Introduction
Polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), commonly known as dioxins and furans respectively, and polychlorinated biphenyls (PCBs) are persistent compounds with a high potential for accumulating in biological tissues. They have been found in all compartments of the ecosystem, including water. Although as many as 209 PCB congeners are theoretically possible, the most significant risk of toxicity has been reported for the coplanar congeners which have “meta” and “para” chlorine substitutions and which are referred to as the ‘dioxin-like’ PCBs. Dioxins and furans comprises a group of 75 PCDDs and 135 PCDFs respectively. The World Heath Organisation (WHO) consider 29 of these compounds (PCDDs, PCDFs and dioxin-like PCBs) to have significant toxicity [
1] and these compounds (listed in
Table 4) are analysed in this study.
PCDDs, PCDFs and dioxin-like PCBs have been reviewed extensively by a variety of experts and organizations [
1–
12]. For the purpose of this paper, a general summary of the key findings of these reviews is presented.
PCDDs consist of a large group of chlorinated organic chemicals. In general, the compounds have low water solubility and low vapour pressure. Most are very chemically stable and tend to bioaccumulate. The number of chlorine atoms attached to the molecule, from one to eight, and their position in the molecule, determines the chemical and physical properties as well as the toxic potency. The most studied and most toxic dioxin is 2,3,7,8-tetrachlorodibenzodioxin (TCDD).
The most relevant physico-chemical property for predicting the environmental fate and toxicity of dioxins and furans is the octanol-water partition coefficient. Congeners with high octanol-water value have low water solubility and tend to accumulate in soils, aquatic sediments, and animal and human fat tissues. The coefficient increases as the number of chlorine atoms in the molecule increases. However, after the log
10 octanol-water coefficient reaches a value of about 6, increased molecular size and decreased solubility usually result in decreased bioconcentration [
3].
The toxicity of different dioxins, furans and dioxin-like PCBs is expressed on a common basis by comparing the toxicity of the 17 most toxic dioxins and furans and the 12 most toxic dioxin-like PCBs to that of TCDD. PCDDs, PCDFs and dioxin-like PCBs congeners become active through the
Ah (aryl hydrocarbon) receptor mechanism and dose additivity is the default assumption in estimating total toxicological potential for dioxins [
5]. The scientific and regulatory communities of many countries have agreed upon a standard set of toxic equivalency factors (TEFs). This system has been broadly corroborated in laboratory studies. The remaining dioxins, furans and PCBs usually contribute comparatively little to the overall toxicity of a complex mixture. The TEFs concept was originally introduced as a method for evaluating the health risks associated with closely related chemicals that have identical mechanisms of action but differing potencies. In 1987, the U.S. EPA proposed TEFs for the dioxin and furan congeners. In 1990, TEFs were also proposed for coplanar PCBs congeners and these TEFs are based on
in vivo and
in vitro potencies relative to TCDD. The TEF values for the dioxins and furans have been refined several times by different regulatory and health agencies as new data have become available. The WHO proposed the most recent version in 2006 [
1].
As part of the Premiers Collaborative Research Project (PCRP): “Characterising treated wastewater for drinking purposes following reverse osmosis treatment, in Perth, Western Australia”, levels of dioxins and dioxin-like compounds in the secondary effluent and after the reverse osmosis treatment were analysed to investigate their occurrence and the removal during treatment. This paper documents results of 29 dioxin, furans and dioxin-like PCBs from wastewater, recycled water and groundwater. Groundwater in this manuscript refers to an underground water source that has been extracted to be treated for use as a drinking water supply. A screening health risk assessment was performed using toxic equivalents (TEQ) to determine whether increased human dioxin exposure occurs when wastewater is treated to augment drinking water supplies.
Occurrence
Dioxins, furans and dioxin-like PCBs are ubiquitously distributed throughout the environment and they can be detected in air, water, soil, sediment and biota. They are released into the environment as a result of combustion activities, including power generation, waste incineration, metal smelting, as well as from natural sources such as bushfires. Other dioxin sources are the impurities formed in the manufacture of chlorinated compounds, such as the wood preservative pentachlorophenol, the herbicides 2,4D and 2,4,5-T as well as pulp and paper mills using chlorine for the bleaching process [
3]. They enter the environment as complex mixtures mainly from incineration, accidental fires or spills involving PCBs. It is estimated that over 96% of dioxins in the environment have originated from emissions to air [
2] which are then deposited on plant, soil and water surfaces.
