1. Introduction
Propiconazole (
Figure 1) is a broad-spectrum 1,2,4-triazole fungicide used for the control of fungal diseases on cereals, bananas, turf, rice, peanuts, stone fruit and maize. It is a systemic foliar fungicide that acts by interfering with ergosterol biosynthesis and inhibition of steroid demethylation [
1]. Propiconazole is enzymatically oxidized at the side chain attached to the dioxolane ring and by deketalization with the loss of the dioxolane moiety in plants, soils and fungi.
Since propiconazole is used as an agricultural fungicide, there is concern regarding potential human and wildlife exposure from residues and metabolites in the environment, including soil, sediment, and water receiving soil runoff. Kahle
et al. [
2] detected propiconazole at concentrations of 4–27 ng/L in the influents of all ten wastewater treatment plants examined; its concentration was largely unaffected by wastewater treatment. Kreuger [
3], in a study of pesticides in stream water in an agricultural catchment in southern Sweden, found propiconazole at maximum concentrations of 2.8, 20 and 0.6 μg/L at three sites. Propiconazole was one of the pesticides detected in the highest concentrations (130 μg/Kg) in the top 1–2 cm of streambed sediments in 30 Danish lowland streams [
4]. A Norwegian study [
5] showed that due to its high sorbtivity and mobility, particles of <2 μm can be important carriers of propiconazole in runoff suspensions entering water bodies.
Propiconazole is known to be degraded in soils by hydroxylation of the
n-propyl side chain and the dioxolane ring as well as with formation of 1,2,4-triazole with DT
50 (loss of 50% of the parent) values of 40–70 days under aerobic conditions at 25 °C [
1]. Another source [
6] gives a range of soil half life of 30 to 112 days in an aerobic soil under controlled conditions. In a study of laboratory degradation of propiconazole and three other pesticides in four Norwegian soils, propiconazole was shown to be more persistent than the other pesticides, with persistence depending upon the soil properties [
7]. Riise [
8], also in Norway, showed that different size fractions of soil possessed different physical and chemical properties and, therefore, different capacities to associate with propiconazole. In laboratory studies on formation and loss of bound residues of propiconazole in soils, Kim
et al. [
9] showed that the half-life of the bound fungicide was about 315 days in sandy loam soil, but was beyond the experimental time limit for silty clay loam soil. Finally, these same investigators showed in another study that most of the propiconazole applied to the soil surface in a rice-paddy-soil lysimeter remained in the top 10 cm of the soil, and a large fraction of that was bound to the organic-rich topsoil [
10]).
In addition to possible exposure of these fungicides to humans and wildlife through soil and water residues, their stereoselective transformation to form new and possible more harmful compounds is also of concern. All of the conazole fungicides are chiral, which can be a critical feature in their environmental behavior and toxicity. Many pesticides are chiral and can be metabolized enantioselectively (stereoselectively) by microbes, becoming depleted in one enantiomer while enriched in the other. In addition, the metabolite may be chiral, as is often the case with conazoles [
11]. Established data show that a wide variety of chiral pesticides occur non-racemically—existing as unequal concentrations of their enantiomers (or stereoisomers)—in various environmental compartments, are transformed enantioselectively in environmental microcosms, produce chiral metabolites which may themselves be enantioselective, or have enantioselective toxic effects on various organisms [
12].
A frequently occurring complication, common to many conazoles, is that many chiral pesticides have more than one chiral center; this results in four or more stereoisomers, each possibly having different biological properties, which leads to stereoselectivity in biotransformation rates, persistence, and toxicity to both target and non-target organisms. This complicates both chemical analysis and risk assessment. For example, since it is known that diastereomer A of the fungicide triadimenol is ten times more acutely toxic to rats (oral LD
50) than is diastereomer B [
13], the stereoselective formation of triadimenol from triadimefon is an important issue for both human health and ecological risk assessment [
11].
