Effect of Medicaid Coverage of Tobacco-Dependence Treatments on Smoking Cessation
Abstract
:1. Introduction
2. Medicaid Background
3. Literature Review
4. Data and Model
5. Results and Discussion
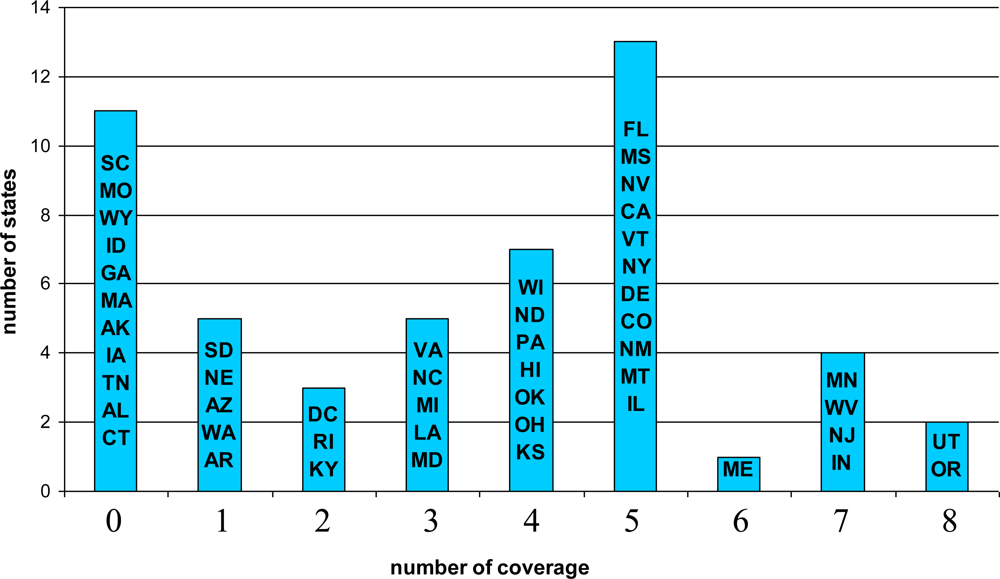
5.1. Descriptive Results
5.2. Multivariate Regression Results
5.3. Robustness Check
5.4. Effect of TDT Coverage on Initiation
6. Conclusions and Discussions
Acknowledgments
References and Notes
- Lillard, DR; Plassmann, V; Kenkel, D; Mathios, A. Who kicks the habit and how they do it: socioeconomic differences across methods of quitting smoking in the USA. Soc. Sci. Med 2007, 64, 2504–2519. [Google Scholar]
- Etter, JF; Stapleton, JA. Nicotine replacement therapy for long-term smoking cessation: a meta-analysis. Tob. Control 2006, 15, 280–285. [Google Scholar]
- Hughes, JR; Goldstein, MG; Hurt, RD; Shiffman, S. Recent advances in the pharmacotherapy of smoking. JAMA 1999, 281, 72–76. [Google Scholar]
- US Department of Health and Human Services. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am. J. Prev. Med 2008, 35, 158–176. [Google Scholar]
- Cummings, KM; Hyland, A. Impact of nicotine replacement therapy on smoking behavior. Annu Rev. Public Health 2005, 26, 583–599. [Google Scholar]
- Bansal, MA; Cummings, KM; Hyland, A; Giovino, GA. Stop-smoking medications: who uses them, who misuses them, and who is misinformed about them. Nicotine Tob Res 2004, (6 Suppl 3), S303–310. [Google Scholar]
- Centers for Medicare & Medicaid Services. Medicaid Program—General Information; US Department of Health and Human Services: Washington, DC, USA, 2008. [Google Scholar]
- Miller, LS; Zhang, X; Novotny, T; Rice, DP; Max, W. State estimates of Medicaid expenditures attributable to cigarette smoking, fiscal year 1993. Public Health Rep 1998, 113, 140–151. [Google Scholar]
- American Legacy Foundation. Saving Lives, Saving Money: Why States Should Invest in a Tobacco-Free Future; American Legacy Foundation: Washington, DC, USA, 2002. [Google Scholar]
- Maciosek, MV; Coffield, AB; Edwards, NM; Flottemesch, TJ; Goodman, MJ; Solberg, LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am. J. Prev. Med 2006, 31, 52–61. [Google Scholar]
- Samet, JM. The 1990 report of the surgeon general: the health benefits of smoking cessation. Am. Rev. Respir. Dis 1990, 142, 993–994. [Google Scholar]
- Flynn, BS; Goldstein, AO; Solomon, LJ; Bauman, KE; Gottlieb, NH; Cohen, JE; Munger, MC; Dana, GS. Predictors of state legislators' intentions to vote for cigarette tax increases. Prev Med 1998, 27, 157–165. [Google Scholar]
- Ky. May Allow Medicaid Payment for Stop-Smoking Therapies.