The National Dioxins Program conducted across Australia in 2003–2004 showed that dioxins are widespread, distributed through soils and sediments, with the highest levels found in urban areas [
10]. Nonetheless, most of the dioxin mixtures in Australia were comprised of the less toxic dioxins, and environmental levels were generally low compared with other countries. The total emissions of dioxins to the environment range from 160–1790 g/yr of which uncontrolled combustion contributes around 70%. Other main sources of emissions in Australia are metal production; fossil fuel power generation; waste disposal, and waste incinerators, emitting 420 g/yr or around 23% of the total emissions. Domestic woodheaters contribute around 4% while motor vehicles account for less than 2% of total dioxin emissions [
2].
Dioxins, furans and dioxin-like PCBs have been detected in freshwater, wastewater and drinking water samples, and therefore exposure to dioxins can potentially occur through various sources of contaminated water including wells, surface water and in swimming areas. In Australia, the more commonly detected congeners in freshwater were octachlorodibenzodioxin (OCDD), followed by the 1,2,3,4,6,7,8-heptachlorodibenzodioxin. In Australia, the estimated annual release of dioxins from water is 3.2 g/TEQ (less than 0.2% of the total annual release of 1,778 g/TEQ). For sediment samples, PCDDs and PCDFs accounted for more than 80% of the total TEQ, although in some samples from Western Australia the contributions of PCB congeners exceeded 50% [
10]. Data on dioxins in drinking water are very limited. In Canada, very low dioxins concentrations in drinking water have been reported from no detections to 46 pg/L of OCDD [
3].
Concentrations of dioxins in secondary effluent are low compared to the influent to wastewater treatment plants (WWTPs) because they are largely removed with sludge solids, to which they are strongly bound [
13,
14]. Most of the occurrence of dioxins is therefore reported in sludge from sewage treatment plants where they are often detected despite the fact that they have been highly regulated since the 1970s [
14–
16]. In Italy, dioxin concentration in the influent of a WWTP has been reported between 0.024–16.9 μg/L. The estimated dioxin emission in the UK in 1998 from industrial processes to wastewater was 4.5 μg TEQ, whereas emissions to air from these processes were estimated to be 1.1 kg TEQ [
14].
With the development of regulations, it has been estimated that dioxins and furans emissions have undergone a 90% reduction in all environmental compartments in less than 20 years. The dioxin emission level as TEQ was approximately 14,000 g TEQ/year in 1987, 3,250 g TEQ/year in 1995 and 1,100 g TEQ/year in 2004 [
11]. Concentrations of dioxins and furans in sludge from a major London WWTP decreased by more than 97% in the past 40 years: from 166 ng/kg TEQ in 1960 to 4.2 ng/kg TEQ in 1998 [
14].
The main sources of water pollution are open water dumping of sewage, medical waste incineration, pulp and paper production, and leaching from landfills and waste dumps [
2]. Dioxins may also be formed in water during chlorination of wastewater in WWTPs. The following dioxin like substances have been reported in Australian sewage effluent: OCDD, PCB 77, PCB 105, PCB 118, PCB 156, PCB 167, and PCB 169 [
17]. Recycled water schemes for indirect potable reuse in California reported concentrations below the reportable detection limit of 0.5 μg/L for PCB 1016, PCB 1221, PCB 1232, PCB 1242, PCB 1248, PCB 1254 and PCB 1260; as well as for total PCBs. Similarly concentrations of TCDD have been below the reportable detection limit of 5 pg/L in a range of samples from different recycled water schemes [
18–
20].
Toxicity
Many dioxin and dioxin-like compounds have been tested for their toxic effects using
in vivo and
in vitro studies. Studies of chronic exposure in mammals with TCDD have demonstrated that the compound is associated with adverse reproduction outcomes, birth defects, hepatotoxicity, immunological suppression and carcinogenicity. Mice exposed orally for a lifetime to TCDD developed cancer of the liver and thyroid. Rats similarly exposed developed cancer of the liver, lung, tongue, hard palate and nose [
3]. The no observed adverse effect level (NOAEL) for TCDD for chronic exposure in rats (cancer and reproduction) is approximately 1 ng/kg bw/day. Based on this NOAEL and a 100-fold uncertainty factor, it is concluded that human intakes should be below 10 pg TEQ/kg bw/day averaged over a lifetime [
3]. Several authoritative agencies and scientific organizations have concluded that 1–4 pg TEQ kg/day of TCDD is unlikely to cause adverse health effects (
Table 1). Conversely, the U.S. EPA using the default linear extrapolation policy for carcinogenic compounds has suggested that TCDD doses in the range of 1 pg/kg bw/day, and even lower, may pose a significant health risk [
11]. The U.S. EPA recommended an intake limit of 0.006 TEQ pg/kg bw/day for a lifetime additional cancer risk of 10
−6 (
Table 1) and in its dioxin reassessment recommends an intake limit of 0.001 TEQ pg/kg bw/day [
21].
