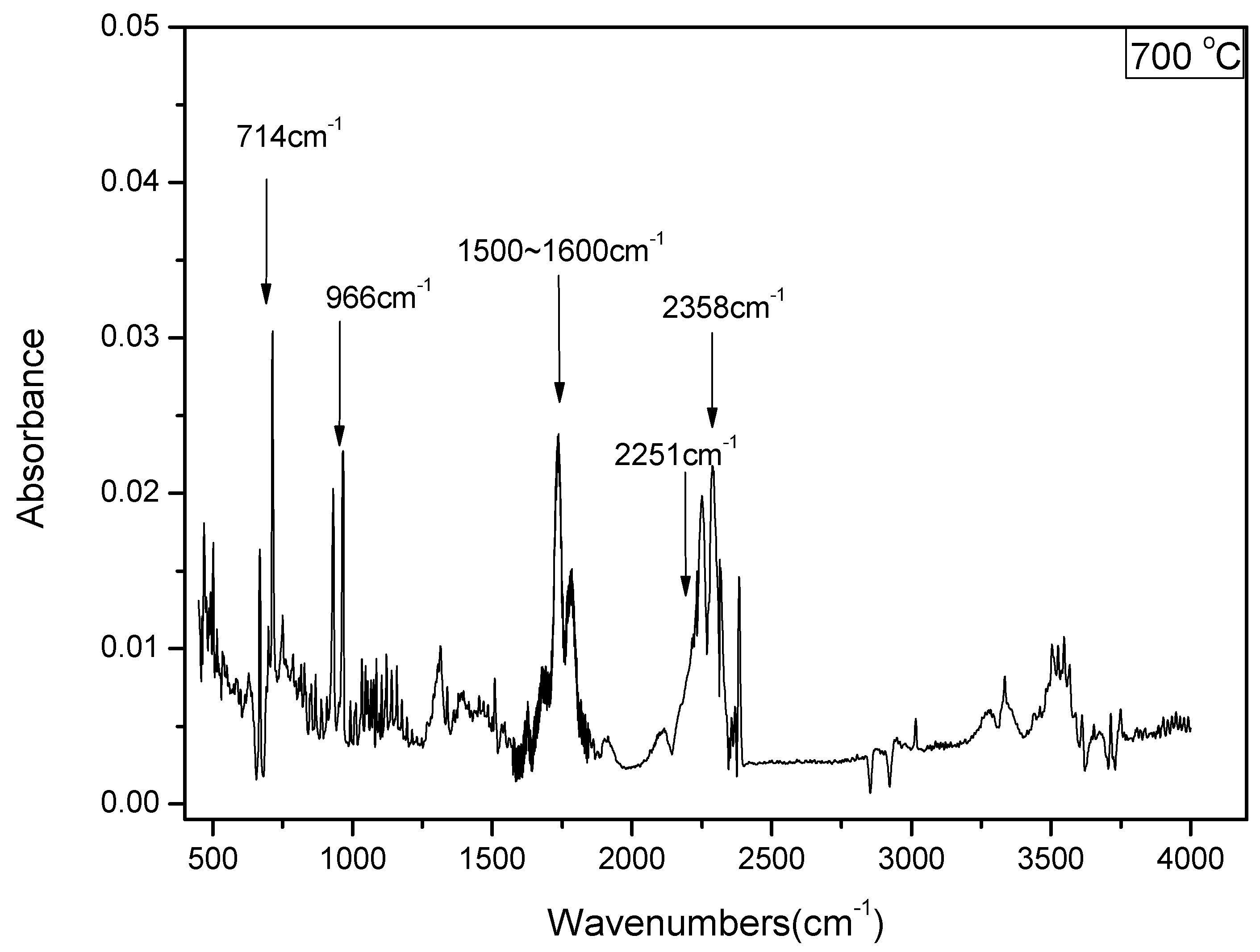
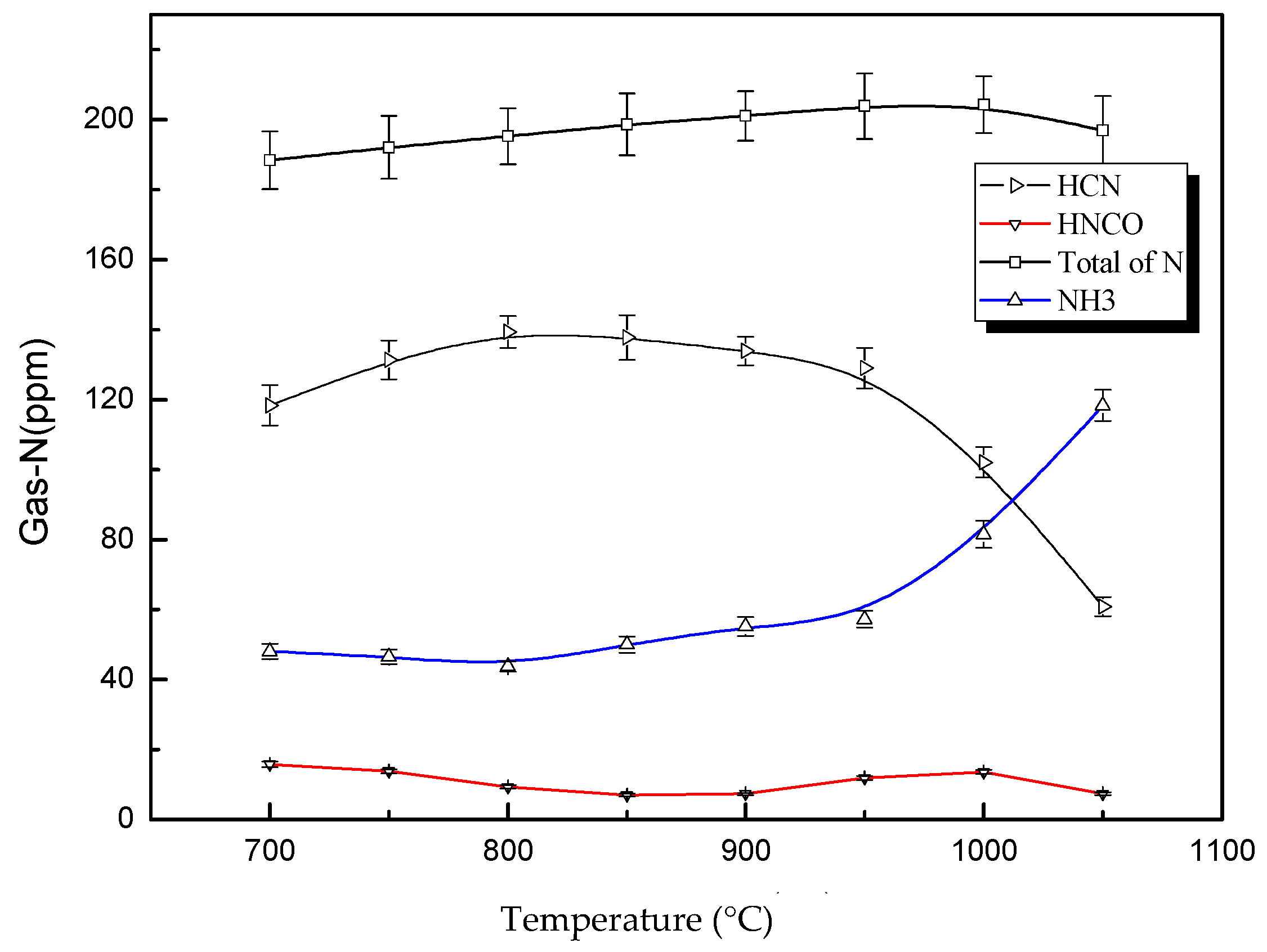
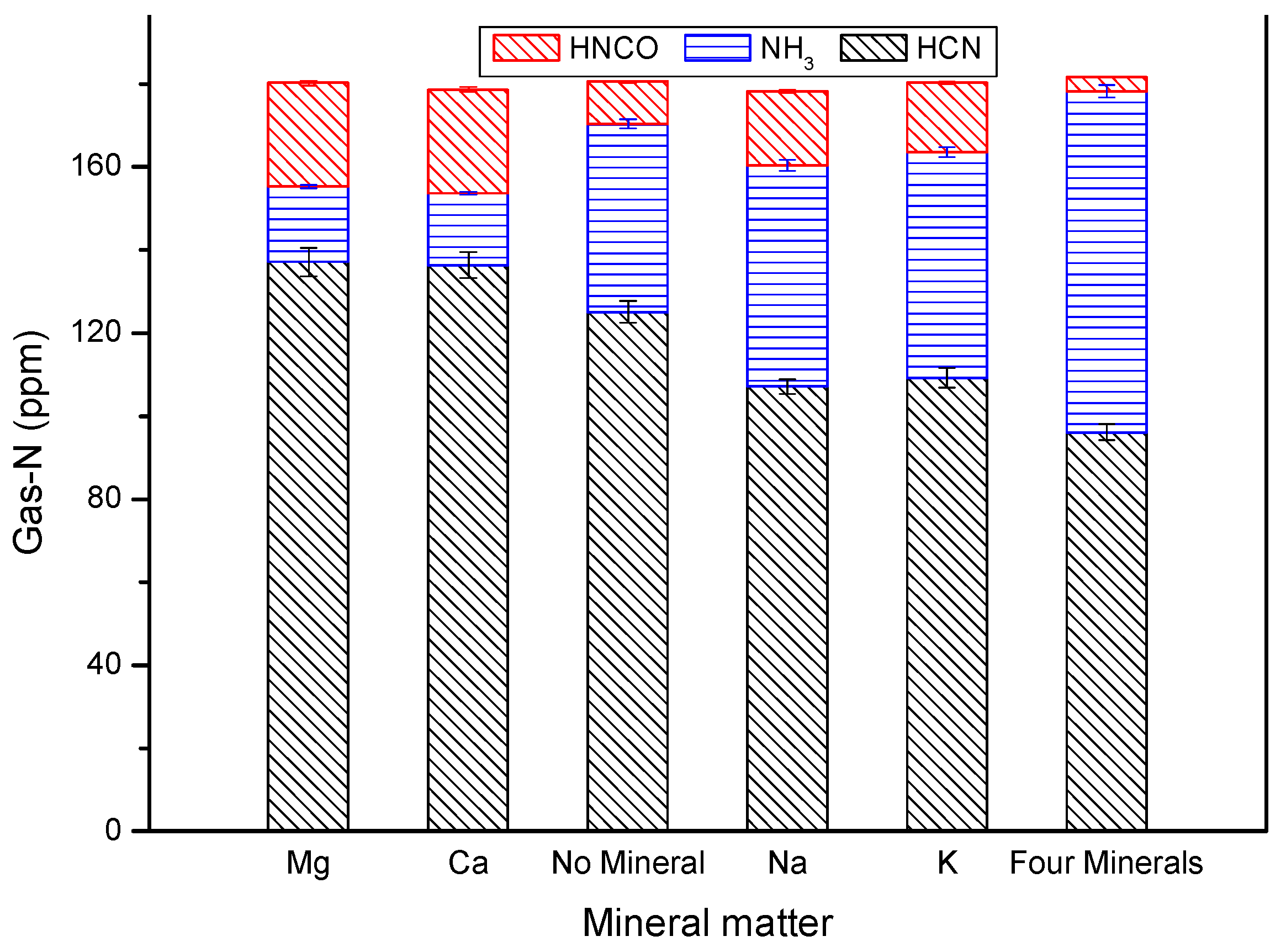
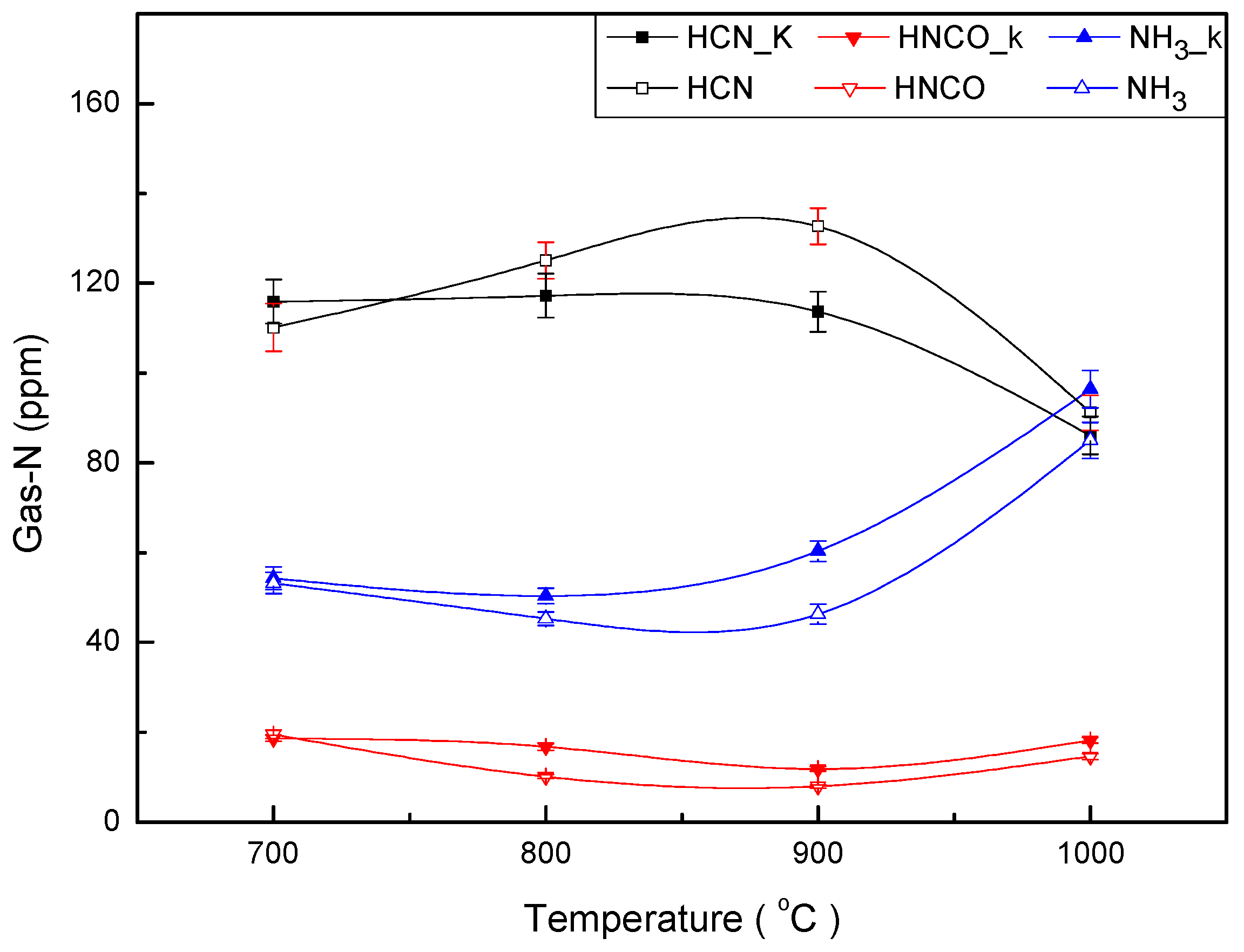
3.1. Distribution of Gas-N Products for Fast DKP Pyrolysis under Stable Temperature
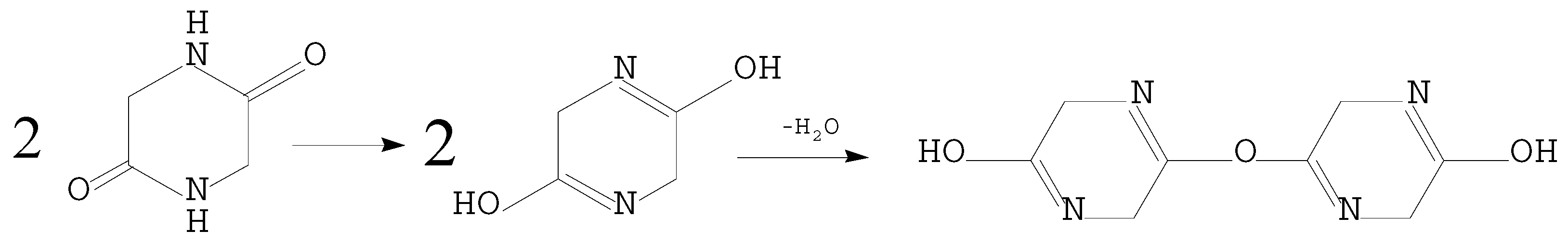
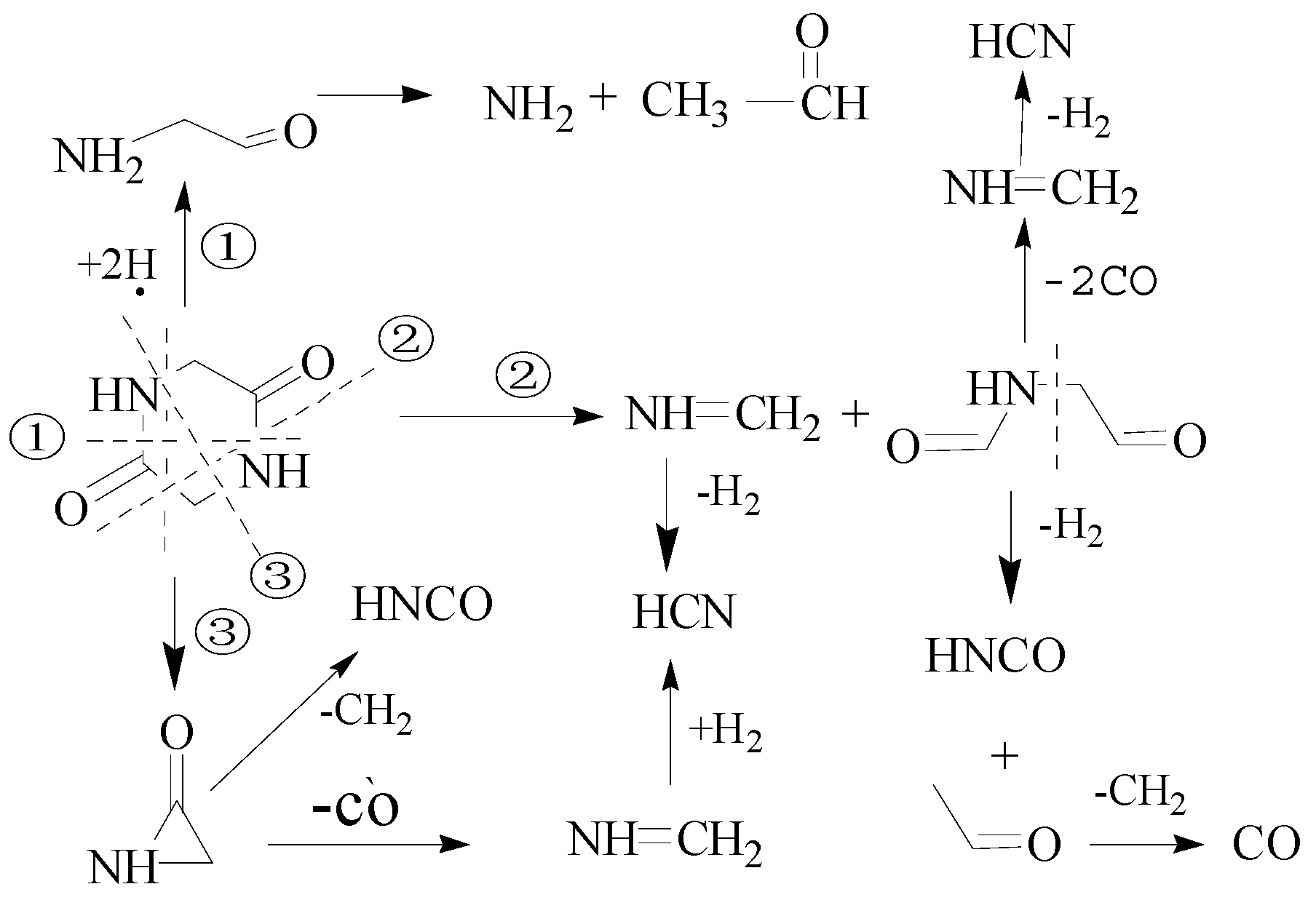
3.2. Decomposition Path Analysis of Fast Pyrolysis DKP
3.3. Analysis of the Catalytic Effect of Minerals on Rapid Pyrolysis of DKP
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jones, J.M.; Lea-Langton, A.R.; Ma, L.; Pourkashanian, M.; Williams, A. Pollutants Generated by the Combustion of Solid Biomass Fuels; Springer: London, UK, 2014. [Google Scholar]
- Chen, H.; Si, Y.; Chen, Y.; Yang, H.; Chen, D.; Chen, W. NOx precursors from biomass pyrolysis: Distribution of amino acids in biomass and Tar-N during devolatilization using model compounds. Fuel 2017, 187, 367–375. [Google Scholar] [CrossRef]
- Zhou, J.-Q.; Gao, P.; Dong, C.-Q.; Yang, Y.-P. TG-FTIR analysis of nitrogen conversion during straw pyrolysis: A model compound study. J. Fuel Chem. Technol. 2015, 43, 1427–1432. [Google Scholar] [CrossRef]
- Yang, J.; Chen, H.; Zhao, W.; Zhou, J. TG-FTIR-MS study of pyrolysis products evolving from peat. J. Anal. Appl. Pyrolysis 2016, 117, 296–309. [Google Scholar] [CrossRef]
