Constitutive, but Not Challenge-Induced, Interleukin-10 Production Is Robust in Acute Pre-Pubescent Protein and Energy Deficits: New Support for the Tolerance Hypothesis of Malnutrition-Associated Immune Depression Based on Cytokine Production in vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing Facilities
2.2. Diets
2.3. Experimental Design
2.4. In vivo Stimulation of IL-10 and Assessment of Cytokine Production by in vivo Capture Assay
2.5. Blood Sampling Procedure for the in vivo Capture Assay
2.6. Procedure to Obtain Mononuclear Cell Suspensions for in vitro Stimulation
2.7. In vitro Stimulation of Mononuclear Cells with LPS to Elicit IL-10 Production
2.8. In vitro Stimulation of T Cells with Anti-CD3 to Elicit IL-10 Production
2.9. Assay of IL-10 Concentrations Generated in vitro
2.10. Assessment of Percentage CD3+ Cells in Mononuclear Cell Suspensions from Spleen and Lymph Nodes
2.11. Assessment of Carcass Composition
2.12. Statistical Analyses
3. Results
3.1. Distinct Weight Loss Pathologies Were Elicited by the Malnutrition Protocols
3.2. IL-10 Production in vivo and in vitro in Response to Either LPS or Anti-CD3
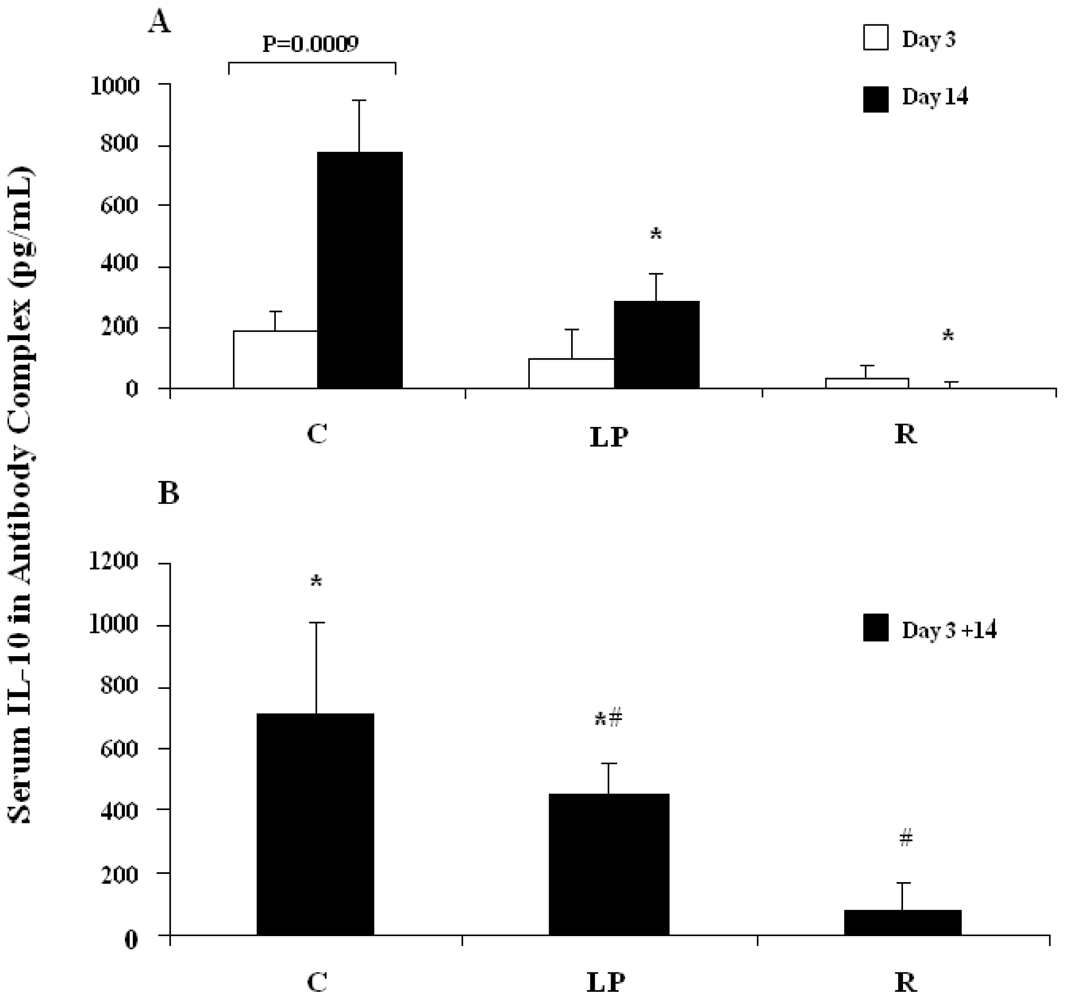
3.2.1. IL-10 capture in vivo following stimulation with LPS
3.2.2. IL-10 capture in vivo following stimulation with anti-CD3
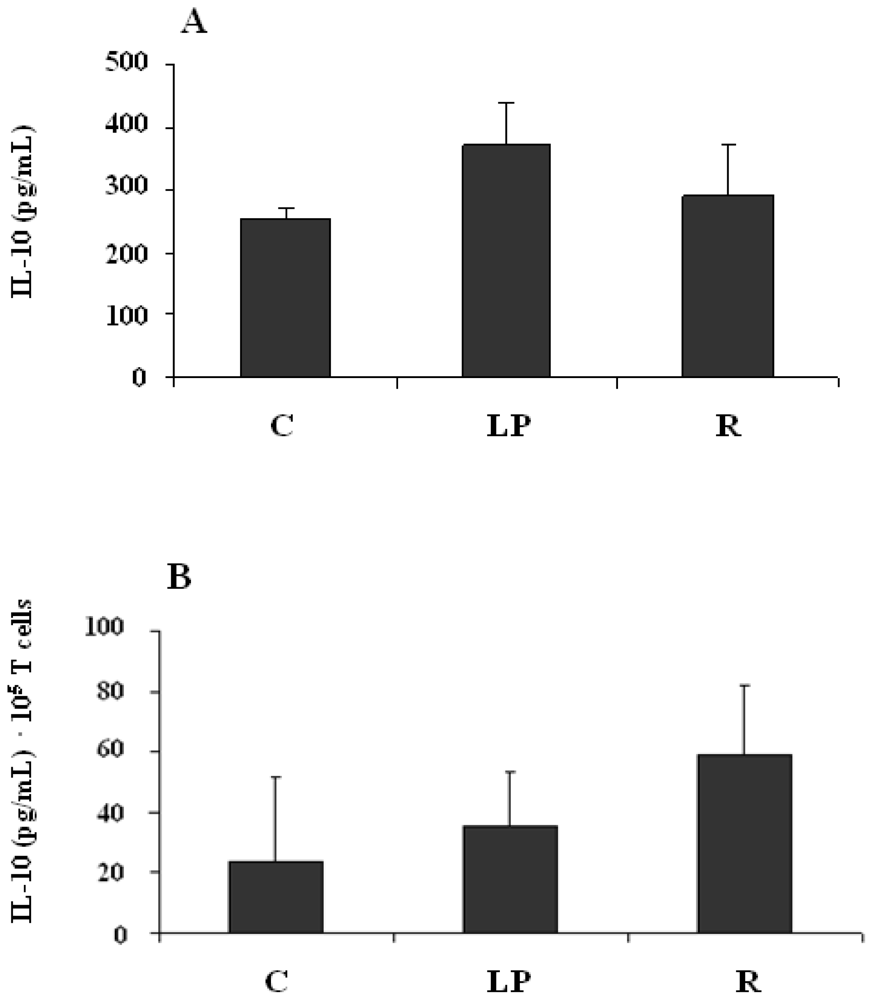
3.2.3. IL-10 production by mononuclear cells stimulated in vitro with LPS
3.2.4. IL-10 production by mononuclear cells stimulated in vitro with anti-CD3
4. Discussion
Acknowledgements
References
- Woodward, B. Chandra, RK, Ed.; Depressed adaptive immune competence in acute protein-energy malnutrition: A regulated pathophysiology controlled by endocrine hormones and cytokines. In Nutrition and Immunology in the 21st Century; TSAR Health: Toronto, Canada, 2004; pp. 23–38. [Google Scholar]
- Woodward, B. Suskind, RM, Tontisirin, K, Eds.; The effect of protein-energy malnutrition on immune competence. In Nutrition, Immunity, and Infection in Infants and Children; Vevey/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001; pp. 89–120. [Google Scholar]
- Woodward, B; Filteau, SM. Immunoenhancement in wasting protein-energy malnutrition: assessment of present information and proposal of a new concept. Adv. Nutr. Res 1990, 8, 11–34. [Google Scholar]
- Hillyer, L; Whitley, C; Olver, A; Webster, M; Steevels, T; Woodward, B. Adoptively transferred dendritic cells restore primary cell-mediated inflammatory competence to acutely malnourished weanling mice. Am. J. Pathol 2008, 172, 378–385. [Google Scholar]