- Curry, SJ; Grothaus, LC; McAfee, T; Pabiniak, C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N. Engl. J. Med 1998, 339, 673–679. [Google Scholar]
- Boyle, RG; Solberg, LI; Magnan, S; Davidson, G; Alesci, NL. Does insurance coverage for drug therapy affect smoking cessation? Health Aff 2002, 21, 162–168. [Google Scholar]
- Centers for Disease Control and Prevention. State Medicaid coverage for tobacco-dependence treatments—United States, 2005. MMWR 2006, 55, 1194–1197. [Google Scholar]
- Schauffler, HH; McMenamin, S; Olson, K; Boyce-Smith, G; Rideout, JA; Kamil, J. Variations in treatment benefits influence smoking cessation: results of a randomised controlled trial. Tob. Control 2001, 10, 175–180. [Google Scholar]
- Petersen, R; Garrett, JM; Melvin, CL; Hartmann, KE. Medicaid reimbursement for prenatal smoking intervention influences quitting and cessation. Tob. Control 2006, 15, 30–34. [Google Scholar]
- Centers for Disease Control and Prevention. State Medicaid coverage for tobacco-dependence treatments—United States, 1994–2002. MMWR 2004, 53, 54–57. [Google Scholar]
- Greene, WH. Econometric Analysis, 3rd ed; Prentice Hall: Upper Saddle River, NJ, USA, 1997; pp. 418–427. [Google Scholar]
- Kennedy, P. A Guide to Econometrics, 3rd ed; Blackwell Publishers: Oxford, UK, 1992; pp. 183–184. [Google Scholar]
- Decicca, P; Kenkel, D; Mathios, A; Shin, YJ; Lim, JY. Youth smoking, cigarette prices, and anti-smoking sentiment. Health Econ 2008, 17, 733–749. [Google Scholar]
- Liu, F. Cutting through the smoke: separating the effect of price on smoking initiation, relapse and cessation. Appl Econ, 2009; doi: 10.1080/00036840801964880. [Google Scholar]
- Curry, SJ; Sporer, AK; Pugach, O; Campbell, RT; Emery, S. Use of tobacco cessation treatments among young adult smokers: 2005 National Health Interview Survey. Am. J. Public Health 2007, 97, 1464–1469. [Google Scholar]
- Shiffman, S; Brockwell, SE; Pillitteri, JL; Gitchell, JG. Individual differences in adoption of treatment for smoking cessation: demographic and smoking history characteristics. Drug Alcohol Dependence 2008, 93, 121–131. [Google Scholar]
- Kaper, J; Wagena, EJ; Willemsen, MC; van Schayck, CP. Reimbursement for smoking cessation treatment may double the abstinence rate: results of a randomized trial. Addiction 2005, 100, 1012–1020. [Google Scholar]
- Ibrahim, JK; Schauffler, HH; Barker, DC; Orleans, CT. Coverage of tobacco dependence treatments for pregnant women and for children and their parents. Am. J. Public Health 2002, 92, 1940–1942. [Google Scholar]
- Adams, EK; Miller, VP; Ernst, C; Nishimura, BK; Melvin, C; Merritt, R. Neonatal health care costs related to smoking during pregnancy. Health Econ 2002, 11, 193–206. [Google Scholar]