- Ghouma, I.; Jeguirim, M.; Sager, U.; Limousy, L.; Bennici, S.; Däuber, E.; Asbach, C.; Ligotski, R.; Schmidt, F.; Ouederni, A. The potential of activated carbon made of agro-industrial residues in nox immissions abatement. Energies 2017, 10, 1508. [Google Scholar] [CrossRef]
- Sánchez, E.; Rivero, R.M.; Ruiz, J.M.; Romero, L. Changes in biomass, enzymatic activity and protein concentration in roots and leaves of green bean plants (Phaseolus vulgaris L. Cv. Strike) under high NH4NO3 application rates. Sci. Hortic. 2004, 99, 237–248. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Yang, X.; Hu, L.; Liu, Y.; Wang, C. Evaluate the pyrolysis pathway of glycine and glycylglycine by TG-FTIR. J. Anal. Appl. Pyrolysis 2007, 80, 247–253. [Google Scholar] [CrossRef]
- Jie, L.; Yuwen, L.; Jingyan, S.; Zhiyong, W.; Ling, H.; Xi, Y.; Cunxin, W. The investigation of thermal decomposition pathways of phenylalanine and tyrosine by TG-FTIR. Thermochim. Acta 2008, 467, 20–29. [Google Scholar] [CrossRef]
- Hansson, K.-M.; Samuelsson, J.; Åmand, L.-E.; Tullin, C. The temperature’s influence on the selectivity between HNCO and HCN from pyrolysis of 2,5-diketopiperazine and 2-pyridone. Fuel 2003, 82, 2163–2172. [Google Scholar] [CrossRef]
- Hansson, K.-M.; Samuelsson, J.; Tullin, C.; Åmand, L.-E. Formation of HNCO, HCN, and NH3 from the pyrolysis of bark and nitrogen-containing model compounds. Combust. Flame 2004, 137, 265–277. [Google Scholar] [CrossRef]
