Rare Earth Borohydrides—Crystal Structures and Thermal Properties
Abstract
:1. Introduction
2. Synthesis of Metal Borohydrides
3. Crystal Structures
3.1. Monometallic RE-Borohydrides
3.1.1. Distorted ReO3-Type (α-RE(BH4)3)
3.1.2. ReO3-Type (β-RE(BH4)3)
3.1.3. Alkaline Earth Borohydride-Types
3.2. Halide-Containing RE-Borohydrides
3.3. Bi- and Trimetallic RE-Borohydrides
4. Thermal Properties of Rare Earth Borohydrides
4.1. Monometallic and Halide Substituted Rare Earth Borohydrides
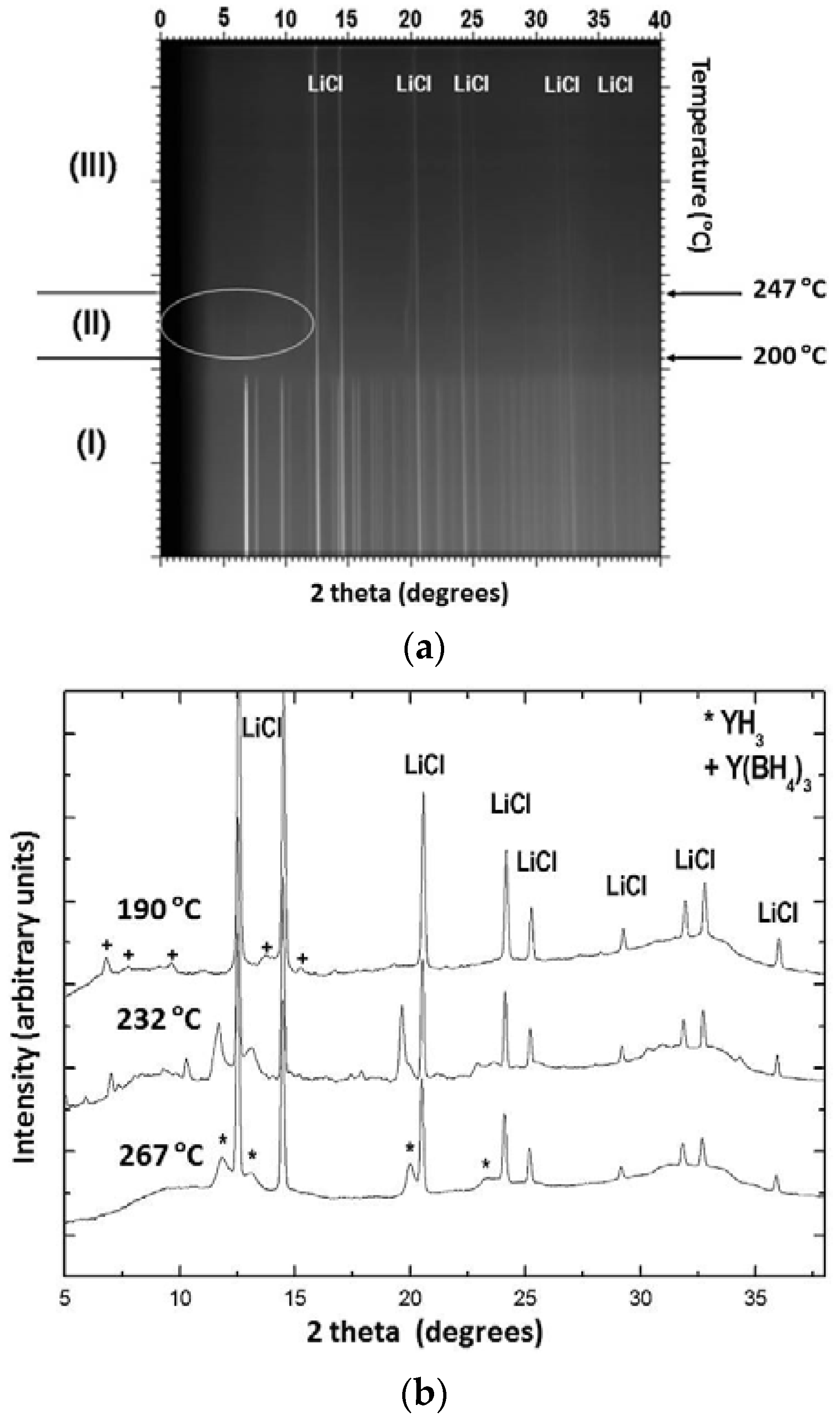
4.1.1. Yttrium borohydride
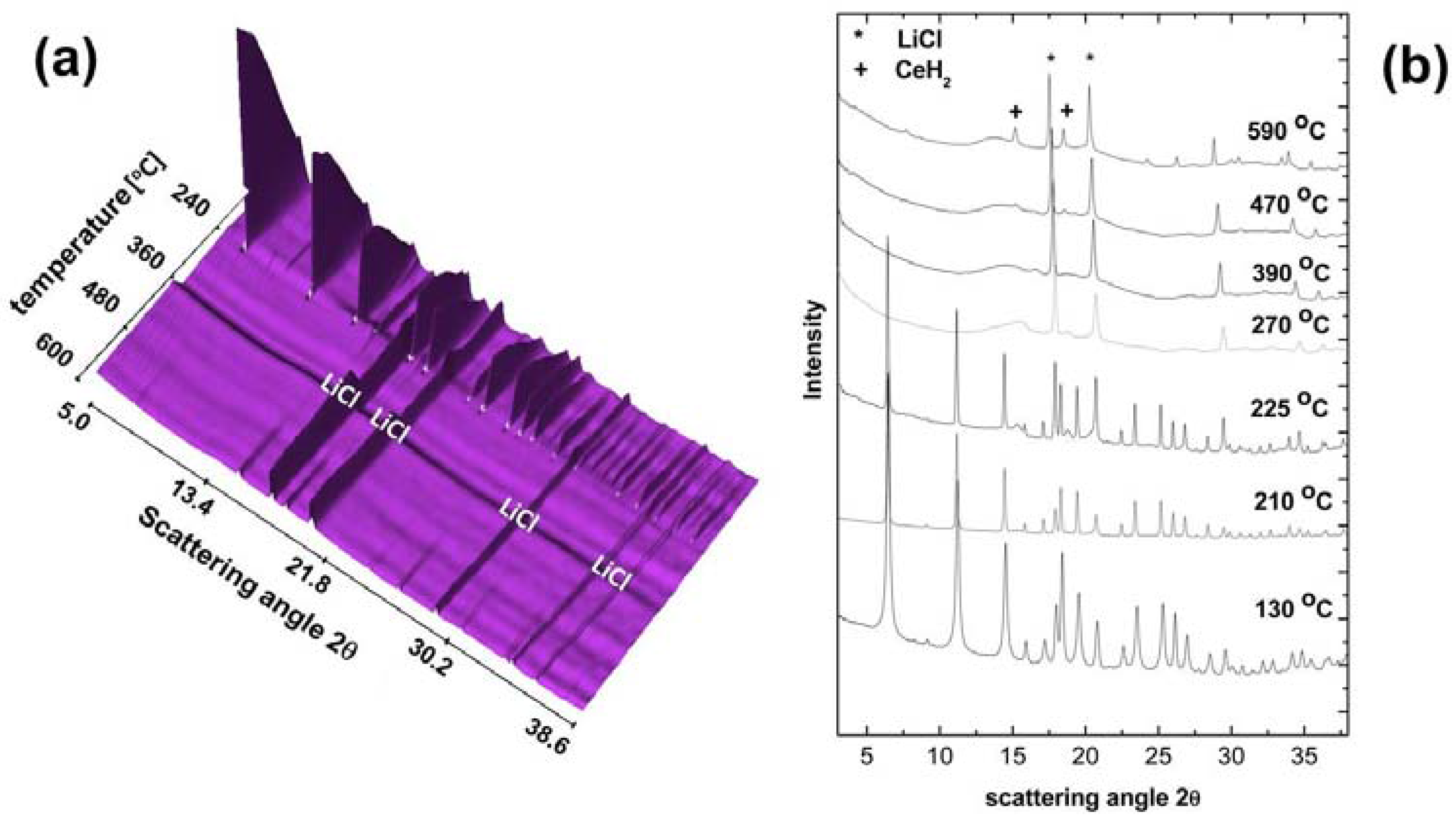
4.1.2. Lithium Cerium Borohydride Chloride
4.2. Composites Containing RE-Borohydrides and LiBH4
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Cerny, R.; Ravnsbæk, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacherf, F.; Jensen, T.R. Metal borohydrides and derivatives—Synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Mohtadi, R.; Orimo, S.I. The renaissance of hydrides as energy materials. Nat. Rev. Mater. 2017, 2, 16091. [Google Scholar] [CrossRef]
- Callini, E.; Atakli, Z.O.K.; Hauback, B.C.; Orimo, S.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A Mater. Sci. Process. 2016, 122, 353. [Google Scholar]
- Li, H.W.; Yan, Y.G.; Orimo, S.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Züttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, D.A.; Paskevicius, M.; Humphries, T.D.; Felderhoff, M.; Capurso, G.; von Colbe, J.B.; Dornheim, M.; Klassen, T.; Ward, P.A.; Teprovich, J.A.; et al. Metal hydrides for concentrating solar thermal power energy storage. Appl. Phys. A Mater. Sci. Process. 2016, 122, 395. [Google Scholar] [CrossRef]
- Møller, K.; Sheppard, D.; Ravnsbæk, D.; Buckley, C.; Akiba, E.; Li, H.-W.; Jensen, T. Complex Metal Hydrides for Hydrogen, Thermal and Electrochemical Energy Storage. Energies 2017, 10, 1645. [Google Scholar] [CrossRef]
- Ley, M.B.; Ravnsbæk, D.B.; Filinchuk, Y.; Lee, Y.S.; Janot, R.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. LiCe(BH4)3Cl, a New Lithium-Ion Conductor and Hydrogen Storage Material with Isolated Tetranuclear Anionic Clusters. Chem. Mater. 2012, 24, 1654–1663. [Google Scholar] [CrossRef]
- Ley, M.B.; Boulineau, S.; Janot, R.; Filinchuk, Y.; Jensen, T.R. New Li Ion Conductors and Solid State Hydrogen Storage Materials: LiM(BH4)3Cl, M = La, Gd. J. Phys. Chem. C 2012, 116, 21267–21276. [Google Scholar] [CrossRef]
- Roedern, E.; Lee, Y.S.; Ley, M.B.; Park, K.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. Solid state synthesis, structural characterization and ionic conductivity of bimetallic alkali-metal yttrium borohydrides MY(BH4)4 (M = Li and Na). J. Mater. Chem. A 2016, 4, 8793–8802. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Ley, M.B.; Jensen, T.R.; Filinchuk, Y. Nuclear Magnetic Resonance Studies of BH4 Reorientations and Li Diffusion in LiLa(BH4)3Cl. J. Phys. Chem. C 2013, 117, 14965–14972. [Google Scholar] [CrossRef]
- Nakamori, Y.; Miwa, K.; Ninomiya, A.; Li, H.W.; Ohba, N.; Towata, S.I.; Züttel, A.; Orimo, S.I. Correlation between thermodynamical stabilities of metal borohydrides and cation electronegativites: First-principles calculations and experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef]
- Wiberg, E.; Bauer, R. Zur Kenntnis eines Magnesium-Bor-Wasserstoffs Mg(BH4)2. Z. Fur Naturforschung Sect. B J. Chem. Sci. 1950, 5, 397–398. [Google Scholar] [CrossRef]