Occupational and accidental exposures to dioxins and furans indicate that dioxins can lead to a variety of effects on skin, eyes, and sensory and behavioral processes. Many other disorders have been reported, including: fluctuations in serum levels of liver enzymes; pulmonary dysfunction; sensory changes such as numbness, nausea, headaches, loss of hearing, sleep disturbances, tiredness; sexual dysfunction; depression; and loss of appetite. Exposure of women to several milligrams of furans in contaminated rice oil in Japan and Taiwan may have been also responsible for reproductive anomalies and infant mortality [
3]. However, the most persistent effect reported from contact with dioxins and furans is chloracne.
The U.S. EPA, International Agency for Research on Cancer, and the WHO list 2,3,7,8-TCDD as a Group 1 human carcinogen [
22]. Most studies suggest that TCDD acts only as a promoter and not as an initiator of cancer. Dioxins are considered to be non-genotoxic carcinogens with a threshold in their dose-response relationships, therefore the calculation of theoretical upper bound risks using linearised models based on animal carcinogenicity bioassays is inappropriate and irrelevant [
23]. To date, there is inconsistent evidence that human populations exposed to dioxins have suffered excess cancer. Although some epidemiological studies found that the exposure to dioxins and dioxin-like substances result in a range of cancers, others have reported no positive association. Thus, evidence is conflicting and data are confounded by exposure to other chemicals, incomplete health records, inadequate case identification and small sample size [
2].
The majority of the human health effects reported in the literature are related to acute exposures to dioxins, furans and PCBs. An epidemiological study conducted in Missouri on people exposed to low concentrations of dioxins over longer periods of time concluded that people chronically exposed do not reported any clinical impacts, although there were indications of an effect on the cell-mediated immune system [
12].
Evidence for endocrine disrupting effects of dioxins, furans and PCBs are also inconclusive. Some studies reported earlier age at menarche after exposure to PCBs and polybrominated biphenyls (PBBs), while other studies found no effect of these compounds on age at menarche or pubertal Tanner stages [
24]. A review conducted by Ross (2004) concluded that there is no reliable evidence of endocrine disrupting properties for PCBs based on the weight of the scientific evidence, the low levels of environmental exposure to PCBs and their weak endocrine activity. Published epidemiological studies are confounded by the exposure to several different chemicals with antagonistic effects (oestrogenic, anti-oestrogenic, anti-androgenic), the exposure to low concentrations of chemical mixtures and the limited knowledge about the most critical window for exposure (prenatal, perinatal and pubertal). Study results are therefore not always comparable and identification of the active agent (if any) is a complex task.
The International Agency for Research on Cancer has determined that PCBs are probably carcinogenic to humans (Group 2A). The U.S. EPA considers PCBs to be “probable human carcinogens” (Group B2) and the California Office of Environmental Health Hazard Assessment (OEHHA) concluded that there was sufficient evidence that PCBs are carcinogenic to animals and that PCBs are reasonably anticipated to be carcinogenic in humans [
25]. The TEF approach is limited to dioxin-like PCBs and assumes that each component will act in an additive manner through a common
Ah-receptor initial mechanism. However, some antagonistic interactions between non coplanar PCBs congeners and between some PCB congeners and TCDD have been reported.
Regulations
In Australia, under the National Pollutant Inventory program, facilities must report on dioxins and furans if they use more than 2,000 tonnes of fuel, or 60,000 megawatt hours of energy per year. Moreover, the limit for total dioxins in the effluent stream to be discharged to ambient waters is set at 15 pg/L. The Government of Tasmania has recommended a maximum limit of 10 pg/L for discharge [
2].
The National Health and Medical Research Council and the Therapeutic Goods Administration have concluded that a tolerable intake of 70 pg TEQ/kg bw/month from all sources (including dioxins, furans and dioxin-like PCBs) could be established on the basis that a threshold exists for all observed adverse effects, including cancer [
6]. This recommended tolerable maximum intake is equivalent to that set by the Joint Expert Committee on Food Additives of the United Nations Food and Agriculture Organization and the WHO. Assuming a body weight of 70 kg, 2 L of water consumption per day and an allocation of 20% TEQ intake to water this corresponds to 16 pg TEQ/L, which is also the guideline value in the Australian Guidelines for Water Recycling (AGWR) - Phase 2 - Augmentation of Drinking Water Supplies [
17].