- Voorhees, K.J.; Zhang, W.; Hendricker, A.D.; Murugaverl, B. An investigation of the pyrolysis of oligopeptides by curie-point pyrolysis—tandem mass spectrometry. J. Anal. Appl. Pyrolysis 1994, 30, 1–16. [Google Scholar] [CrossRef]
- Langhammer, M.; Lüderwald, I.; Simons, A. Analytical pyrolysis of proteins. Fresenius J. Anal. Chem. 1986, 324, 5–8. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, L.; Yang, X. Pyrolysis characteristics and pathways of protein, lipid and carbohydrate isolated from microalgae Nannochloropsis sp. Bioresour. Technol. 2017, 229, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Chiavari, G.; Galletti, G.C. Pyrolysis—Gas chromatography/mass spectrometry of amino acids. J. Anal. Appl. Pyrolysis 1992, 24, 123–137. [Google Scholar] [CrossRef]
- Rodante, F.; Marrosu, G.; Catalani, G. Thermal analysis of some α-amino acids with similar structures. Thermochim. Acta 1992, 194, 197–213. [Google Scholar] [CrossRef]
- Tsuge, S.; Matsubara, H. High-resolution pyrolysis-gas chromatography of proteins and related materials. J. Anal. Appl. Pyrolysis 1985, 8, 49–64. [Google Scholar] [CrossRef]
- Simmonds, P.G.; Medley, E.E.; Ratcliff, M.A.; Shulman, G.P. Thermal decomposition of aliphatic monoaminomonocarboxylic acids. Anal. Chem. 1972, 44, 2060–2066. [Google Scholar] [CrossRef]
- Cao, J.-P.; Li, L.-Y.; Morishita, K.; Xiao, X.-B.; Zhao, X.-Y.; Wei, X.-Y.; Takarada, T. Nitrogen transformations during fast pyrolysis of sewage sludge. Fuel 2013, 104, 1–6. [Google Scholar] [CrossRef]