- Goerrig, D. Verfahren zur Herstellung von Boranaten. German Patent DBP 1,077,644, 27 December 1958. [Google Scholar]
- Chlopek, K.; Frommen, C.; Leon, A.; Zabara, O.; Fichtner, M. Synthesis and properties of magnesium tetrahydroborate, Mg(BH4)2. J. Mater. Chem. 2007, 17, 3496–3503. [Google Scholar] [CrossRef]
- Koester, R. Neue herstellungsmethoden für metallborhydride. Angew. Chem. 1957, 3, 94. [Google Scholar] [CrossRef]
- Zanella, P.; Crociani, L.; Masciocchi, N.; Giunchi, G. Facile high-yield synthesis of pure, crystalline Mg(BH4)2. Inorg. Chem. 2007, 46, 9039–9041. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Miwa, K.; Nakamori, Y.; Ohoyama, K.; Li, H.W.; Noritake, T.; Aoki, M.; Towata, S.I.; Orimo, S.I. Experimental and computational studies on solvent-free rare-earth metal borohydrides R(BH4)3 (R = Y, Dy, and Gd). Phys. Rev. B 2008, 77, 104114. [Google Scholar] [CrossRef]
- Olsen, J.E.; Frommen, C.; Jensen, T.R.; Riktor, M.D.; Sørby, M.H.; Hauback, B.C. Structure and thermal properties of composites with RE-borohydrides (RE = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Er, Yb or Lu) and LiBH4. RSC Adv. 2014, 4, 1570–1582. [Google Scholar] [CrossRef]
- Jaroń, T.; Grochala, W. Y(BH4)3-an old-new ternary hydrogen store aka learning from a multitude of failures. Dalton Trans. 2010, 39, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Frommen, C.; Aliouane, N.; Deledda, S.; Fonneløp, J.E.; Grove, H.; Lieutenant, K.; Llamas-Jansa, I.; Sartori, S.; Sørby, M.H.; Hauback, B.C. Crystal structure, polymorphism, and thermal properties of yttrium borohydride Y(BH4)3. J. Alloys Compd. 2010, 496, 710–716. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Filinchuk, Y.; Cerny, R.; Ley, M.B.; Haase, D.; Jakobsen, H.J.; Skibsted, J.; Jensen, T.R. Thermal Polymorphism and Decomposition of Y(BH4)3. Inorg. Chem. 2010, 49, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.G.; Li, H.W.; Sato, T.; Umeda, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding properties of yttrium borohydride Y(BH4)3 prepared by liquid-phase synthesis. Int. J. Hydrogen Energy 2009, 34, 5732–5736. [Google Scholar] [CrossRef]
- Jaroń, T.; Kozminskia, W.; Grochala, W. Phase transition induced improvement in H2 desorption kinetics: The case of the high-temperature form of Y(BH4)3. Phys. Chem. Chem. Phys. 2011, 13, 8847–8851. [Google Scholar] [CrossRef] [PubMed]
- Gennari, F.C.; Esquivel, M.R. Synthesis and dehydriding process of crystalline Ce(BH4)3. J. Alloys Compd. 2009, 485, L47–L51. [Google Scholar] [CrossRef]
- Zhang, B.J.; Liu, B.H.; Li, Z.P. Destabilization of LiBH4 by (Ce, La)(Cl, F)3 for hydrogen storage. J. Alloys Compd. 2011, 509, 751–757. [Google Scholar] [CrossRef]
- Humphries, T.D.; Ley, M.B.; Frommen, C.; Munroe, K.T.; Jensen, T.R.; Hauback, B.C. Crystal structure and in situ decomposition of Eu(BH4)2 and Sm(BH4)2. J. Mater. Chem. A 2015, 3, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Gennari, F.C.; Albanesi, L.F.; Puszkiel, J.A.; Larochette, P.A. Reversible hydrogen storage from 6LiBH4-MCl3 (M = Ce, Gd) composites by in-situ formation of MH2. Int. J. Hydrogen Energy 2011, 36, 563–570. [Google Scholar] [CrossRef]
- Olsen, J.E.; Frommen, C.; Jensen, T.R.; Riktor, M.D.; Sørby, M.H.; Hauback, B.C. Crystal structures and properties of solvent-free LiYb(BH4)4−xClx, Yb(BH4)3 and Yb(BH4)2−xClx. RSC Adv. 2013, 3, 10764–10774. [Google Scholar] [CrossRef]