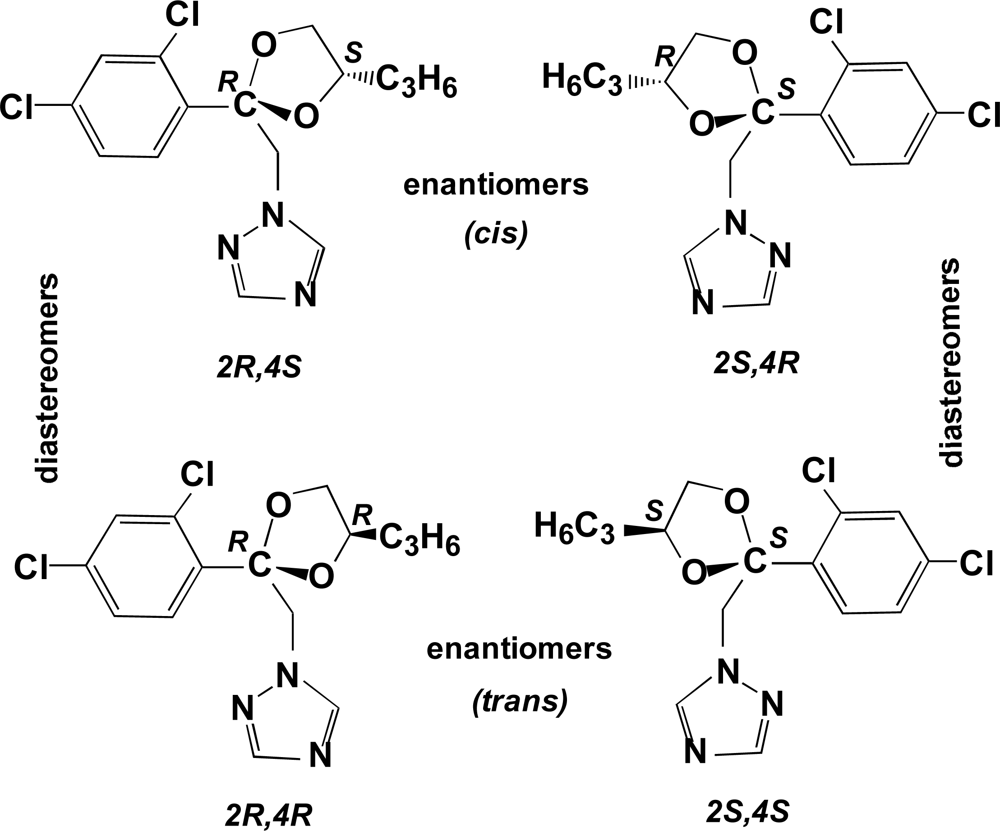
Propiconazole has two chiral centers, at the 2- and 4-positions of the dioxolane ring, and thus exists as two pairs of diastereomers and two pairs of enantiomers for a total of four stereoisomers (
Figure 1). The two pairs of enantiomers are referred to as
cis [(2R,4S) and (2S,4R)] and
trans [(2R,4R) and 2S,4S)] [
14,
15]. The isomers with absolute configuration 2S are more efficient inhibitors of ergosterol biosynthesis than the corresponding 2R isomers. In addition, field trials of the individual stereoisomers showed that each of three different pathogenic fungi species discriminated in its biological activity toward each stereoisomer, and that the fungicidal activity as well as the small plant growth regulating effects of the different isomers varied for different target organisms [
14]. In soils, several metabolic products result from hydroxylation of the propyl side chain or of the dioxolane ring. Previous research showed that the metabolism of propiconazole in winter wheat to produce the three side-chain hydroxylation products was stereoselective;
i.e., each of the four stereoisomers formed the products at different rates [
1,
16].