- Chen, H.; Namioka, T.; Yoshikawa, K. Characteristics of tar, NOx precursors and their absorption performance with different scrubbing solvents during the pyrolysis of sewage sludge. Appl. Energy 2011, 88, 5032–5041. [Google Scholar] [CrossRef]
- Neves, D.; Thunman, H.; Matos, A.; Tarelho, L.; Gómez-Barea, A. Characterization and prediction of biomass pyrolysis products. Prog. Energy Combust. Sci. 2011, 37, 611–630. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Xu, G.; Yoshikawa, K. Fuel-n evolution during the pyrolysis of industrial biomass wastes with high nitrogen content. Energies 2012, 5, 5418–5438. [Google Scholar] [CrossRef]
- Sharma, R.K.; Chan, W.G.; Hajaligol, M.R. Product compositions from pyrolysis of some aliphatic α-amino acids. J. Anal. Appl. Pyrolysis 2006, 75, 69–81. [Google Scholar] [CrossRef]
- Hao, J.; Guo, J.; Ding, L.; Xie, F.; Xia, Q.; Xie, J. TG-FTIR, Py-two-dimensional GC–MS with heart-cutting and LC–MS/MS to reveal hydrocyanic acid formation mechanisms during glycine pyrolysis. J. Therm. Anal. Calorim. 2014, 115, 667–673. [Google Scholar] [CrossRef]
- Ratcliff, M., Jr.; Medley, E.; Simmonds, P. Pyrolysis of amino acids. Mechanistic considerations. J. Organ. Chem. 1974, 39, 1481–1490. [Google Scholar] [CrossRef]
- Becidan, M. Experimental Studies on Municipal Solid Waste and Biomass Pyrolysis. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2007. [Google Scholar]