- Heere, M.; GharibDoust, S.H.P.; Frommen, C.; Humphries, T.D.; Ley, M.B.; Sørby, M.H.; Jensen, T.R.; Hauback, B.C. The influence of LiH on the rehydrogenation behavior of halide free rare earth (RE) borohydrides (RE = Pr, Er). Phys. Chem. Chem. Phys. 2016, 18, 24387–24395. [Google Scholar] [CrossRef] [PubMed]
- Ravnsbæk, D.B.; Ley, M.B.; Lee, Y.S.; Hagemann, H.; D’Anna, V.; Cho, Y.W.; Filinchuk, Y.; Jensen, T.R. A mixed-cation mixed-anion borohydride NaY(BH4)2Cl2. Int. J. Hydrogen Energy 2012, 37, 8428–8438. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Grochala, W. Polymorphism and hydrogen discharge from holmium borohydride, Ho(BH4)3, and KHo(BH4)4. Int. J. Hydrogen Energy 2014, 39, 20024–20030. [Google Scholar] [CrossRef]
- Cerny, R.; Schouwink, P. The crystal chemistry of inorganic metal borohydrides and their relation to metal oxides. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 619–640. [Google Scholar] [CrossRef] [PubMed]
- Ley, M.B.; Jørgensen, M.; Cerny, R.; Filinchuk, Y.; Jensen, T.R. From M(BH4)3 (M = La, Ce) Borohydride Frameworks to Controllable Synthesis of Porous Hydrides and Ion Conductors. Inorg. Chem. 2016, 55, 9748–9756. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Didelot, E.; Spyratou, A.; Daku, L.M.L.; Cerny, R.; Hagemann, H. Halide Free M(BH4)2 (M = Sr, Ba, and Eu) Synthesis, Structure, and Decomposition. Inorg. Chem. 2016, 55, 7090–7097. [Google Scholar] [CrossRef] [PubMed]
- Frommen, C.; Sørby, M.H.; Ravindran, P.; Vajeeston, P.; Fjellvåg, H.; Hauback, B.C. Synthesis, Crystal Structure, and Thermal Properties of the First Mixed-Metal and Anion-Substituted Rare Earth Borohydride LiCe(BH4)3Cl. J. Phys. Chem. C 2011, 115, 23591–23602. [Google Scholar] [CrossRef] [Green Version]
- GharibDoust, S.P.; Brighi, M.; Sadikin, Y.; Ravnsbæk, D.B.; Cerny, R.; Skibsted, J.; Jensen, T.R. Synthesis, Structure, and Li-Ion Conductivity of LiLa(BH4)3X, X = Cl, Br, I. J. Phys. Chem. C 2017, 121, 19010–19021. [Google Scholar] [CrossRef]
- Sadikin, Y.; Stare, K.; Schouwink, P.; Ley, M.B.; Jensen, T.R.; Meden, A.; Cerny, R. Alkali metal—Yttrium borohydrides: The link between coordination of small and large rare-earth. J. Solid State Chem. 2015, 225, 231–239. [Google Scholar] [CrossRef]
- Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Cerny, R.; Penin, N.; Sørby, M.H.; Hauback, B.C.; Severa, G.; Jensen, C.M. LiSc(BH4)4: A novel salt of Li+ and discrete Sc(BH4)4− complex anions. J. Phys. Chem. A 2008, 112, 7551–7555. [Google Scholar] [CrossRef] [PubMed]
- Jaroń, T.; Wegner, W.; Fijałkowski, K.J.; Leszczyński, P.J.; Grochala, W. Facile Formation of Thermodynamically Unstable Novel Borohydride Materials by a Wet Chemistry Route. Chem. A Eur. J. 2015, 21, 5689–5692. [Google Scholar] [CrossRef] [PubMed]
- Cerny, R.; Severa, G.; Ravnsbæk, D.B.; Filinchuk, Y.; D’Anna, V.; Hagemann, H.; Haase, D.; Jensen, C.M.; Jensen, T.R. NaSc(BH4)4: A Novel Scandium-Based Borohydride. J. Phys. Chem. C 2010, 114, 1357–1364. [Google Scholar] [CrossRef]
- GharibDoust, S.H.P.; Ravnsbæk, D.B.; Cerny, R.; Jensen, T.R. Synthesis, structure and properties of bimetallic sodium rare-earth (RE) borohydrides, NaRE(BH4)4, RE = Ce, Pr, Er or Gd. Dalton Trans. 2017, 46, 13421–13431. [Google Scholar] [CrossRef] [PubMed]
- Jaroń, T.; Grochala, W. Probing Lewis acidity of Y(BH4)(3) via its reactions with MBH4 (M = Li, Na, K, NMe4). Dalton Trans. 2011, 40, 12808–12817. [Google Scholar] [CrossRef] [PubMed]