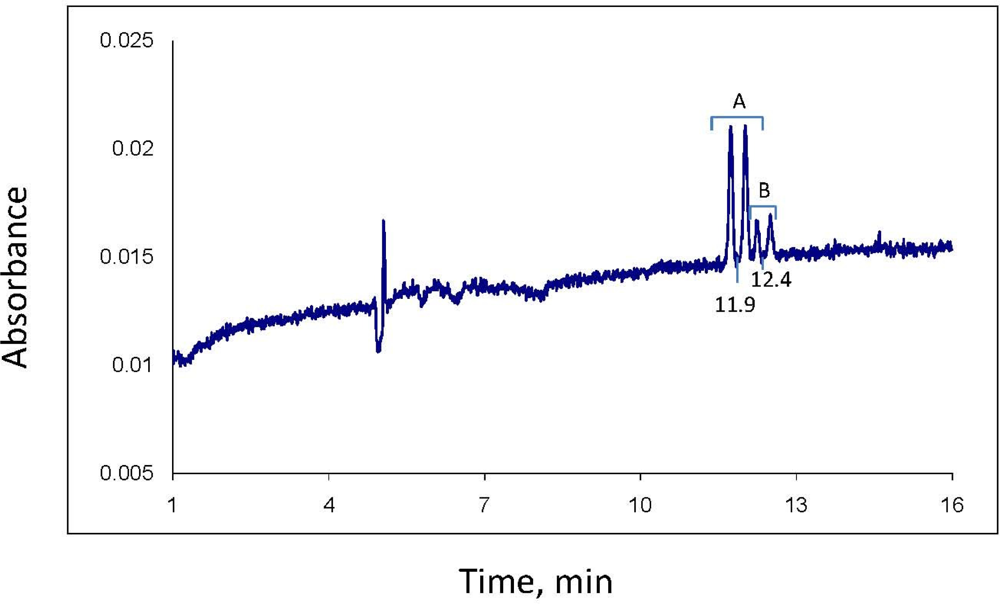
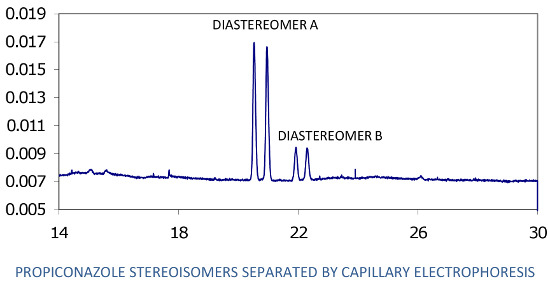
Analysis of the separate stereoisomers of chiral pesticides is usually accomplished by HPLC or GC using chromatography columns with chiral solid phases. Such analysis is also possible using capillary electrophoresis (CE). The analytical approach used here involves the mode of CE known as MEKC (micellar electrokinetic chromatography) [
17], which was developed a few decades ago expressly for the CE analysis of neutral analytes. In MEKC, a typical CE buffered electrolyte, such as is used in capillary zone electrophoresis for analysis of ionic analytes, is prepared and a micelle forming compound such as sodium dodecyl sulfate (SDS) for complexing neutral analytes and giving them a charge and resultant mobility in the electrolyte-filled column is added. Finally, for analysis of enantiomers, a chiral selector, e.g., a cyclodextrin, is added to selectively complex the enantiomers of the chiral analyte, creating diastereomers that, in turn, complex with the SDS to different degrees and allow separation by the system. Several articles provide the basic methodology, even recipes, for separation of pesticide enantiomers, including those in environmental samples [
17–
20].
In this research, a MEKC method was developed for CE separation and analysis of the four stereoisomers of propiconazole and applied to follow the loss of the stereoisomers from the water phase of two different aerobic soil-water slurries over about five months. Gas chromatography-mass spectrometry using a column with a chiral phase was employed as an auxiliary technique. Goals of this research were to demonstrate the application of CE to the analysis of chiral pesticides and their stereoisomers in environmental matrices, as well as to determine whether the loss of propiconazole from soil is stereoselective.