- Bassilakis, R.; Zhao, Y.; Solomon, P.; Serio, M. Sulfur and nitrogen evolution in the Argonne coals. Experiment and modeling. Energy Fuels 1993, 7, 710–720. [Google Scholar] [CrossRef]
- Zvicevičius, E.; Raila, A.; Čiplienė, A.; Černiauskienė, Ž.; Kadžiulienė, Ž.; Tilvikienė, V. Effects of moisture and pressure on densification process of raw material from Artemisia dubia wall. Renew. Energy 2018, 119, 185–192. [Google Scholar] [CrossRef]
- Kogan, A. Direct solar thermal splitting of water and on site separation of the products I. Theoretical evaluation of hydrogen yield. Int. J. Hydrogen Energy 1997, 22, 481–486. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Wu, X.; Liang, C.; Chen, X.; Shen, J.; Tang, G.; Wang, Z. Effect of mineral matter on the formation of NOx precursors during biomass pyrolysis. J. Anal. Appl. Pyrolysis 2009, 85, 447–453. [Google Scholar] [CrossRef]
- Qin, J.; Yu, C.; Li, S.; Ma, X.; Wang, Q.; Fang, M.; Luo, Z. The transportation and transformation of alkali metals in Straw-Fired CFB boilers. Acta Energiae Sol. Sin. 2009, 5, 667–673. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C.; Chen, X.; Duan, L.; Li, Y.; Ma, C. NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis: Co-pyrolysis of amino acids and cellulose, hemicellulose and lignin. Proc. Combust. Inst. 2011, 33, 1715–1722. [Google Scholar] [CrossRef]