- Heere, M.; GharibDoust, S.H.P.; Sørby, M.H.; Frommen, C.; Jensen, T.R.; Hauback, B.C. In situ investigations of bimetallic potassium erbium borohydride. Int. J. Hydrogen Energy 2017, 42, 22468–22474. [Google Scholar] [CrossRef]
- Wegner, W.; Jaroń, T.; Grochala, W. MYb(BH4)4 (M = K, Na) from laboratory X-ray powder data. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 1289–1291. [Google Scholar] [CrossRef] [PubMed]
- Cerny, R.; Ravnsbæk, D.B.; Severa, G.; Filinchuk, Y.; D’ Anna, V.; Hagemann, H.; Haase, D.; Skibsted, J.; Jensen, C.M.; Jensen, T.R. Structure and Characterization of KSc(BH4)4. J. Phys. Chem. C 2010, 114, 19540–19549. [Google Scholar] [CrossRef]
- Aeberhard, P.C.; Refson, K.; Edwards, P.P.; David, W.I.F. High-pressure crystal structure prediction of calcium borohydride using density functional theory. Phys. Rev. B 2011, 83, 174102. [Google Scholar] [CrossRef]
- Brighi, M.; Schouwink, P.; Sadikin, Y.; Cerny, R. Fast ion conduction in garnet-type metal borohydrides Li3K3Ce2(BH4)12 and Li3K3La2(BH4)12. J. Alloys Compd. 2016, 662, 388–395. [Google Scholar] [CrossRef]
- GharibDoust, S.H.P.; Heere, M.; Sørby, M.H.; Ley, M.B.; Ravnsbæk, D.B.; Hauback, B.C.; Cerny, R.; Jensen, T.R. Synthesis, structure and properties of new bimetallic sodium and potassium lanthanum borohydrides. Dalton Trans. 2016, 45, 19002–19011. [Google Scholar] [CrossRef] [PubMed]
- Jaroń, T.; Wegner, W.; Grochala, W. M Y(BH4)4 and M2LiY(BH4)6−xClx (M = Rb, Cs): New borohydride derivatives of yttrium and their hydrogen storage properties. Dalton Trans. 2013, 42, 6886–6893. [Google Scholar] [CrossRef] [PubMed]
- Møller, K.T.; Jørgensen, M.; Fogh, A.S.; Jensen, T.R. Perovskite alkali metal samarium borohydrides: Crystal structures and thermal decomposition. Dalton Trans. 2017, 46, 11905–11912. [Google Scholar] [CrossRef] [PubMed]
- Schouwink, P.; Didelot, E.; Lee, Y.S.; Mazet, T.; Cerny, R. Structural and magnetocaloric properties of novel gadolinium borohydrides. J. Alloys Compd. 2016, 664, 378–384. [Google Scholar] [CrossRef]
- Schouwink, P.; Ley, M.B.; Tissot, A.; Hagemann, H.; Jensen, T.R.; Smrcok, L.; Cerny, R. Structure and properties of complex hydride perovskite materials. Nat. Commun. 2014, 5, 5706. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.H.; Chen, Y.Y.; Xie, R.W.; Hu, Z.; Song, Y. Influence of alloying elements on the stability and dehydrogenation properties on Y(BH4)3 by first principles calculations. Int. J. Hydrogen Energy 2016, 41, 1662–1671. [Google Scholar] [CrossRef]
- Remhof, A.; Borgschulte, A.; Friedrichs, O.; Mauron, P.; Yan, Y.; Züttel, A. Solvent-free synthesis and decomposition of Y(BH4)3. Scr. Mater. 2012, 66, 280–283. [Google Scholar] [CrossRef]
- Park, K.; Lee, H.S.; Remhof, A.; Lee, Y.S.; Yan, Y.G.; Kim, M.Y.; Kim, S.J.; Züttel, A.; Cho, Y.W. Thermal properties of Y(BH4)3 synthesized via two different methods. Int. J. Hydrogen Energy 2013, 38, 9263–9270. [Google Scholar] [CrossRef]
- Riktor, M.D.; Filinchuk, Y.; Vajeeston, P.; Bardaji, E.G.; Fichtner, M.; Fjellvåg, H.; Sørby, M.H.; Hauback, B.C. The crystal structure of the first borohydride borate, Ca3(BD4)3(BO3). J. Mater. Chem. 2011, 21, 7188–7193. [Google Scholar] [CrossRef]
- Schrauzer, G.N. Ermittlung des Ionencharakters mit Hilfe der Stabilitatsquotiententheoriet. Naturwissenschaften 1955, 42, 43. [Google Scholar]