2. Experimental
2.1. Chemicals
The propiconazole standard was obtained from the U.S. Environmental Protection Agency National Pesticide Standard Repository (Ft. Meade, MD, USA); its purity was 99.6%. Solutions of the fungicide were prepared at 5 μg/mL in methyl tert-butyl ether (MTBE) and analyzed by GG-MS to check for purity, which nominally matched the given percentage purity. Methanol, MTBE, other organic solvents, sodium hydroxide and inorganic buffer salts were of analytical grade from Fisher Chemicals (Fair Lawn, NJ, USA). CE reagents, including 2-hydroxypropyl γ-cyclodextrin (the chiral selector) and sodium dodecyl sulfate (for micelle formation) were obtained from Sigma-Aldrich. Reagent water for all experiments was produced by a Barnstead Nanopure Infinity water purification system (Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Soil Sources, Collection Data and Physiocochemical Characteristics
Soil 1 (USDA): J. Phil Campbell Natural Resource Conservation Center, Watkinsville, GA, USA. This is a composite agricultural soil collected from the A horizon of a cropped field on this USDA property. After collection, the soil was air-dried, sieved to <2 mm and riffled for homogenization.
Soil 2 (UGA): Univ. of Georgia Horticulture farm, Watkinsville, GA. This is a composited soil collected from the top 10 cm of the peach tree area of the horticulture farm. It was air-dried, sieved to <1 mm and homogenized (this soil was collected at a different time from soil 1 and inadvertently sieved to a smaller particle size.)
Physicochemical characteristics of soils [CEC = cation exchange capacity (meq/100g) and OM = organic matter]:
Soil 1: pH, 6.2; CEC, 8.9; sand, 70%; soil, 19%; clay, 11%; OM, 3.0%.
Soil 2: pH, 6.3; CEC, 13.9; sand, 72%; soil, 18%; clay, 10%; OM, 5.4%.
Soil pH was measured in a slurry of 1:2.5 soil to 0.01 M CaCl2 solution (wt:vol). The other soil parameters were measured by a contractor using standard methods.
2.3. Propiconazole Exposure to Soils
The experiment required preparation of 50 μg/mL of propiconazole in 1:5 (wt:vol) soil-water slurries of each soil in mineral/nutrient water. For this, 25 mg of propiconazole was dispensed in methanol solution onto the bottom of an autoclaved (121 °C for 20 min) Erlenmeyer flask; the solvent was allowed to evaporate under ambient conditions. Simultaneously, 500 mL of an aqueous solution of minerals and nutrients used to support growth of standard microbial cultures [
21] was autoclaved, covered with foil, and cooled. This solution was added to the flask containing the propiconazole and the contents were stirred overnight with a magnetic stirrer at room temperature to produce 50 μg/mL of the fungicide in the mineral/nutrient water, as verified by the GC/MS analytical method described below. Aliquots of 20 mL of this aqueous solution were transferred to each of several 50 mL amber serum vials which had been autoclaved. Four grams of soil 1 (not autoclaved) were then added to each of these vials. This process was repeated with soil 2. These vials were then stoppered and shaken overnight, and constituted the active (spiked) treatments.
There were also produced, by autoclaving the soils, a corresponding number of controls. For this, 4 g of soil 1 was wetted with 2.0 mL of water and transferred to each of several amber serum vials; this was repeated for soil 2. These soils were autoclaved (121 °C for 20 min) once a day for 3 days; then 20 mL of the above autoclaved mineral/nutrient solution containing 50 ug/mL of propiconazole was added to each vial and the vials were shaken overnight. All vials, of both active and control treatments, for these kinetic experiments were shaken continuously in the dark in a thermostated shaker at 20 °C for the course of the experiment.