Few countries have set guidelines for dioxins in water (
Table 1). The Ontario Ministry of the Environment set an interim Drinking Water Objective of 15 pg/L TCDD TEQ [
3]. The Maximum Contaminant Level (MCL) for TCDD established by U.S. EPA is 0.03 ng/L, and is above the proposed public health goal of 1 pg/L proposed by the OEHHA based on carcinogenic effects in animals [
22]. The human intake limits in the U.S. are far lower than those established by WHO and other countries (
Table 1). The differences arise because of disagreement on fundamental issues including the likelihood of a threshold for carcinogenic dose-response and the degree of safety factors needed in deriving a protective exposure limit.
In this study only TEQs were calculated for dioxin-like PCBS. However, there are also regulations for total concentrations of PCBs. For example, the AGWR recommend a guideline value for total PCBs of 0.14 μg/L derived from the ADI of Aroclor 1254 of 0.02 μg/kg/day (
http://www.epa.gov/ncea/iris/subst/0389.htm) and an allocation to water of 20% [
17]. The U.S. EPA has established a maximum contaminant level (MCL) of 0.5 μg/L for total PCBs in drinking water [
26] and the OEHHA has developed a Public Health Goal of 0.09 μg/L for water-soluble polychlorinated biphenyls (PCBs) [
25].
Estimated Intake
Human exposure to dioxins, furans and dioxin-like PCBs through drinking water is considered negligible compared to diet [
11,
27]. For example, 96% of the average daily Canadian intake of dioxins over a lifetime (2.0 – 4.2 pg TEQ/kg bw/day) is estimated to be from food. In comparison, the contribution from drinking water is estimated to be less than 0.05 TEQ/kg bw/day for an adult and less than 0.11 TEQ/kg bw/day for an infant [
3]. Levels of dioxins in the Australian population are low by international standards. For Australians aged two years or older, the monthly intake of dioxins from food was between 3.9–15.8 pg TEQ/kg bw/month [
2]. Toddlers aged 2–4 years were estimated to have the highest exposure to dioxins (6.2–36.7 pg TEQ/kg bw/month, lower to upper bound respectively) as a result of their higher food consumption relative to body weight.
Dietary intake of PCDDs, PCDFs and dioxin-like PCBs is decreasing over time. The U.S. EPA mean dietary intake level estimated in the dioxin-reassessment in 2000 was 0.6 pg TEQ/kg bw/day, whereas in 1994 the level was assessed at 1.7 pg TEQ/kg bw/day [
11]. Similarly, studies reported decreased dioxins concentrations in human samples. The TEQ levels for dioxins decreased to half of the original values over 14 years (from 1987 to 2001) in Germany [
11] and by 40% in human milk over 10 years (from 1993 to 2003) in Australia [
28].
Some groups in the population are more exposed than others to dioxins. It is estimated that smoking produces approximately 1.8 ng/m
3 TEQ dioxins, and therefore smokers are often more exposed through inhalation. Burning wood in fireplaces and barbecuing also produce small amounts of dioxins that may increase exposure to dioxins through inhalation [
2].
Given the concerns regarding the potential exposure to dioxins in recycled water, an assessment of their likely toxicity was undertaken for the three major WWTPs in Perth, Western Australia. The objectives of this analysis were:
To determine the occurrence and concentration of dioxin and dioxin-like compounds in the secondary effluent of the three main WWTPs;
To determine the occurrence and concentration of dioxin and dioxin-like compounds, before and after the advanced treatment process at the Kwinana Water Reclamation Plant (KWRP) and at the Beenyup Pilot Plant (BPP).