- Schrauzer, G.N. Die chemische Bestimmung des Ionencharakters im Lithiumborhydridt. Naturwissenschaften 1955, 42, 43–44. [Google Scholar]
- Ley, M.B.; Paskevicius, M.; Schouwink, P.; Richter, B.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Novel solvates M(BH4)3S(CH3)2 and properties of halide-free M(BH4)3 (M = Y or Gd). Dalton Trans. 2014, 43, 13333–13342. [Google Scholar] [CrossRef] [PubMed]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Züttel, A. Stability and reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Bosenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; et al. Hydrogen sorption properties of MgH2-LiBH4 composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Lim, J.H.; Shim, J.H.; Lee, Y.S.; Suh, J.Y.; Cho, Y.W.; Lee, J. Rehydrogenation and cycle studies of LiBH4-CaH2 composite. Int. J. Hydrogen Energy 2010, 35, 6578–6582. [Google Scholar] [CrossRef]
- Jin, S.A.; Lee, Y.S.; Shim, J.H.; Cho, Y.W. Reversible hydrogen storage in LiBH4-MH2 (M = Ce, Ca) composites. J. Phys. Chem. C 2008, 112, 9520–9524. [Google Scholar] [CrossRef]
- Gennari, F.C. Destabilization of LiBH4 by MH2 (M = Ce, La) for hydrogen storage: Nanostructural effects on the hydrogen sorption kinetics. Int. J. Hydrogen Energy 2011, 36, 15231–15238. [Google Scholar] [CrossRef]
- Jin, S.A.; Shim, J.H.; Cho, Y.W.; Yi, K.W.; Zabara, O.; Fichtner, M. Reversible hydrogen storage in LiBH4-Al-LiH composite powder. Scr. Mater. 2008, 58, 963–965. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q.F.; Zhang, J.; Liu, S.S.; Sun, L.X. The Dehydrogenation Reactions and Kinetics of 2LiBH4-Al Composite. J. Phys. Chem. C 2009, 113, 18424–18430. [Google Scholar] [CrossRef]
- Vajo, J.J.; Salguero, T.T.; Gross, A.E.; Skeith, S.L.; Olson, G.L. Thermodynamic destabilization and reaction kinetics in light metal hydride systems. J. Alloys Compd. 2007, 446, 409–414. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A.R.; Spencer, W.A.; Anton, D.L.; Pinkerton, F.E.; Hwang, S.J.; Kim, C.; Bowman, R.C. Stability and Reversibility of Lithium Borohydrides Doped by Metal Halides and Hydrides. J. Phys. Chem. C 2008, 112, 18661–18671. [Google Scholar] [CrossRef]
- Zhang, B.J.; Liu, B.H. Hydrogen desorption from LiBH4 destabilized by chlorides of transition metal Fe, Co, and Ni. Int. J. Hydrogen Energy 2010, 35, 7288–7294. [Google Scholar] [CrossRef]
- Frommen, C.; Heere, M.; Riktor, M.D.; Sørby, M.H.; Hauback, B.C. Hydrogen storage properties of rare earth (RE) borohydrides (RE = La, Er) in composite mixtures with LiBH4 and LiH. J. Alloys Compd. 2015, 645, S155–S159. [Google Scholar] [CrossRef] [Green Version]
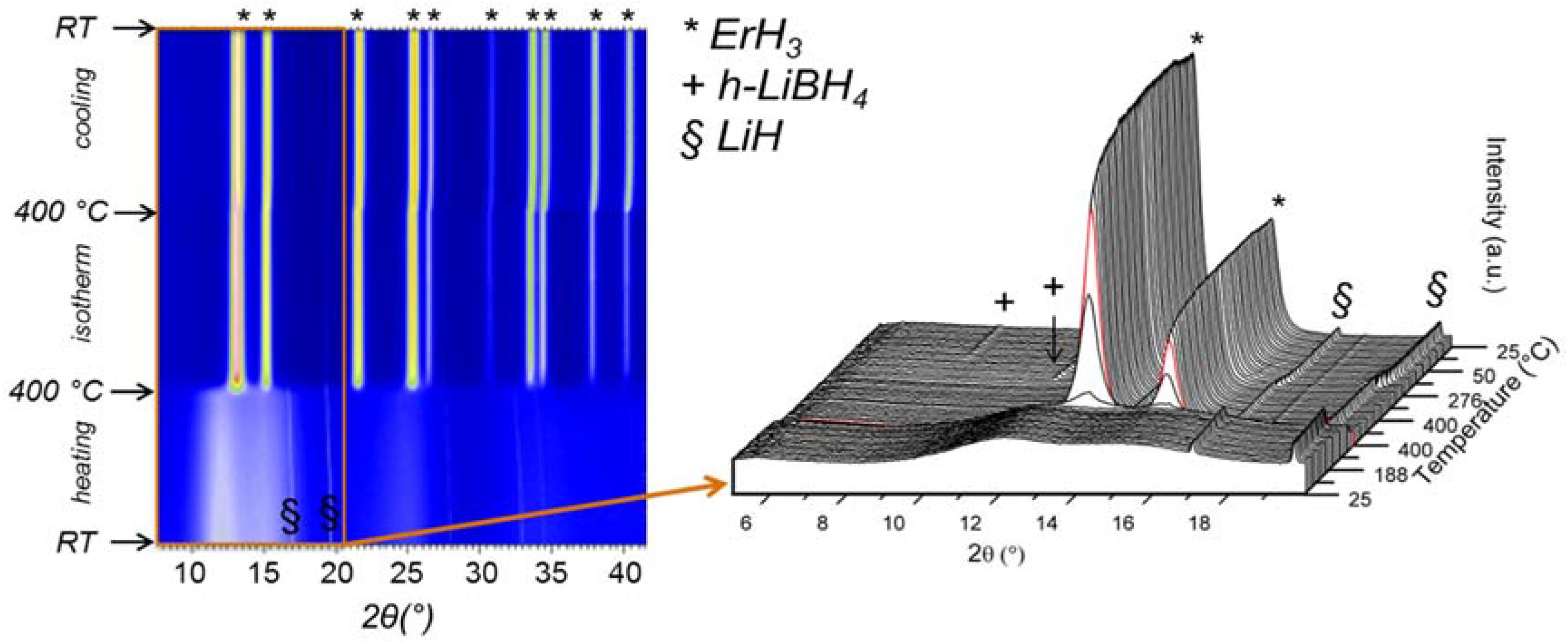
- Heere, M.; GharibDoust, S.H.P.; Brighi, M.; Frommen, C.; Sørby, M.H.; Cerny, R.; Jensen, T.R.; Hauback, B.C. Hydrogen Sorption in Erbium Borohydride Composite Mixtures with LiBH4 and/or LiH. Inorganics 2017, 5, 31. [Google Scholar] [CrossRef]
- Gennari, F.C. Mechanochemical synthesis of erbium borohydride: Polymorphism, thermal decomposition and hydrogen storage. J. Alloys Compd. 2013, 581, 192–195. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected kinetic effect of MgB2 in reactive hydride composites containing complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Dhliwayo, T. Development of advanced shield systems for fast neutrons. Int. Nuclear Saf. J. 2014, 3, 49–53. [Google Scholar]