- Hillyer, L; Dao, B; Niemiec, P; Lee, S; Doidge, M; Bemben, I; Neyestani, T; Woodward, B. Elevated bioactivity of the tolerogenic cytokines, interleukin-10 and transforming growth factor-β, in the blood of acutely malnourished weanling mice. Exp. Biol. Med 2006, 231, 1439–1447. [Google Scholar]
- Monk, JM; Woodward, B. The blood level of transforming growth factor-beta rises in the early stages of acute protein and energy deficit in the weanling mouse. Br. J. Nutr 2010, 103, 886–889. [Google Scholar]
- Monk, JM; Woodward, B. Elevated blood interleukin-10 levels and undiminished systemic interleukin-10 production rate prevail throughout acute protein-energy malnutrition in the weanling mouse. Cytokine 2009, 47, 126–131. [Google Scholar]
- Li, MO; Flavell, RA. Contextual regulation of inflammation: a duet by transforming growth factor-β and interleukin-10. Immunity 2008, 28, 468–476. [Google Scholar]
- Li, MO; Wan, YY; Sanjabi, S; Robertson, AK; Flavell, RA. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol 2006, 24, 99–146. [Google Scholar]
- Karagiannidis, C; Akdis, M; Holopainen, P; Woolley, HJ; Hense, G; Rücker, B; Mantel, PY; Menz, G; Akdis, CA; Blaser, K; Schmidt-Weber, CB. Glucocorticoids upregulate FOXP3 expression and regulatory T cells in asthma. J. Allergy Clin. Immunol 2004, 114, 1425–1433. [Google Scholar]
- Navarro, J; Aristimuño, C; Sánchez-Ramón, S; Vigil, D; Martínez-Ginés, ML; Fernández-Cruz, E; de Andrés, C. Circulating dendritic cell subsets and regulatory T-cells at multiple sclerosis relapse: Differential short-term changes on corticosteroids therapy. J. Neuroimmunol 2006, 176, 153–161. [Google Scholar]
- Zhang, L; Yi, H; Xia, X-P; Zhao, Y. Transforming growth factor-beta: An important role in CD4+CD25+ regulatory T cells and immune tolerance. Autoimmunity 2006, 39, 269–276. [Google Scholar]
- Vignali, DAA; Collison, LW; Workman, CJ. How regulatory T cells work. Nat. Rev. Immunol 2008, 8, 523–531. [Google Scholar]
- Monk, JM; Makinen, K; Shrum, B; Woodward, B. Blood corticosterone concentration reaches critical illness levels early during acute malnutrition in the weanling mouse. Exp. Biol. Med 2006, 231, 264–268. [Google Scholar]
- Ha, C-L; Wong, SSL; Gray, MM; Watt, J; Hillyer, LM; Woodward, BD. Overabundance of CD45RA+ (quiescent-phenotype) cells within the involuted CD4+ T-cell population follows initiation of immune depression in energy-deficient weanling mice and reflects involution exclusive to the CD45RA− subset. J. Nutr 2001, 131, 1812–1818. [Google Scholar]
- Hillyer, LM; Maliwichi, HE; Woodward, B. Blood serum interferon-gamma bioactivity is low in weanling mice subjected to acute deficits of energy or both protein and energy. Br. J. Nutr 2007, 97, 528–534. [Google Scholar]