Methodology
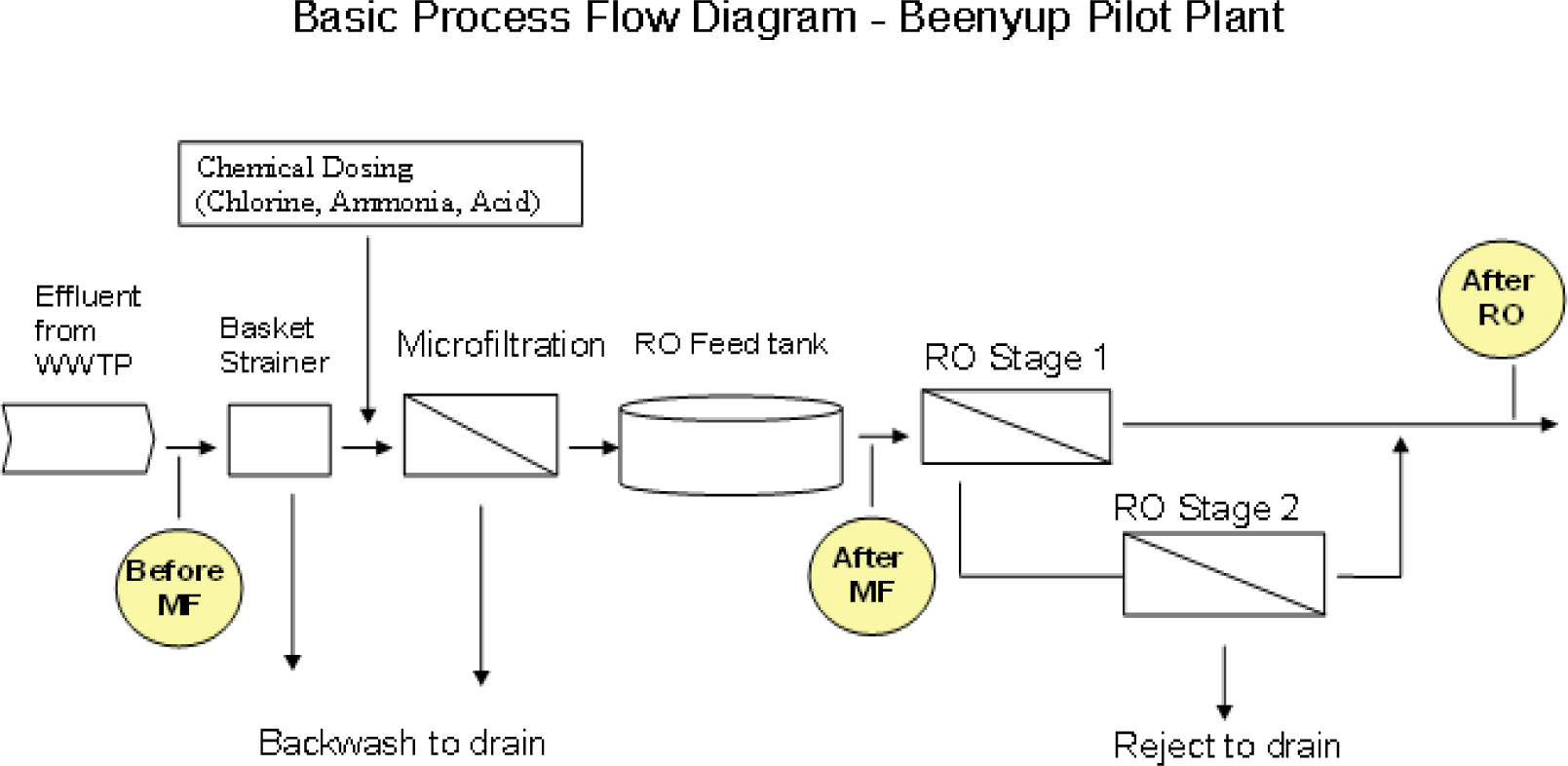
All water samples were collected by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) personnel using consistent protocols and procedures designed to obtain (i) grab samples from groundwater and WWTPs, and (ii) 24-hour composite samples from KWRP and BPP. Standard protocols were used to ensure adequate sample preparation, preservation and transportation to the laboratory. Samples taken in 2005 (event 0) were analyzed by ALS Environmental. All other samples were analyzed by the National Measurement Institute (NMI). The location of the sampling points in the water reclamation plants is depicted in
Figure 1 and the distribution of sampling days by location is presented in
Table 2. Data were analyzed in Stata version 10 [
29].
The NMI analytical methodology for the determination of PCDDs & PCDFs and PCBs are based on U.S. EPA methods 1613B and 1668A, respectively. The methods are NATA accredited and provides data on 7 dioxins, 10 furan isomers and 12 dioxin-like PCBs. The detection limits and quantification levels are usually dependent on the level of interferences rather than instrumental limitations. Samples were spiked with a range of isotopically labelled surrogate standards and clean-up was achieved by partitioning with sulfuric acid then distilled water. Further purification was performed using column chromatography on acid and base modified silica gels, neutral alumina and carbon dispersed on celite. After cleanup, the extract was concentrated to near dryness. Immediately prior to injection, internal standards were added to each extract, and an aliquot of the extract was injected into the gas chromatograph. The analytes were separated by the gas chromatography (GC) and detected by a high-resolution (≥10,000) mass spectrometer (MS). The quality of the analysis was assured through reproducible calibration and testing of the extraction, cleanup, and GC/MS systems.