- Fantidis, J.G. The comparison between simple and advanced shielding materials for the shield of portable neutron sources. Int. J. Radiat. Res. 2015, 13, 287–295. [Google Scholar]

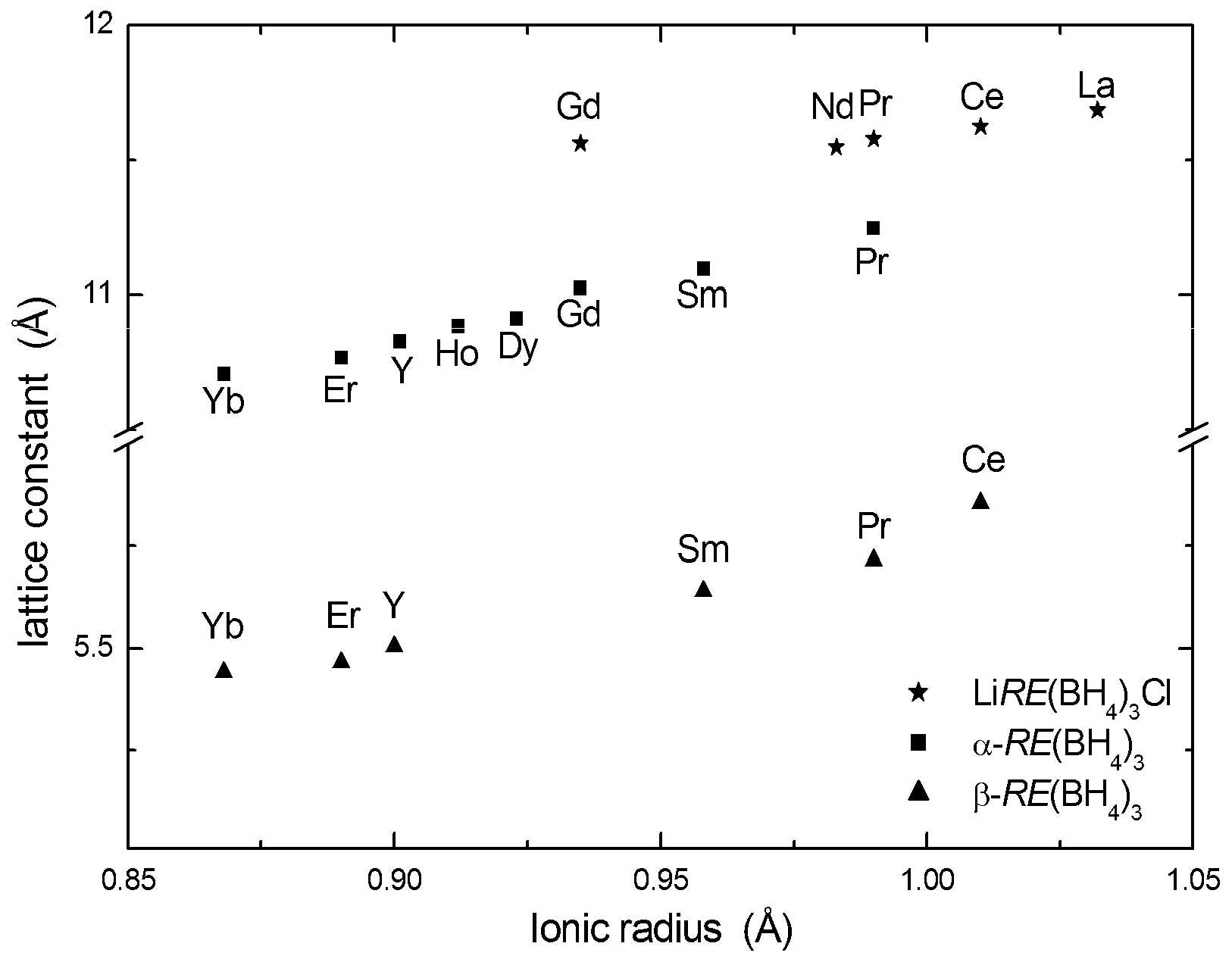
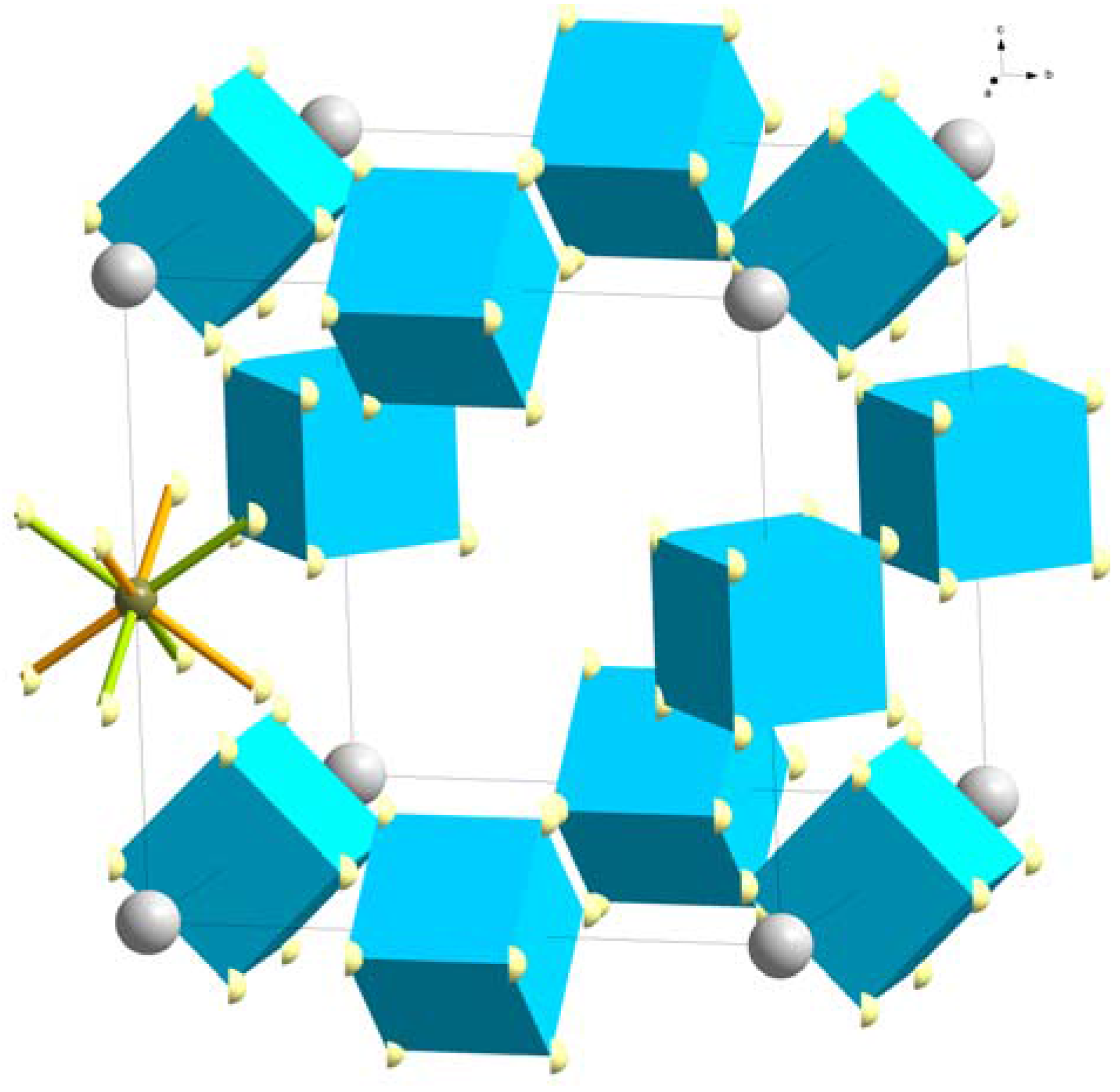
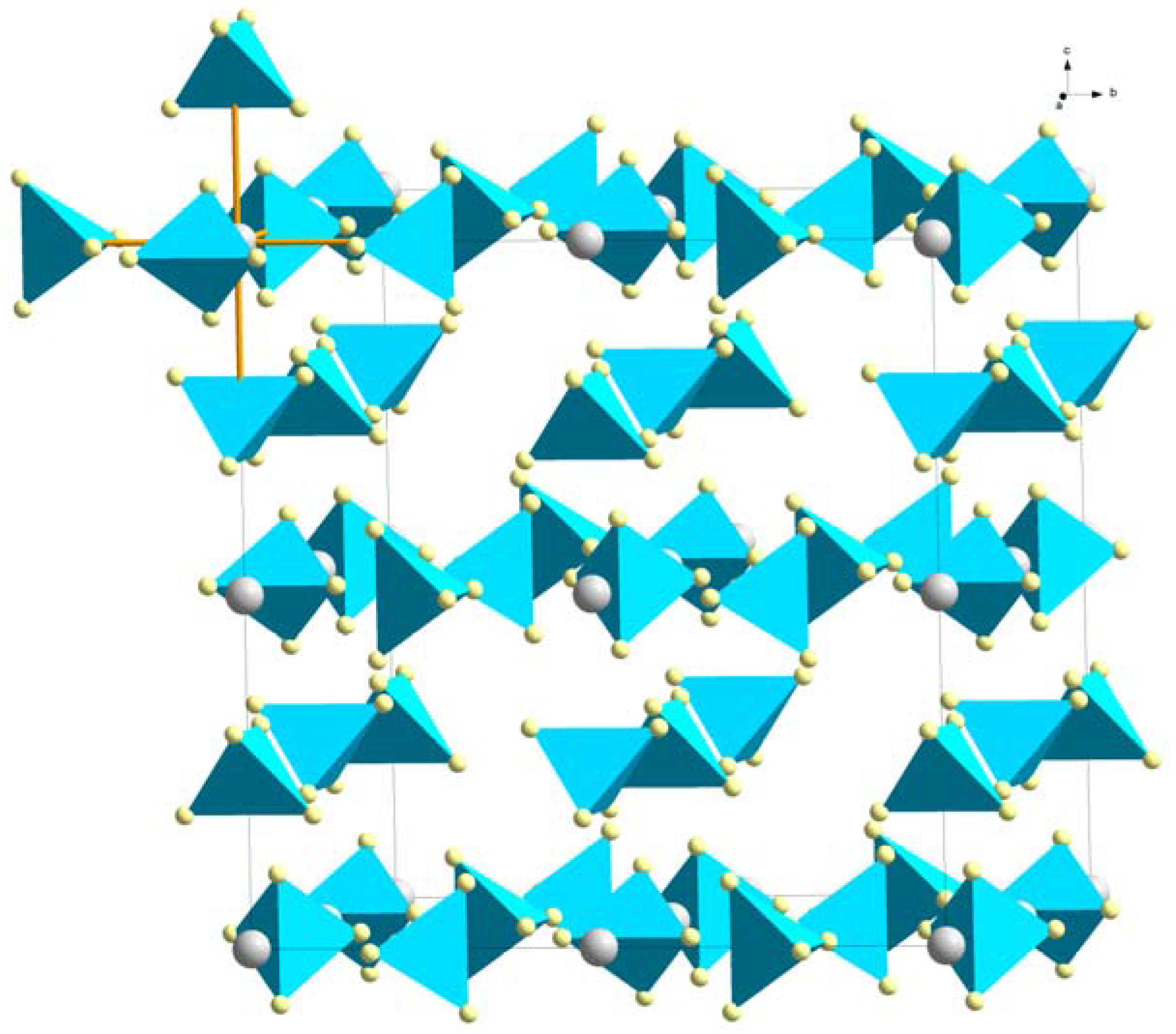
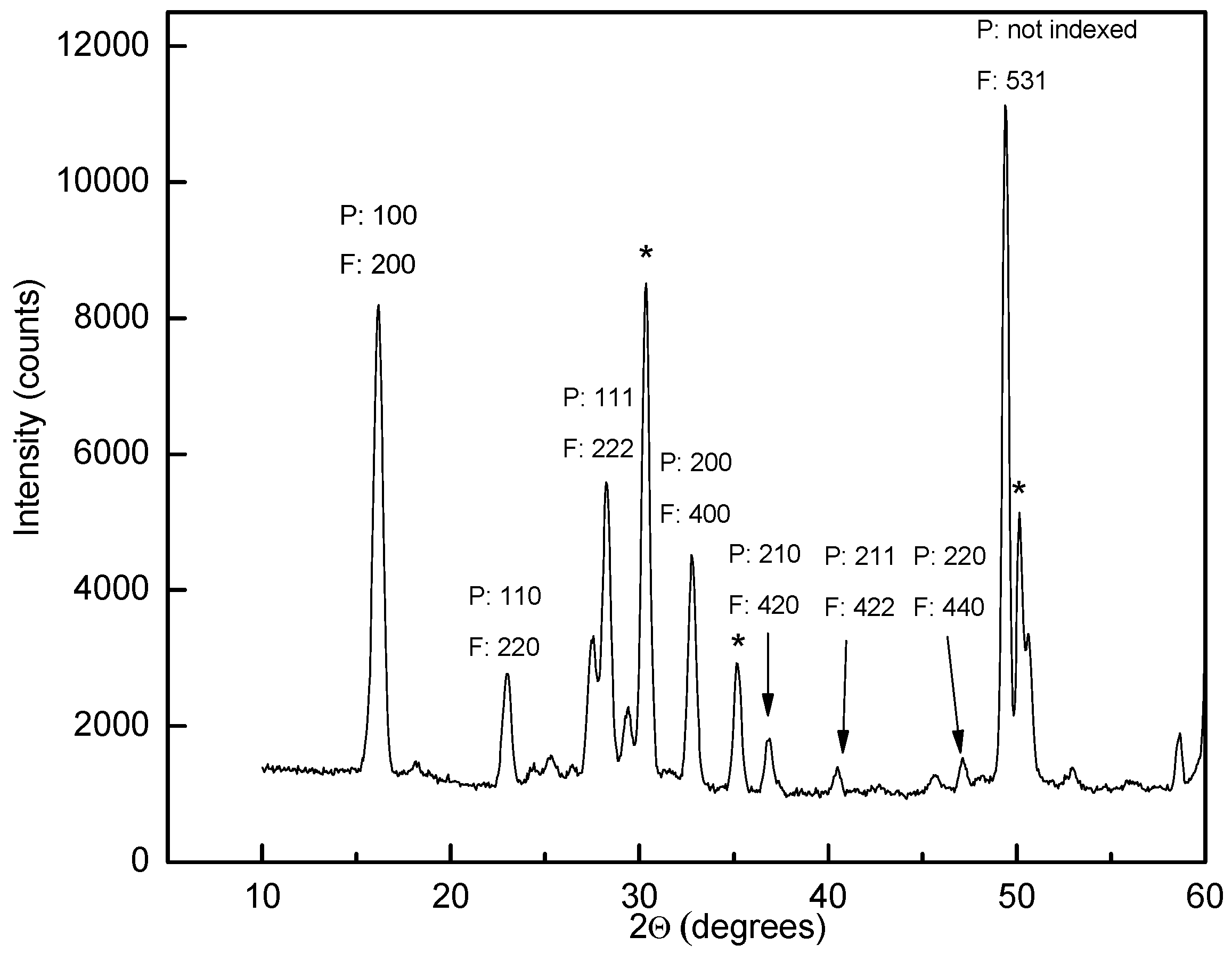
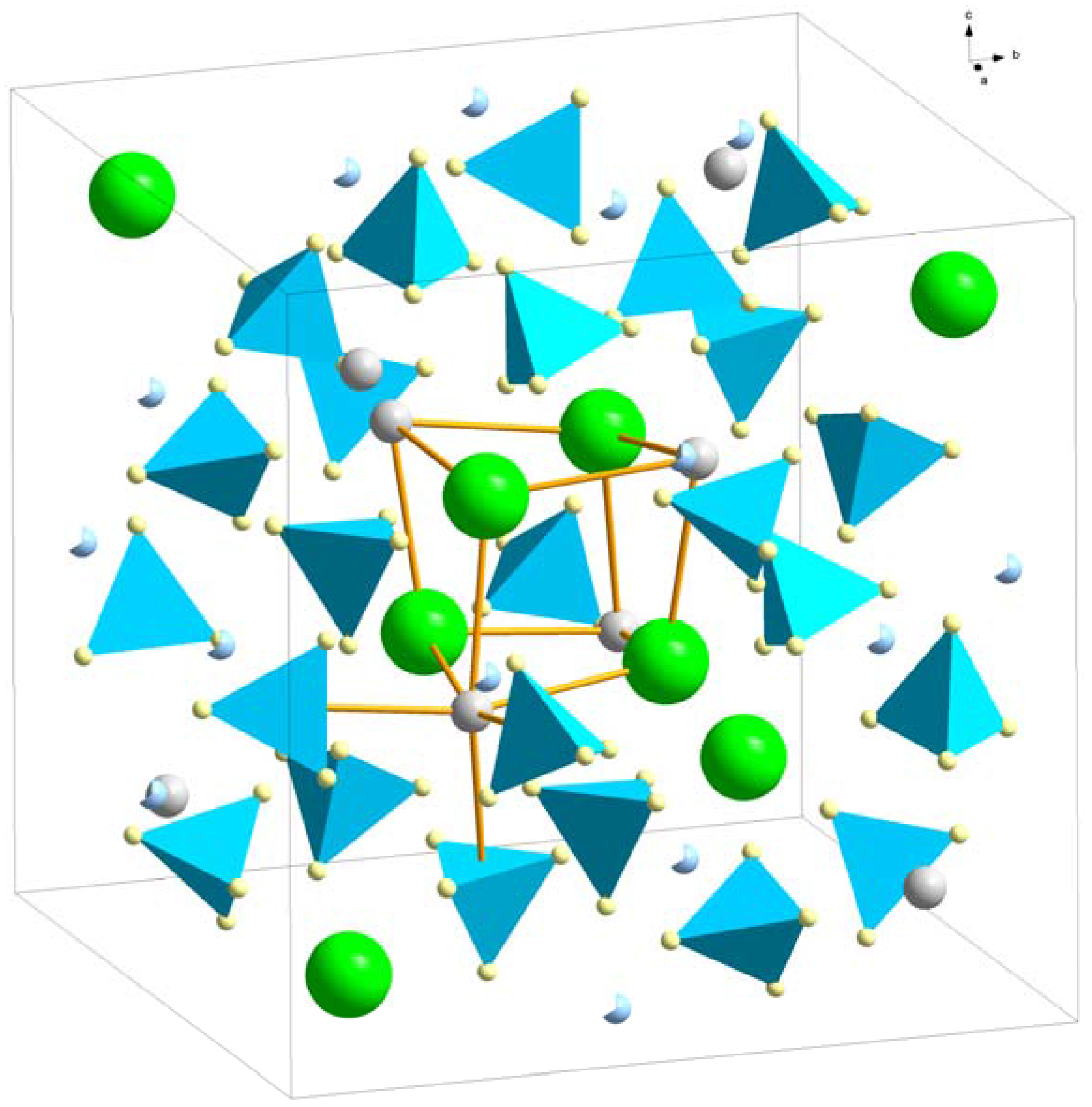
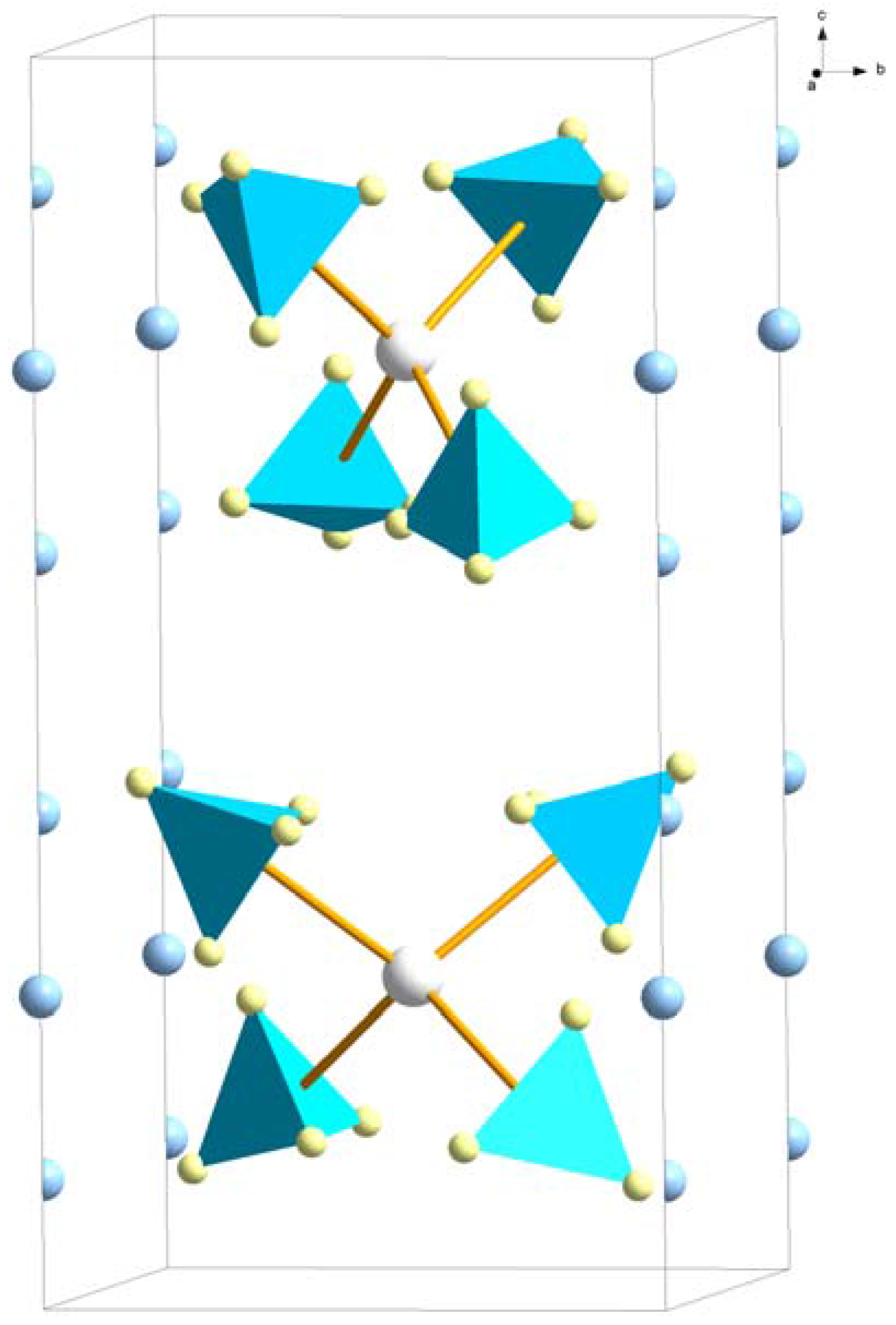

| RE Ion | Ion Radius [Å] | LiRE(BH4)4 (CuAuCl4) | α-RE(BH4)3 (ReO3) | β-RE(BH4)3 (ReO3) | LiRE(BH4)3Cl (Spinel) |
|---|---|---|---|---|---|
| Sc3+ | 0.745 | X | |||
| Y3+ | 0.90 | X | X | ||
| La3+ | 1.172 | X | |||
| Ce3+ | 1.15 | X | X | ||
| Pr3+ | 1.13 | X | X | X | |
| Nd3+ | 1.123 | X | |||
| Sm3+ | 1.098 | X | X | X | |
| Gd3+ | 1.078 | X | X | ||
| Tb3+ | 1.063 | X | |||
| Dy3+ | 1.052 | X | |||
| Ho3+ | 1.041 | X | X | ||
| Er3+ | 1.03 | X | X | ||
| Yb3+ | 1.008 | X | X | X | |
| Lu3+ | 1.001 | X |
| Cation | Polymorph | Hydrogen Capacity (wt %) | Stability | Crystal System | Space Group | Structure Type | Ref. |
|---|---|---|---|---|---|---|---|
| Y3+ | α-Y(BH4)3 β-Y(BH4)3 | 9.1 | RT RT, HT | Cubic Cubic | Pa Fmc | Distorted ReO3 ReO3 | [18] [21] |
| La3+ | La(BH4)3 | 6.6 | RT | Trigonal | Rc | Distorted ReO3 | [34] |
| Ce3+ | Ce(BH4)3 | 6.6 | RT | Cubic Trigonal | Fmc Rc | ReO3 Distored ReO3 | [34] [34] |
| Pr3+ | Pr(BH4)3 | 6.5 | RT | Cubic | Pa Pmm | Distorted ReO3 ReO3 | [30] |
| Sm2+ | o-Sm(BH4)2 | 4.5 | ~500 K | Orthorhombic | Pbcn | ort-Sr(BH4)2 | [19,27] |
| Sm3+ | α-Sm(BH4)3 β-Sm(BH4)3 | 6.2 | RT RT | Cubic Cubic | Pa Pmm | Distorted ReO3 ReO3 | [19] [19] |
| Eu2+ | o-Eu(BH4)2 t-Eu(BH4)2 c-Eu(BH4)2 | 4.4 | ~430 K >668 K >668 K | Orthorhombic Tetragonal Cubic | Pbcn P41212 Pmm | orthorombic Sr(BH4)2 tetragonal Sr(BH4)2 cubic Sr(BH4)2 | [27] [35] [35] |
| Gd3+ | Gd(BH4)3 | 6.0 | RT | Cubic | Pa | Distorted ReO3 | [18,19] |
| Tb3+ | Tb(BH4)3 | 5.9 | RT | Cubic | Pa | Distorted ReO3 | [19] |
| Dy3+ | Dy(BH4)3 | 5.8 | RT | Cubic | Pa | Distorted ReO3 | [18] |
| Ho3+ | α-Ho(BH4)3 β-Ho(BH4)3 | 5.8 | RT RT | Cubic Cubic | Pa Fmc | Distorted ReO3 ReO3 | [32] [32] |
| Er3+ | α-Er(BH4)3 β-Er(BH4)3 | 5.7 | RT RT | Cubic Cubic | Pa Pmm | Distorted ReO3 ReO3 | [19] [19] |
| Tm3+ | α-Tm(BH4)3 | 5.7 | Cubic | Pa | Distorted ReO3 | [33] | |
| Yb2+ | α-Yb(BH4)2 β-Yb(BH4)2 γ-Yb(BH4)2 | 4.0 | RT >523 K 473–573 K | Orthorhombic Tetragonal Orthorhombic | F2dd P Pbca | α-Ca(BH4)2 β-Ca(BH4)2 γ-Ca(BH4)2 | [1] [29] [29] |
| Yb3+ | α-Yb(BH4)3 β-Yb(BH4)3 | 5.6 | RT RT, meta | Cubic Cubic | Pa Pmm | Distorted ReO3 ReO3 | [29] [29] |
| Cation | Composition | Crystal System | Space Group | Ref. | |
|---|---|---|---|---|---|
| Li+ | La3+ | LiLa(BH4)3Cl | Cubic | I3m | [9,19,37] |
| Li+ | La3+ | LiLa(BH4)3Br | Cubic | I3m | [37] |
| Li+ | La3+ | LiLa(BH4)3I | Cubic | I3m | [37] |
| Li+ | Ce3+ | LiCe(BH4)3Cl | Cubic | I3m | [8,19,36] |
| Li+ | Pr3+ | LiPr(BH4)3Cl | Cubic | I3m | [19] |
| Li+ | Nd3+ | LiNd(BH4)3Cl | Cubic | I3m | [19] |
| Li+ | Sm3+ | LiSm(BH4)3Cl | Cubic | I3m | [19] |
| Li+ | Gd3+ | LiGd(BH4)3Cl | Cubic | I3m | [9] |
| Na+ | Y3+ | NaY(BH4)2−xCl2+x | Monoclinic | P2/c | [31,38] |
| Li+ | Yb3+ | LiYb(BH4)4−xClx | Tetragonal (x ~ 1.0) | P2c | [29] |
| Yb2+ | Yb(BH4)2−xClx | Orthorhombic (x = 0.3) | Pbca | [29] | |
| Tetragonal (x = 0.76) | P | [29] | |||