2.4. Extraction and Sample Preparation Methods
At t0 and each successive time point, aliquots of the water phase were taken from all vials, active and control, for CE and, in some cases, GC-MS analysis. First, each vial was manually shaken to mix the slurry, then the stopper was immediately removed and about 1mL of the slurry was collected with a wide-mouth pipet and dispensed into a 2 mL centrifuge tube. The tube and contents were centrifuged at 14,000 rpm for 20 min (Eppendorf centrifuge 5418). As much as possible of the aqueous phase was removed with an automatic pipet. For CE analysis, 40 μL of this was fitered through a 0.45 μm syringe filter into a CE analysis vial. For GC-MS, 0.5 mL of this phase was extracted with 0.5 mL of methyl tert-butyl ether (MTBE) by vibration with a vortex mixer; 100 μL of this extract was diluted with 900 μL of MTBE for final GC-MS analysis.
2.5. Propiconazole Recovery Data
Recovery from deionized water spiked at 50 μg/mL; average of duplicates: 97%.
Recovery from spiked soil-water slurries: a slurry of 5 mL of water and 0.5 g of soil 1 containing 50 μg/mL propiconazole was prepared as described above (section 2.3) and shaken overnight at 24 °C. The slurry was vortexed, a 1 mL sample of the slurry was collected, and the water was extracted as described above (see Extraction and Sample Preparation Methods) for subsequent analysis by CE. The solid (soil phase) remaining in the centrifuge tube was washed with 1.0 mL water, which was discarded. (This step was included to remove most of the soil pore water, which may have contained dissolved propiconazole, from the soil phase to allow for more accurate soil analysis.) The wet soil was extracted by vortexing with 1.0 mL of MeOH 2 times; extracts were then combined and evaporated to 0.5 mL. MeOH was selected as the extraction solvent because of its water miscibility. This extract was diluted with 1.5 mL of water to achieve an appropriate 25% MeOH aqueous solution for CE analysis; 40 μL of this was filtered through a 0.45 μm syringe filter. Both water and soil phases were analyzed by CE (see section 2.6). This procedure was repeated for soil 2, in duplicate.
Average % recovery (duplicates) of propiconazole from water phase of spiked soil:water slurry: Soil 1, 34.7%; Soil 2, 30.9%.
Average % recovery (duplicates) of propiconazole from soil phase of spiked soil:water slurry: Soil 1, 40.6%; Soil 2, 34.1%.
Total recoveries (from water + soil): Soil 1, 75.3%; Soil 2, 65.0%. Apparently, about 25% and 35%, respectively, of propiconazole was irreversibly sorbed to the soil, lost in the 1 mL of water used to wash the soil, or lost by sorption to glassware during the extraction process. The total recoveries are considered adequate for these experiments.
2.6. CE Analysis
CE analysis was with a liquid cooled Beckman P/ACE System 5500 CE, with diode array UV detector, hydrodynamic injection, power supply up to 30 kV, and System Gold version 8.1 chromatography software (Beckman Instruments, Fullerton, CA, USA). The CE column was uncoated fused silica: 75 μm id, 300 μm od, 57 cm total length, and 50 cm effective length (MicroSolv Technology Corp., Long Branch, NJ, USA). Samples were contained in vial inserts for small volumes (40 μL or 400 μL, Beckman Instruments). The CE samples prepared during the sample extraction procedure described above were filtered through a 0.45 μm nylon syringe filter (or equivalent) just before transfer to the CE vials.