The WHO 2005 toxic equivalency factors (TEF) were used to calculate the toxic equivalents (TEQs) [
1]. The middle bound of the TEQs which define all congeners values reported below the limit of detection (LOD) as equal to half the LOD are presented unless otherwise specified.
Results
The TEQ of each date and location was calculated and
Table 3 illustrates the summary statistics of the middle and upper bound TEQ. None of the 33 samples taken from any of the sampling locations referred to in
Table 2 was above the health standard of 16 pg TEQ/L using either the middle bound or the upper bound TEQ calculated from the 29 congeners (
Table 3). The mean TEQ from the groundwater samples was slightly lower than the mean TEQ from the three WWTPs
For the water reclamation plants, the TEQ of the influent (before MF) was lower than for the pooled secondary effluent from the three WWTPs (3.34 pg TEQ/L and 4.5 pg TEQ/L respectively). Concentrations of TEQs of dioxin, furans and dioxin-like PCBs were higher before MF compared to concentrations after RO, indicating that the advanced treatment is able to further reduce the concentrations of these contaminants in the product water. For the BPP, the presumptive percentage of removal ranged from 24% to 43% while for the KWRP the presumptive percentage of removal was more variable (range from 4% to 47%). Risk quotients (RQ) were all below 1, even when the upper bound TEQ was used as a “worst case” scenario for the screening health risk assessment.
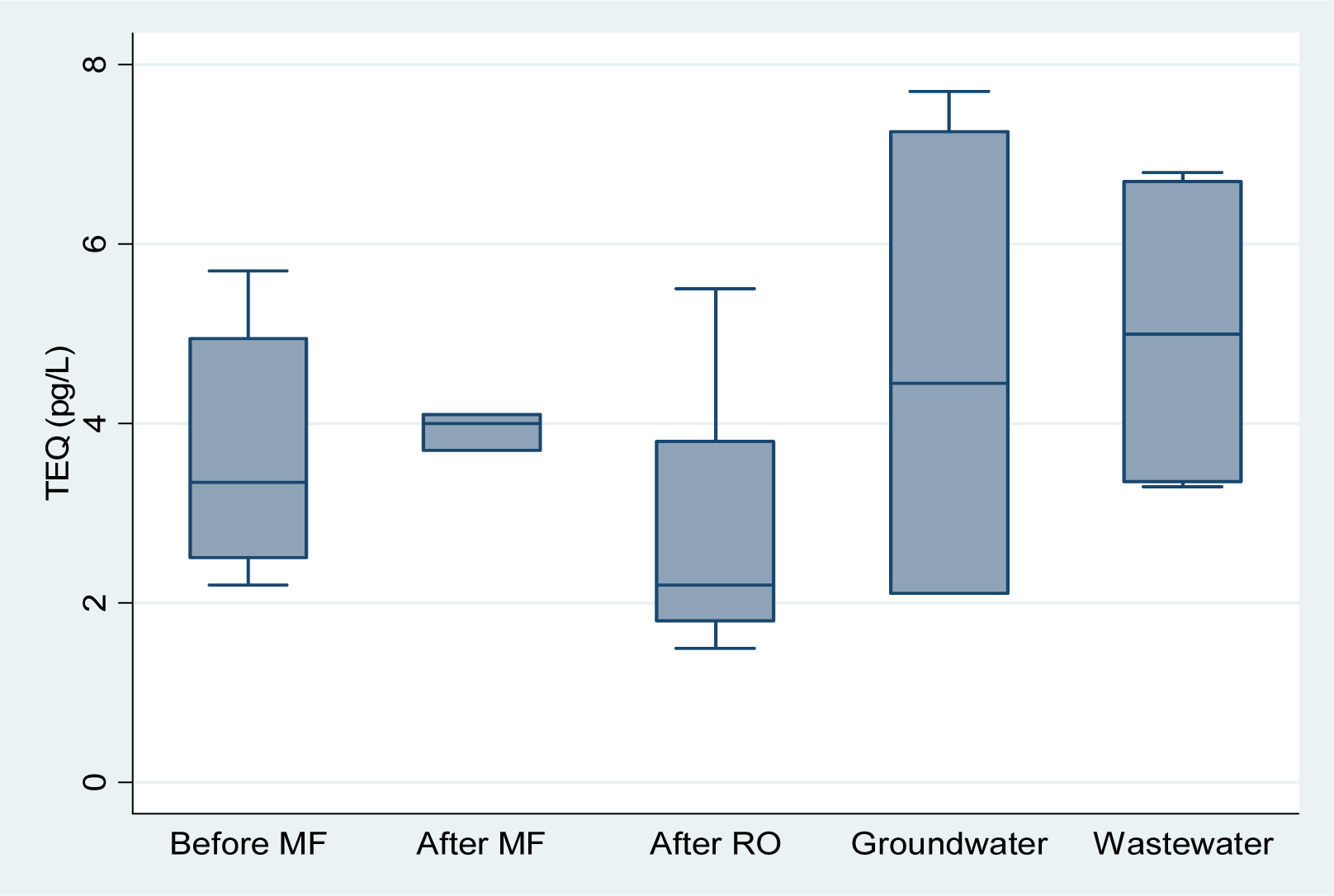
The lowest mean TEQ was observed in the product water of the KWRP and BPP (
Figure 2). Variability was higher in the groundwater and wastewater samples due in part to the smaller number of samples analysed from these locations (4 and 6 respectively). The mean TEQ were similar in the product water of both plants.