- Lee, WH; Woodward, BD. The CD4/CD8 ratio in the blood does not reflect the response of this index in secondary lymphoid organs of weanling mice in models of protein-energy malnutrition known to depress thymus-dependant immunity. J. Nutr 1996, 126, 849–859. [Google Scholar]
- Woodward, B; Hillyer, L; Hunt, K. T cells with a quiescent phenotype (CD45RA+) are overabundant in the blood and involuted lymphoid tissues in wasting protein and energy deficiencies. Immunology 1999, 96, 246–253. [Google Scholar]
- Anderson, P; Delgado, M. Endogenous anti-inflammatory neuropeptides and pro-resolving lipid mediators: a new therapeutic approach for immune disorders. J. Cell. Mol. Med 2008, 12, 1830–1847. [Google Scholar]
- O’Garra, A; Vieira, P. Regulatory T cells and mechanisms of immune system control. Nat. Med 2004, 10, 801–805. [Google Scholar]
- Moore, KW; de Waal-Malefyt, R; Coffman, RL; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol 2001, 19, 683–765. [Google Scholar]
- Mosser, DM; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev 2008, 226, 205–218. [Google Scholar]
- Medzhitov, R. Toll-like receptors and innate immunity. Nat. Rev. Immunol 2001, 1, 135–145. [Google Scholar]
- Filteau, SM; Woodward, B. The effect of severe protein deficiency on serum zinc concentration of mice fed a requirement levels or a very high level of dietary zinc. J. Nutr 1982, 121, 1974–1977. [Google Scholar]
- Marchant, A; Bruyns, C; Vandenabeele, P; Ducarme, M; Géard, C; Delvaux, A; De Groote, D; Abramowicz, D; Velu, T; Goldman, M. Interleukin-10 controls interferon-γ and tumor necrosis factor production during experimental endotoxemia. Eur. J. Immunol 1994, 24, 1167–1171. [Google Scholar]
- Durez, P; Abramowicz, D; Gerard, C; Van Mechelen, M; Amraoui, Z; Dubois, C; Leo, O; Velu, T; Goldman, M. In vivo induction of interleukin-10 by anti-CD3 monoclonal antibody or bacterial lipopolysaccharide: differential modulation by cyclosporin A. J. Exp. Med 1993, 177, 551–555. [Google Scholar]
- Hillyer, LM; Woodward, B. Interleukin-10 concentration determined by sandwich enzyme-linked immunosorbent assay is unrepresentative of bioactivity in murine blood. Am. J. Physiol. Regul. Integr. Comp. Physiol 2003, 285, R1514–R1519. [Google Scholar]
- Zhang, X; Hillyer, LM; Woodward, BD. The capacity of non-inflammatory (steady state) dendritic cells to present antigen in the primary response is preserved in acutely protein-or energy-deficient weanling mice. J. Nutr 2002, 132, 2748–2756. [Google Scholar]
- Steevels, TAM; Hillyer, LM; Monk, JM; Fisher, ME; Woodward, B. Effector/Memory T cells of the weanling mouse exhibit type 2 Cytokine polarization in vitro and in vivo in the advanced stages of acute energy deficit. J. Nutr. Biochem 2010, 21, 504–511. [Google Scholar]