| Cations | Polymorph | wt % H | Crystal System | Space Group | Structure Type | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| Li+ | Sc3+ | LiSc(BH4)4 | 14.5 | Tetragonal | P2c | New | [39] | |
| Li+ | Y3+ | LiY(BH4)4 | 10.4 | Tetragonal | P2c | LiSc(BH4)4 | [10,40] | |
| Li+ | Yb3+ | LiYb(BH4)4 | 6.7 | Tetragonal | P2c | LiSc(BH4)4 | [29] | |
| Li+ | Lu3+ | LiLu(BH4)4 | 6.7 | Tetragonal | P2c | LiSc(BH4)4 | [19] | |
| Na+ | Sc3+ | NaSc(BH4)4 | 12.7 | Orthorhombic | Cmcm | HT-CrVO4 | [41] | |
| Na+ | Y3+ | NaY(BH4)4 | 9.4 | Orthorhombic | Cmcm | NaSc(BH4)4 | [10,40] | |
| Na+ | La3+ | NaLa(BH4)4 | 7.3 | Orthorhombic | Pbcn | New | [49] | |
| Na+ | Ce3+ | NaCe(BH4)4 | 7.3 | Orthorhombic | Pbcn | NaLa(BH4)4 | [42] | |
| Na+ | Pr3+ | NaPr(BH4)4 | 7.2 | Orthorhombic | Pbcn | NaLa(BH4)4 | [42] | |
| Na+ | Er3+ | NaEr(BH4)4 | 6.5 | Orthorhombic | Cmcm | NaSc(BH4)4 | [42] | |
| Na+ | Yb3+ | NaYb(BH4)4 | 6.3 | Orthorhombic | Cmcm | NaSc(BH4)4 | [45] | |
| K+ | Sc3+ | KSc(BH4)4 | 11.3 | Orthorhombic | Pnma | BaSO4 | [46] | |
| K+ | Y3+ | o-KY(BH4)4 m-KY(BH4)4 | 8.6 | Orthorhombic Monoclinic | Cmcm C2/c | NaSc(BH4)4 rt-LaNbO4 | [38,43] [38] | |
| K+ | La3+ | K3La(BH4)6 | 7.0 | Monoclinic | P21/n | Double-perovskite | [49] | |
| K+ | Ce3+ | K3Ce(BH4)6 | 7.0 | Monoclinic | P21/c | Double-perovskite | [48] | |
| K+ | Sm2+ | KSm(BH4)3 | 5.2 | Monoclinic | P21cn | Perovskite-related | [51] | |
| K+ | Gd3+ | KGd(BH4)4 K2Gd(BH4)5 K3Gd(BH4)6 | 6.3 6.5 6.7 | Monoclinic Monoclinic Monoclinic | P21/c P21/m P21/c | LiMnF4 New Double-perovskite | [52] [52] [52] | |
| K+ | Ho3+ | KHo(BH4)4 | 6.1 | Orthorhombic | Cmcm | NaSc(BH4)4 | [32] | |
| K+ | Er3+ | KEr(BH4)4 | 6.1 | Orthorhombic | Cmcm | NaSc(BH4)4 | [44] | |
| K+ | Yb2+ | LT-KYb(BH4)3 HT-KYb(BH4)3 | 4.7 | Cubic Orthorhombic | P3m Pmc21 | Perovskite-related Perovskite-related | [53] [53] | |
| K+ | Yb3+ | KYb(BH4)4 | 5.9 | Orthorhombic | Cmcm | NaSc(BH4)4 | [45] | |
| Rb+ | Y3+ | o-RbY(BH4)4 m-RbY(BH4)4 Rb3Y(BH4)6 | 6.9 6.9 5.6 | Orthorhombic Monoclinic Cubic | Pnma P21/c Fm | KSc(BH4)4 Distorted KSc(BH4)4 Double-perovskite | [38] [50] [38,53] | |
| Rb+ | Ce3+ | Rb3Ce(BH4)6 | 5.0 | Monoclinic | P21/c | Double-perovskite | [53] | |
| Rb+ | Sm2+ | RbSm(BH4)3 | 4.3 | Orthorhombic | Pbn21 | Perovskite-related | [51] | |
| Rb+ | Eu2+ | RbEu(BH4)3 | 4.3 | Orthorhombic | Pna21 | Perovskite-related | [53] | |
| Cs+ | Y3+ | CsY(BH4)4 Cs2Y(BH4)5 Cs3Y(BH4)6 | 5.7 4.2 | Tetragonal Hexagonal Cubic | I41/a P63cm Fm | CaWO4 new Double-perovskite | [50] [38] [38,53] | |
| Cs+ | Sm2+ | α -CsSm(BH4)3 α’-CsSm(BH4)3 β-CsSm(BH4)3 | 3.7 | Orthorhomic Tetragonal Cubic | P21212 Pm2 Pmm | Perovskite-related Perovskite-related Ideal perovskite | [51] [51] [51] | |
| Cs+ | Eu2+ | CsEu(BH4)3 | 3.7 | Tetragonal | P4/mbm | Perovskite-related | [53] | |
| Cs+ | Gd3+ | Cs3Gd(BH4)6 | 3.8 | Cubic | Fm | Double-perovskite | [53] | |
| Li+ | K+ | La3+ | Li3K3La2(BH4)12 | 8.1 | Cubic | Iad | Garnet | [48] |
| Li+ | K+ | Ce3+ | Li3K3Ce2(BH4)12 | 8.1 | Cubic | Iad | Garnet | [48] |
| Li+ | Rb+ | Y3+ | Rb2LiY(BH4)6 | 6.8 | Cubic | Fm | Double-perovskite | [38,50] |
| Li+ | Cs+ | Y3+ | Cs2LiY(BH4)6 | 5.4 | Cubic | Fm | Double-perovskite | [38,50] |
| Li+ | Cs+ | Ce3+ | Cs2LiCe(BH4)6 | 4.8 | Cubic | Fm | Double-perovskite | [53] |
| Li+ | Cs+ | Gd3+ | Cs2LiGd(BH4)6 | 4.7 | Cubic | Fm | Double-perovskite | [53] |
| Cation | Compound | Tdec. (°C) | Decompositon Products | Comments | Ref. |
|---|---|---|---|---|---|