The minimum, maximum, and mean of the pooled wastewater and pooled groundwater samples in pg/L as well as the contribution to total TEQ (middle bound) of each congener is reported in
Table 4. None of the PCDDs, PCDFs and dioxin-like PCBs was detected in groundwater. Mean dioxin-like PCBs concentrations were in general higher in the groundwater compared to wastewater samples, except for PCB 126 and PCB 169. Mean concentrations of dioxins and furans were lower in groundwater than in wastewater, in particular for OCDD and octachlorodibenzofuran (OCDF). Nevertheless, the RQs for wastewater and groundwater were similar 0.27 and 0.28 respectively. The TEQ (middle bound) for both groundwater and wastewater were below 5 pg TEQ/L.
The contribution to total TEQ of each congener during the advanced treatment is presented in
Table 5. RQs before MF and after RO were below 1 when applying the TEF to the mean concentrations of all dioxin-like compounds. The results indicate that applying the TEFs to the mean concentrations (i.e. TEQ middle bound) of all dioxin-like compounds reported in
Table 5 produces a combined TEQ of 3.34 pg TEQ/L before MF and a 2.45 pg TEQ/L after RO. Expressed as risk quotients these values are also below 1 (RQ before MF=0.21 and RQ after RO=0.15).
The dioxin-like compounds with detections in wastewater and product water included PCB 77, 81, 105, 118, 126, 156, 167, 169, OCDD, 1,2,3,4,6,7,8-Heptachlorodibenzofuran and OCDF (
Table 6). All detected dioxins in
Table 6, except PCB 169, have TEFs of 0.01 or lower, which signifies very low toxicity relative to TEQ in the additive model. The average treatment efficiency expressed as TEQ was 26% between the influent and the product water in both plants.
Bootstrap simulations were performed in Stata in order to infer the 95% confidence intervals (CI). The observations were assumed to be from an independent and identically distributed population and re-samples of equal size of the observed dataset were obtained by random sampling with replacement from the original dataset. TEQs before MF and after RO were re-sampled with 250 and 1000 replacements and the estimated 95% CI are presented in
Table 7. The calculated 95% CI suggest that if wastewater conditions remain unchanged, the middle bound TEQ will be between 3.24 pg TEQ/L and 5.03 pg TEQ/L in the plant influent, which is below the health value of 16 pg TEQ/L.
Discussion
Samples from the influent and product water at KWRP and BPP were analysed to identify any potential human health risks from dioxins in the recycled water. Results indicate that the concentrations of these compounds in the recycled water are of low health significance. For all sampling points calculated RQs were below 1 even when the upper bound TEQ was used as a worst case scenario.
Samples from the three WWTPs were analysed separately from the plant influent (before MF) because they were grab and composite respectively. The calculated TEQ were lower before MF (3.34 pg TEQ/L) than in the secondary effluent from the three WWTPs (4.5 pg TEQ/L) despite the fact that the water quality is the same. These differences may be explained by one sample from Subiaco WWTP with a middle bound TEQ of 6.8 pg TEQ/L (data not shown): this sample effectively increases the mean value of the wastewater samples. Nevertheless, the low dioxin and dioxin-like compounds concentrations observed in the secondary effluent may be due to good removal during conventional wastewater treatment given that dioxins have high affinity to sludge solids and low water solubility. These results are also consistent with other studies in which the release of dioxins in sewage treatment plants was small [
2] and with data from indirect potable reuse projects around the world. Reported concentrations of PCBs and TCDD are below the guideline values after the advanced treatment in water recycling schemes in the U.S. and Singapore [
18–
20].