- Ness, RB; Grisso, JA; Hirschinger, N; Markovic, N; Shaw, LM; Day, NL; Kline, J. Cocaine and tobacco use and the risk of spontaneous abortion. N. Engl. J. Med 1999, 340, 333–339. [Google Scholar]
- Oncken, C; Kranzler, H; O'Malley, P; Gendreau, P; Campbell, WA. The effect of cigarette smoking on fetal heart rate characteristics. Obstet. Gynecol 2002, 99, 751–755. [Google Scholar]
- Centers for Disease Control and Prevention. Preventing Smoking and Exposure to Secondhand Smoke Before, During, and After Pregnancy; National Center for Chronic Disease Prevention and Health Promotion: Atlanta, GA, USA, 2007. [Google Scholar]
- West, R; McNeill, A; Raw, M. Smoking cessation guidelines for health professionals: an update. Health Education Authority. Thorax 2000, 55, 987–999. [Google Scholar]
- McMenamin, SB; Halpin, HA; Bellows, NM. Knowledge of Medicaid coverage and effectiveness of smoking treatments. Am. J. Prev. Med 2006, 31, 369–374. [Google Scholar]
- Murphy, JM; Mahoney, MC; Hyland, AJ; Higbee, C; Cummings, KM. Disparity in the use of smoking cessation pharmacotherapy among Medicaid and general population smokers. J. Public Health Manag. Pract 2005, 11, 341–345. [Google Scholar]
| Variable | Whole sample | Women Age 18–44 | Women Age 45+ | Men Age 18–44 | Men Age 45+ |
|---|---|---|---|---|---|
| Quit | 0.098 | 0.102 | 0.101 | 0.087 | 0.098 |
| TDT coverage (Tobacco Dependence Treatment) | 1.916 (2.38) | 1.712 (2.31) | 2.155 (2.46) | 1.852 (2.34) | 2.250 (2.47) |
| Age | 41.464 (15.45) | 30.913 (7.14) | 58.364 (9.92) | 32.560 (7.66) | 57.644 (9.86) |
| Family income (in 1,000 dollars) | 13.894 (13.63) | 13.660 (13.02) | 12.486 (12.92) | 15.784 (15.05) | 14.601 (14.53) |
| Household size | 2.960 (1.74) | 3.564 (1.59) | 2.035 (1.46) | 3.290 (1.83) | 2.139 (1.52) |
| Number of years smoked | 24.648 (14.99) | 14.806 (7.39) | 39.284 (10.94) | 16.771 (8.19) | 41.229 (10.89) |
| Married | 0.272 | 0.221 | 0.198 | 0.403 | 0.378 |
| Employed | 0.275 | 0.349 | 0.128 | 0.372 | 0.160 |
| Female | 0.674 | 1 | 1 | 0 | 0 |
| Race | |||||
| • White (omitted) | 0.708 | 0.699 | 0.718 | 0.734 | 0.710 |
| • Black | 0.169 | 0.186 | 0.178 | 0.114 | 0.148 |
| • Hispanic | 0.063 | 0.056 | 0.056 | 0.074 | 0.078 |
| • Others | 0.060 | 0.059 | 0.048 | 0.078 | 0.064 |
| Education | |||||
| • Less than high school (omitted) | 0.398 | 0.346 | 0.461 | 0.390 | 0.458 |
| • High school | 0.370 | 0.411 | 0.316 | 0.383 | 0.319 |
| • Some college | 0.197 | 0.225 | 0.171 | 0.190 | 0.167 |
| • College + | 0.035 | 0.018 | 0.052 | 0.037 | 0.056 |
| Year | |||||
| • 1993 (omitted) | 0.203 | 0.238 | 0.162 | 0.207 | 0.152 |
| • 1996 | 0.215 | 0.224 | 0.210 | 0.230 | 0.182 |
| • 1999 | 0.174 | 0.162 | 0.190 | 0.167 | 0.196 |
| • 2001 | 0.035 | 0.031 | 0.031 | 0.034 | 0.048 |
| • 2002 | 0.164 | 0.151 | 0.171 | 0.152 | 0.208 |