- Woods, JW; Woodward, BD. Enhancement of primary systemic acquired immunity by exogenous triiodothyronine in wasted, protein-energy malnourished weanling mice. J. Nutr 1991, 121, 1425–1432. [Google Scholar]
- Woodward, BD; Woods, JW; Crouch, DA. Direct evidence that acquired cell-mediated immunity is less resistant than is primary thymus-dependent humoral immunity to the depressive influence of wasting protein-energy malnutrition in weanling mice. Am. J. Clin. Nutr 1992, 55, 1180–1185. [Google Scholar]
- Baker, PJ. Regulation of magnitude of antibody response to bacterial polysaccharide antigens by thymus-derived lymphocytes. Infec. Immun 1990, 58, 3465–3468. [Google Scholar]
- Bosisio, D; Polentarutti, N; Sironi, M; Bernasconi, S; Miyake, K; Webb, GR; Martin, MU; Mantovani, A; Muzio, M. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-g: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 2002, 99, 3427–3431. [Google Scholar]
- Komai-Koma, M; Jones, L; Ogg, GS; Xu, D; Liew, FY. TLR2 is expressed on activated T cells as a costimulatory receptor. Proc. Nat. Acad. Sci. USA 2004, 101, 3029–3034. [Google Scholar]
- Sabroe, I; Jones, EC; Usher, LR; Whyte, MKB; Dower, SK. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: A critical role for monocytes in leukocyte lipopolysaccharide responses. J. Immunol 2002, 168, 4701–4710. [Google Scholar]
- Dasari, P; Nicholson, IC; Hodge, G; Dandie, GW; Zola, H. Expresion of toll-like receptors on B lymphocytes. Cell. Immunol 2005, 236, 140–145. [Google Scholar]
- O’Garra, A; Vieira, P. TH1 cells control themselves by producing interleukin-10. Nat. Rev. Immunol 2007, 7, 425–428. [Google Scholar]
- Rogers, PR; Dubey, C; Swain, SL. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol 2000, 164, 2338–2346. [Google Scholar]
- Kaech, SM; Wherry, EJ; Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol 2002, 2, 251–262. [Google Scholar]
- Dubey, C; Croft, M; Swain, SL. Naive and effector CD4 T cells differ in their requirements for T cell receptor versus costimulatory signals. J. Immunol 1996, 157, 3280–3289. [Google Scholar]
- Rosenblum, MG; Unger, BW; Gutterman, JU; Hersh, EM; David, GS; Frincke, JM. Modification of human leukocyte interferon pharmacology with a monoclonal antibody. Cancer Res 1985, 45, 2421–2424. [Google Scholar]
- Jones, AT; Ziltener, HJ. Enhancement of the biologic effects of interleukin-3 in vivo by anti-interleukin-3 antibodies. Blood 1993, 82, 1133–1141. [Google Scholar]