| La3+ | La(BH4)3 | 258 | Not specified | Material obtained from thermolysis of La(BH4)3 nS(CH3)2; 8% mass loss due to simultaneous release of B2H6 and H2 | [34] |
| Ce3+ | Ce(BH4)3 | 251 | Not specified | Material obtained from thermolysis of Ce(BH4)3 nS(CH3)2; 8.5% mass loss due to simultaneous release of B2H6 and H2 | [34] |
| Pr3+ | Pr(BH4)3 | 275 | No crystalline products | Sample synthesized in toluene and DMS. In situ SR-PXD showed no crystalline decomposition product until 500 °C. | [30] |
| Gd3+ | Gd(BH4)3 | 260 | Not specified | Sample obtained from thermal decomposition of Gd(BH4)3*nS(CH3)2; DSC shows endothermic event at 260 °C related to hydrogen desorption. MS shows minor contributions of B2H6 between 225 and 275 °C. | [60] |
| Tb3+ | Tb(BH4)3 | 213 and 253 | Not specified | 3LiBH4-TbCl3 mixture after ball milling (2 h). DSC trace shows two endothermic events with peak temperatures of 213 and 253 °C | [19] |
| Dy3+ | Dy(BH4)3 | No data | No data | Only structural information available; decomposition is believed to occur similar to Y(BH4)3 and Gd(BH4)3 | [18] |
| Ho3+ | Ho(BH4)3 | 170 225 255 | No crystalline products | Three distinct endothermic events visible in the DSC up to 400 °C; onset of hydrogen release observed at 170 °C with the fastest rate at 255 °C | [32] |
| Er3+ | Er(BH4)3 | 230 | ErH2 and ErB4 | Hydrogen desorption starts at 230 °C; multiple endothermic events visible in DSC; 3.2% mass loss observed up to 400 °C | [30] |
| Cation1 | Cation 2 | Compound | Tdec. (°C) | Decompositon Products | Comments | Ref |
|---|---|---|---|---|---|---|
| La3+ | Li+ | LiLa(BH4)3Cl | 226 and 266 | LaH2 and LaB6 | TG/DSC shows two endothermic events between 200 and 300 °C and total mass loss of 2.87% | [9] |
| Ce3+ | Li+ | LiCe(BH4)3Cl | a 215, a 246, b 240, b 266 | CeH2 and CeB6 | a 3LiBD4-CeCl3 mixture after ball milling; two endothermic events at 215 and 246 °C observed by DSC b 3LiBH4-CeCl3 mixture after ball milling: major DSC events at 213 (melting), 240, and 266 °C (both decomposition) | [8,36] |
| Pr3+ | Li+ | LiPr(BH4)3Cl | a 176 b 212 and 247 | PrH3 and PrB6 | a Synthesised in (C2H5)2O. Sample is a mixture between Pr(BH4)3 and LiPr(BH4)3Cl; Tdec determined by TG/DSC b 3LiBH4-PrCl3 mixture after ball milling.TG/DSC shows two endothermic events at 212 °C and 247 °C with 0.7% and 2.6% mass loss, respectively | [30] |
| Nd3+ | Li+ | LiNd(BH4)3Cl | 223 and 241 | Not specified | 3LiBH4-NdCl3 mixture after ball milling. TG/DSC shows two endothermic events at 223 °C and 241 °C with 0.4% and 2.4% mass loss respectively | [19] |
| Gd3+ | Li+ | LiGd(BH4)3Cl | 261 | GdB4 | Value determined from thermogravimetric experiment | [9] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frommen, C.; Sørby, M.H.; Heere, M.; Humphries, T.D.; Olsen, J.E.; Hauback, B.C. Rare Earth Borohydrides—Crystal Structures and Thermal Properties. Energies 2017, 10, 2115. https://doi.org/10.3390/en10122115
Frommen C, Sørby MH, Heere M, Humphries TD, Olsen JE, Hauback BC. Rare Earth Borohydrides—Crystal Structures and Thermal Properties. Energies. 2017; 10(12):2115. https://doi.org/10.3390/en10122115
Chicago/Turabian StyleFrommen, Christoph, Magnus H. Sørby, Michael Heere, Terry D. Humphries, Jørn E. Olsen, and Bjørn C. Hauback. 2017. "Rare Earth Borohydrides—Crystal Structures and Thermal Properties" Energies 10, no. 12: 2115. https://doi.org/10.3390/en10122115