| • 2003 | 0.209 | 0.194 | 0.236 | 0.210 | 0.214 |
| Number of observations | 5323 | 2450 | 1139 | 862 | 872 |
| TDT usage | 1 | 2 | 3 | N * |
|---|---|---|---|---|
| Medication | ||||
| - Nicotine gum | 60.9% | 8.2% | 7.3% | 69 |
| - Nicotine patch | 68.0% | 19.3% | 13.6% | 150 |
| - Nicotine nasal spray | 87.5% | 1.4% | 1.3% | 8 |
| - Nicotine inhaler | 60.0% | 3.0% | 2.6% | 25 |
| - Zyban, Buproprion, Wellbutrin | 84.1% | 9.0% | 4.7% | 69 |
| Total | 69.8% | 321 | ||
| Counselling | ||||
| - Telephone | 5.9% | 1.0% | 2.0% | 17 |
| - Group | 11.8% | 1.2% | 2.1% | 17 |
| - Individual | 33.3% | 2.6% | 2.3% | 21 |
| Total | 18.2% | 55 | ||
| Baseline | State FE | Sentiment | Baseline | State FE | Sentiment | |
|---|---|---|---|---|---|---|
| Female | Age 18–44 | Age 45+ | ||||
| TDT coverage | 0.009*** (0.004) | 0.007* (0.004) | 0.007** (0.003) | 0.003 (0.005) | 0.007 (0.006) | 0.003 (0.005) |
| Anti-Smoking sentiment | 0.126*** (0.045) | 0.015 (0.064) | ||||
| N | 2,450 | 1,139 | ||||
| Male | Age 18–44 | Age 45+ | ||||
| TDT coverage | 0.003 (0.005) | −0.005 (0.010) | 0.001 (0.005) | −0.002 (0.006) | −0.011 (0.010) | −0.002 (0.006) |
| Anti-Smoking sentiment | 0.067* (0.038) | −0.008 (0.058) | ||||
| N | 862 | 872 | ||||
| Baseline | State FE | Sentiment | |
|---|---|---|---|
| TDT coverage | 0.015** (0.008) | 0.008 (0.013) | 0.011* (0.006) |
| Pregnant | 0.129*** (0.051) | 0.139** (0.070) | 0.129*** (0.051) |
| Anti-Smoking sentiment | 0.180** (0.079) | ||
| N | 694 |
| Baseline | State FE | Sentiment | Baseline | State FE | Sentiment | |
|---|---|---|---|---|---|---|
| Female | Age 18–44 | Age 45+ | ||||
| TDT coverage | −0.004** (0.002) | −0.005** (0.002) | −0.003** (0.001) | 0.0002 (0.001) | 0.001 (0.001) | 0.0002 (0.001) |
| Anti-Smoking sentiment | −0.018 (0.017) | −0.014 (0.010) | ||||
| N | 5,469 | 5,250 | ||||
| Male | Age 18–44 | Age 45+ | ||||
| TDT coverage | −0.001 (0.002) | 0.001 (0.004) | −0.001 (0.002) | 0.0006 (0.001) | −0.0006 (0.003) | 0.0005 (0.001) |
| Anti-Smoking sentiment | −0.013 (0.019) | 0.008 (0.011) | ||||
| N | 1,658 | 2,209 | ||||
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, F. Effect of Medicaid Coverage of Tobacco-Dependence Treatments on Smoking Cessation. Int. J. Environ. Res. Public Health 2009, 6, 3143-3155. https://doi.org/10.3390/ijerph6123143
Liu F. Effect of Medicaid Coverage of Tobacco-Dependence Treatments on Smoking Cessation. International Journal of Environmental Research and Public Health. 2009; 6(12):3143-3155. https://doi.org/10.3390/ijerph6123143
Chicago/Turabian StyleLiu, Feng. 2009. "Effect of Medicaid Coverage of Tobacco-Dependence Treatments on Smoking Cessation" International Journal of Environmental Research and Public Health 6, no. 12: 3143-3155. https://doi.org/10.3390/ijerph6123143