- Sato, J; Hamaguchi, N; Doken, K; Gotoh, K; Ootsu, K; Iwasa, S; Ogawa, Y; Toguchi, H. Enhancement of anti-tumor activity of recombinant interleukin-2 (rIL-2) by immunocomplexing with a monoclonal antibody against rIL-2. Biotherapy 1993, 6, 225–231. [Google Scholar]
- Courtney, LP; Phelps, JL; Karavodin, LM. An anti-IL-2 antibody increases serum half-life and improves anti-tumor efficacy of human recombinant interleukin-2. Immunopharmacology 1994, 28, 223–232. [Google Scholar]
- Finkelman, FD; Madden, KB; Morris, SC; Holmes, JM; Boiani, N; Katona, IM; Maliszewski, CR. Anti cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J. Immunol 1993, 151, 1235–1244. [Google Scholar]
- Boyman, O; Kovar, M; Rubinstein, MP; Surh, CD; Sprent, J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006, 311, 1924–1927. [Google Scholar]
- Kamimura, D; Sawa, Y; Sato, M; Agung, E; Hirano, T; Murakami, M. IL-2 in vivo activities and antitumor efficacy enhanced by an anti-IL-2 mAb. J. Immunol 2006, 177, 306–314. [Google Scholar]
- Fernandez-Botran, R; Vitetta, ES. Evidence that natural murine soluble interleukin 4 receptors may act as transport proteins. J. Exp. Med 1991, 174, 673–681. [Google Scholar]
- Bendtzen, K; Svenson, M; Jonsson, V; Hippe, E. Autoantibodies to cytokines–friends or foes? Immunol. Today 1990, 11, 167–169. [Google Scholar]
- Fock, RA; Ramirez Vinolo, MA; de Sa Rocha, RM; de Sa Rocha, LC; Borelli, P. Protein-energy malnutrition decreases the expression of TLR-4/MD-2 and CD14 receptors in peritoneal macrophages and reduces the synthesis of TNF-α in response to lipopolysaccharide (LPS) in mice. Cytokine 2007, 40, 105–114. [Google Scholar]
- Woodward, BD; Bezanson, KD; Hillyer, LM; Lee, W-H. The CD45RA+ (quiescent) cellular phenotype is overabundant relative to the CD45RA− phenotype within the involuted splenic T cell population of weanling mice subjected to wasting protein-energy malnutrition. J. Nutr 1995, 125, 2471–2482. [Google Scholar]
- Woodward, B; Hillyer, L; Hunt, K. T cells with a quiescent phenotype (CD45RA+) are overabundant in the blood and involuted lymphoid tissues in wasting protein and energy deficiencies. Immunology 1999, 96, 246–253. [Google Scholar]
| Dietary Group 2 | ||||
|---|---|---|---|---|
| Index | C | LP | R | SEM |
| Day 3 | ||||
| Initial body weight (g/mouse) | 8.5 | 8.4 | 8.6 | 0.10 |
| Final body weight (g/mouse) | 10.4 X | 7.2 Y | 7.4 Y | 0.10 |
| Food intake (g/mouse · 3d) 3 | 7.8 X | 4.5 Y | 3.6 Y | 0.20 |
| Food intake (g/g body weight · d)3 | 0.19 X | 0.13 Y | 0.09 Z | 0.001 |
| Carcass dry matter (g/100g wet weight) | 30.3 X | 28.3 Y | 27.9 Y | 0.30 |
| Carcass lipid (g/100g wet weight)4 | 9.2 X | 5.9 Y | 3.4 Z | 0.03 |
| Day 14 | ||||
| Initial body weight (g/mouse) | 8.3 | 8.5 | 8.6 | 0.08 |