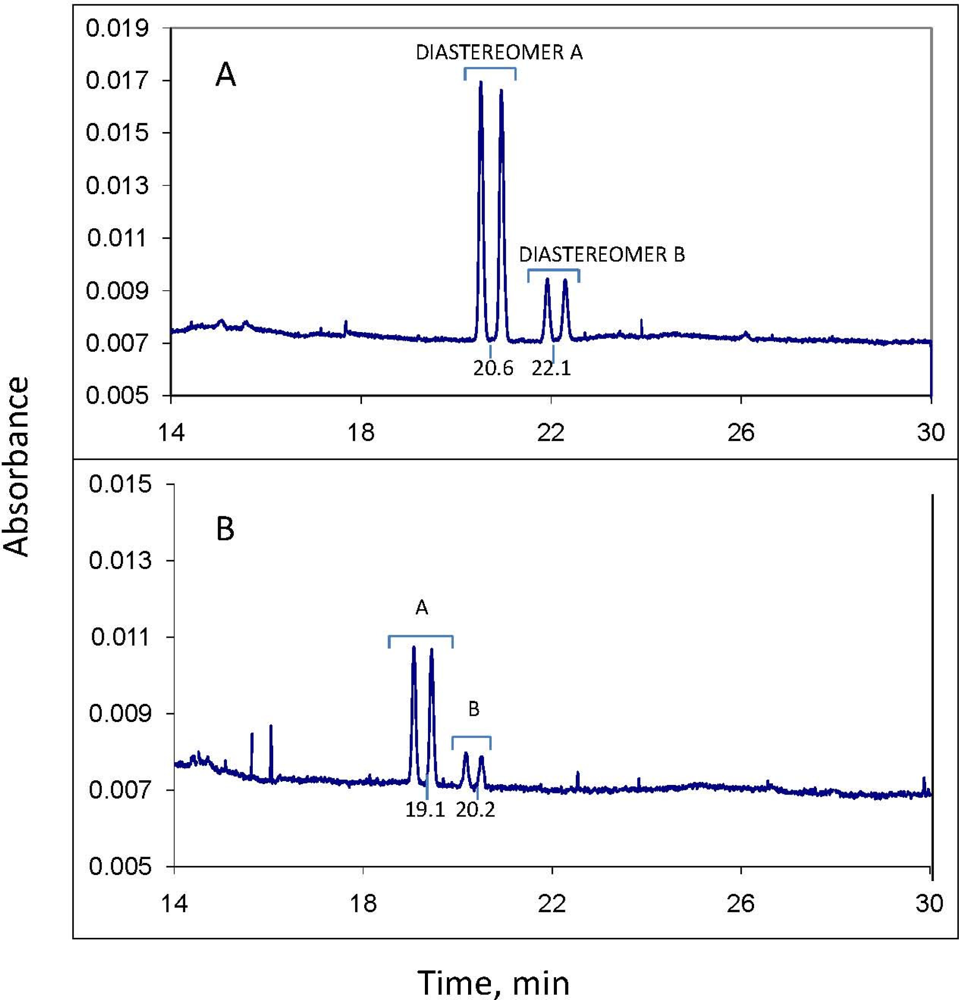
Before analysis of each sample, the column was washed with distilled water for 2 min, 0.10 M NaOH for 2 min, water again for 2 min, and electrolyte solution for 2 min. The column was maintained at 23 °C. Sample injection was hydrodynamic, usually for 6.5 sec; injections of 10 sec were used if more sensitivity was needed. Typical MEKC electrolyte composition was: 25 mM NaPO4 buffer, pH 7; 75 mM sodium dodecyl sulfate; 30 mM 2-hydroxypropyl-γ-cyclodextrin; and 10% methanol and 5% acetonitrile as organic modifiers. Instrumental conditions: temperature, 23 °C; detector wavelength, 190 nm; voltage, 30 kV; and run time, 30 min. The limit of quantitation for each of the 2 minor propiconazole stereoisomers in the water phase of a water-soil slurry was observed to be about 0.3 mg/L. However, for quantitation of all four stereoisomers (total propiconazole), the limit is about 2.5 mg/L.