The results may also suggest that there are limited numbers of industrial sources that produce dioxins and discharge into sewerage systems in Perth, although raw wastewater data were not analysed in this study. Trade waste policies in place may also further limit or exclude dioxins from industrial sources from entering the system. Given that emission controls are already in place in Australia for the main point sources of dioxin-like compounds, the detected dioxins and dioxin-like compounds in wastewater listed in
Table 6 may enter principally from diffuse atmospheric deposition and environmental cycling. Therefore, point source control offers limited scope for further reduction of inputs and concentrations of these persistent organic substances in wastewater. Moreover, the detected congeners such as OCDD or PCB 77 have TEFs of 0.01 or lower, which signifies very low toxicity contribution relative to TEQ.
Despite the fact that none of the congeners were detected in the groundwater, calculated RQs in recycled water after RO were lower that the RQs of the groundwater source for drinking (0.15 and 0.27 respectively). This result is indicative that better LOR were achieved for tertiary wastewater than for groundwater, suggesting the RQ for groundwater will probably be less accurate. Regardless, the importance of dioxins in recycled water are significantly diminished, and there would be little practical or public health benefit gained from adopting frequent regular monitoring for dioxins and dioxin-like compounds after the advanced MF/RO treatment. This conclusion is reinforced by the high cost and specialist analytical requirements of quantifying these compounds in wastewater effluents and recycled water.
There are several uncertainties associated with the approach used in this study. Analysis through a NATA accredited laboratory with adequate sampling preparation, preservation and transportation ensures reported data is good quality. However, the significant number of non-detects will skew results in relation to the LOR achievable in different water matrices, rather than in actual dioxin concentrations. Therefore, the assumption that all non-detects is equal to half their LOR makes the “middle bound” not totally comparable from one type of water to another because the differences in LOR achieved depends on the level of interferences in the different water matrices.
The TEF approach to estimate TEQ of dioxins and dioxin-like compounds is internationally well accepted and simplifies risk assessment of complex mixtures. However, there is uncertainty related to the nature of the endpoint used to establish the toxicity threshold. Although various toxicity endpoints have been assessed, recent studies have indicated that the most sensitive adverse effects attributable to TCDD may be those observed in rodent offspring exposed
in utero during critical gestational periods [
30]. Another uncertainty of using TEQ is related to the establishment of a short-term tolerable intake based on long-term body burden data. The use of body burden as a dose metric does not account for, or eliminate, the substantial differences in sensitivity to dioxin observed across species or between different strains of the same species and, thus, does not eliminate the need to consider the relative sensitivity of humans compared to laboratory animal models in risk assessments. In addition, the distribution of these compounds in the body compartments varies across species, which has implications for the risk assessment on a body-burden basis [
30]. In addition, for some congeners, there is a lack of parallelism of dose-response across toxicity endpoints and of
Ah-receptor occupancy, which may suggest involvement of other mechanisms of action. Finally, there is the inevitable subjectivity in setting one TEF estimate to represent a data base which may contain several studies of the same endpoint and displaying a range of median toxicities [
5].
As discussed in the occurrence section, dioxin concentrations in the water environment have been decreasing due to significant reductions in the primary sources of these compounds, the cessation of production, and the environmental control measures and regulations that have been in place for the past two decades. Consequently inputs to WWTPs from dioxin compounds have decreased and this is expected to continue. The results suggest that current Australian regulations are adequate to control the discharge of dioxins to wastewater. Assuming no changes in the WWTPs effluent, the estimated middle bound TEQ 95% CI for the secondary effluent will be between 3.24 pg TEQ/L and 5.03 pg TEQ/L which corresponds to a RQ from 0.20 to 0.31.
Conclusions
The results indicate that dioxin and dioxin-like compounds are present only at low concentrations, expressed as TEQ, in secondary effluents and that advanced treatment is able to further reduce those concentrations to levels well below health significance.
These findings corroborate the importance of the multiple barrier approach using conventional WWTP processes and advanced treatment to remove these compounds.
Given the low concentrations of dioxins in wastewater and particularly in the product water, it is anticipated that the use of recycled water for drinking purposes will not increase the human exposure to dioxins and dioxin-like compounds through water consumption. Based on the calculated RQs, the decreasing trend in dioxin release and the significant costs and logistic constraints associated with dioxins testing, it is difficult to justify routine ongoing testing of wastewater and recycled water for indirect potable reuse.
Quarterly validation monitoring program, for dioxins and dioxin like compounds, is recommended during the first year for a full scale water reclamation plant. If results are of low health concern, verification monitoring every two or three years is then recommended. The information provided here may help regulators and policy makers make informed decisions about monitoring programs for dioxins and dioxin-like compounds for indirect potable reuse schemes.