| Final body weight (g/mouse) 4 | 17.5 X | 6.1 Y | 6.0 Y | 0.02 |
| Food intake (g/mouse · 14d) 5 | 60.6 X | 18.2 Y | 12.6 Z | 0.01 |
| Food intake (g/g body weight · d) | 0.20 X | 0.11 Y | 0.08 Z | 0.002 |
| Carcass dry matter (g/100g wet weight) | 32.1 X | 28.1 Y | 26.5 Z | 0.26 |
| Carcass lipid (g/100g wet weight) | 10.4 X | 4.5 Y | 2.7 Z | 0.34 |
| Dietary Group 2 | ||||
|---|---|---|---|---|
| Index | C | LP | R | SEM |
| Day 3 | ||||
| Initial body weight (g/mouse) | 8.4 | 8.5 | 8.7 | 0.09 |
| Final body weight (g/mouse) | 10.1 X | 7.4 Y | 7.5 Y | 0.12 |
| Food intake (g/mouse · 3 d) 3 | 7.5 X | 4.9 Y | 3.5 Z | 0.04 |
| Food intake (g/g body weight · d) 4 | 0.18 X | 0.13 Y | 0.09 Z | 0.24 |
| Carcass dry matter (g/100g wet weight)3 | 30.4 X | 29.6 X | 27.4 Y | 0.01 |
| Carcass lipid (g/100g wet weight) | 8.0 X | 6.1 Y | 3.8 Z | 0.23 |
| Day 14 | ||||
| Initial body weight (g/mouse) | 8.8 | 8.6 | 8.8 | 0.10 |
| Final body weight (g/mouse) 3 | 18.5 X | 6.5 Y | 6.2 Y | 0.02 |
| Food intake (g/mouse · 14 d) 3 | 65.6 X | 16.6 Y | 11.2 Z | 0.03 |
| Food intake (g/g body weight · d) 3 | 0.27 X | 0.10 Y | 0.07 Z | 0.02 |
| Carcass dry matter (g/100g wet weight) 3 | 32.8 X | 27.9 Y | 26.8 Y | 0.02 |
| Carcass lipid (g/100g wet weight) 3 | 10.0 X | 4.1 Y | 2.0 Z | 0.04 |
© 2011 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Monk, J.M.; Steevels, T.A.M.; Hillyer, L.M.; Woodward, B. Constitutive, but Not Challenge-Induced, Interleukin-10 Production Is Robust in Acute Pre-Pubescent Protein and Energy Deficits: New Support for the Tolerance Hypothesis of Malnutrition-Associated Immune Depression Based on Cytokine Production in vivo. Int. J. Environ. Res. Public Health 2011, 8, 117-135. https://doi.org/10.3390/ijerph8010117
Monk JM, Steevels TAM, Hillyer LM, Woodward B. Constitutive, but Not Challenge-Induced, Interleukin-10 Production Is Robust in Acute Pre-Pubescent Protein and Energy Deficits: New Support for the Tolerance Hypothesis of Malnutrition-Associated Immune Depression Based on Cytokine Production in vivo. International Journal of Environmental Research and Public Health. 2011; 8(1):117-135. https://doi.org/10.3390/ijerph8010117
Chicago/Turabian StyleMonk, Jennifer M., Tessa A.M. Steevels, Lyn M. Hillyer, and Bill Woodward. 2011. "Constitutive, but Not Challenge-Induced, Interleukin-10 Production Is Robust in Acute Pre-Pubescent Protein and Energy Deficits: New Support for the Tolerance Hypothesis of Malnutrition-Associated Immune Depression Based on Cytokine Production in vivo" International Journal of Environmental Research and Public Health 8, no. 1: 117-135. https://doi.org/10.3390/ijerph8010117