- Chiavari, G.; Fabbri, D.; Prati, S. Gas chromatographic–mass spectrometric analysis of products arising from pyrolysis of amino acids in the presence of hexamethyldisilazane. J. Chromatogr. A 2001, 922, 235–241. [Google Scholar] [CrossRef]
- Tian, F.-J.; Yu, J.-L.; McKenzie, L.J.; Hayashi, J.-I.; Li, C.-Z. Conversion of Fuel-N into HCN and NH3 during the pyrolysis and gasification in steam: A comparative study of coal and biomass. Energy Fuels 2007, 21, 517–521. [Google Scholar] [CrossRef]
- Gokon, N.; Takahashi, S.; Yamamoto, H.; Kodama, T. Thermochemical two-step water-splitting reactor with internally circulating fluidized bed for thermal reduction of ferrite particles. Int. J. Hydrogen Energy 2008, 33, 2189–2199. [Google Scholar] [CrossRef]
- Kita, I.; Matsuo, S.; Wakita, H. H2 generation by reaction between H2O and crushed rock: An experimental study on H2 degassing from the active fault zone. J. Geophys. Res. Solid Earth 1982, 87, 10789–10795. [Google Scholar] [CrossRef]
- Hindiyarti, L. Gas Phase Sulfur, Chlorine and Potassium Chemistry in Biomass Combustion. Ph.D. Thesis, CHEC Research Centre, Department Chemical Engineering, Technical University of Denmark, Kgs. Lyngby, Denmark, 2007. [Google Scholar]
- Gao, P.; Xue, L.; Lu, Q.; Dong, C. Effects of alkali and alkaline earth metals on N-containing species release during rice straw pyrolysis. Energies 2015, 8, 13021–13032. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. A comparative study of nitrogen conversion during pyrolysis of coconut fiber, its corresponding biochar and their blends with lignite. Bioresour. Technol. 2014, 151, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Darvell, L.; Bridgeman, T.; Pourkashanian, M.; Williams, A. An investigation of the thermal and catalytic behaviour of potassium in biomass combustion. Proc. Combust. Inst. 2007, 31, 1955–1963. [Google Scholar] [CrossRef]
- Ren, Q.; Zhao, C. NOx and N2O precursors (NH3 and HCN) from biomass pyrolysis: Interaction between amino acid and mineral matter. Appl. Energy 2013, 112, 170–174. [Google Scholar] [CrossRef]
- Yi, L.L.; Liu, H.; Lu, G.; Zhang, Q.; Wang, J.X.; Hu, H.Y.; Yao, H. Effect of mixed Fe/Ca additives on nitrogen transformation during protein and amino acid pyrolysis. Energy Fuels 2017, 31, 9484–9490. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.Y.; Liu, P.; Hu, X.W.; Li, A.J.; Yao, H. Catalytic role of conditioner Cao in nitrogen transformation during sewage sludge pyrolysis. Proc. Combust. Inst. 2015, 35, 2759–2766. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, W.; Qiu, J.; Wang, X.; Li, B. Influence of Na and Ca on the emission of NOx during coal combustion. Fuel 2006, 85, 601–606. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Ohshima, Y.; Xu, C.; Ohtsuka, Y. Enhancement of N2 formation from the nitrogen in carbon and coal by calcium. Energy Fuels 2001, 15, 158–162. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Zhiheng, W.; Furimsky, E. Effect of alkali and alkaline earth metals on nitrogen release during temperature programmed pyrolysis of coal. Fuel 1997, 76, 1361–1367. [Google Scholar] [CrossRef]
| Sample | Pyrolysis Device | Heating Rate | Temperature/°C | Gas-N/% | Reference | ||
|---|---|---|---|---|---|---|---|
| HCN | NH3 | HNCO | |||||
| Glycine | Quartz tube | Fast | 800 | 43.7 | 46.4 | 9.9 | [31] |
| Glycine | GC-MS | Fast | 500 | 41.0 | 59.0 | n.a. | [17] |
| DKP | TG-FTIR | Slow | 20~800 | 28.6 | 54.3 | 17.1 | [3] |
| DKP | Fluidized bed | Fast | 800 | 79 | 9 | 12 | [9] |
| DKP | Quartz tube | Fast | 800 | 69.2 | 25.1 | 5.7 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Gao, P.; Dong, C.; Yang, Y. Effect of Temperature and Mineral Matter on the Formation of NOx Precursors during Fast Pyrolysis of 2,5-Diketopiperazine. Energies 2018, 11, 629. https://doi.org/10.3390/en11030629
Zhou J, Gao P, Dong C, Yang Y. Effect of Temperature and Mineral Matter on the Formation of NOx Precursors during Fast Pyrolysis of 2,5-Diketopiperazine. Energies. 2018; 11(3):629. https://doi.org/10.3390/en11030629
Chicago/Turabian StyleZhou, Jianqiang, Pan Gao, Changqing Dong, and Yongping Yang. 2018. "Effect of Temperature and Mineral Matter on the Formation of NOx Precursors during Fast Pyrolysis of 2,5-Diketopiperazine" Energies 11, no. 3: 629. https://doi.org/10.3390/en11030629