2.7. GC-MS Analysis
GC-MS analysis of propiconazole standards and extracts was by use of a Hewlett-Packard 5973 mass spectrometer linked to a 6890 gas chromatograph equipped with a BGB 172 (BGB Analytik AG, Switzerland) chiral column. Column description: 30 m × 0.25 mm ID × 0.25 μm film thickness; chiral stationary phase, 20% tert-butyldimethylsilylated-β-cyclodextrin. GC conditions were: injection, splitless at temp. 275 °C; column temp. program, 150–220 °C at 4°/min, followed by temp. hold for 60 min; helium gas flow, 1.5 mL/min; MS inlet temp., 275°; MS source temp., 230°; and fragmentation voltage, 70 eV. Sample injection volume was 1 μL. Detection was by selected ion monitoring (SIM); SIM ions were m/z 173, 259 and 261 for propiconazole.
Quantitation for both CE and GC-MS was by comparison of stereoisomer peak areas to those of propiconazole standards of similar concentration analyzed the same day; quality control included analysis of at least one standard per day as well as analysis of a standard before and after each 10 samples. These standards were in turn referenced to a standard curve of propiconazole stereoisomer peak area vs. concentration. For example, a standard curve was generated by CE using a series of 3, 5, 8, 10, 50 and 100 mg/L standards of propiconazole in water plotted against CE peak area to give a linear plot with R2 of 0.9922. Reproducibility of propiconazole quantitation by CE was measured using a 5 mg/L standard solution in water, with n = 6. The percent relative standard deviation for peak 1 stereoisomer was 5.6%, and for peak 2, 7.3%. The RSD for the 2 minor peaks were higher because of their low concentrations (about 0.5 mg/L each): peak 3 was 31.9% and peak 4 was 36.1%. The RSD for total propiconazole was 18.9%.
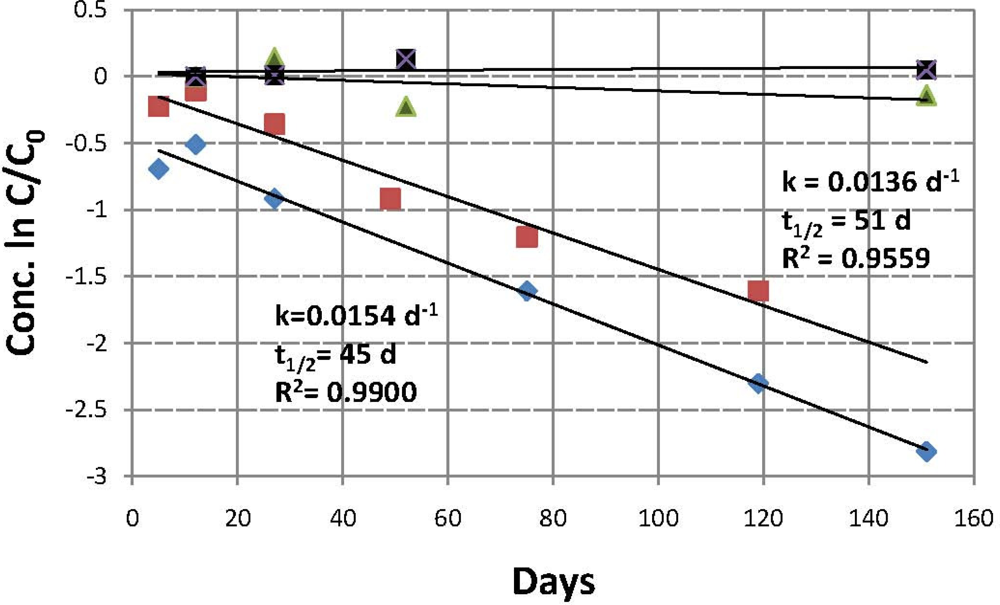
 soil 1, active;
soil 1, active;
 soil 2, active; ▪ soil 1, control;
soil 2, active; ▪ soil 1, control;
 soil 2, control.
soil 2, control.
 soil 1, active;
soil 1, active;
 soil 2, active; ▪ soil 1, control;
soil 2, active; ▪ soil 1, control;
 soil 2, control.
soil 2, control.