Cytotoxicity of Dinitrotoluenes (2,4-DNT, 2,6-DNT ) to MCF-7 and MRC-5 Cells
Abstract
:Introduction
Materials and Methods
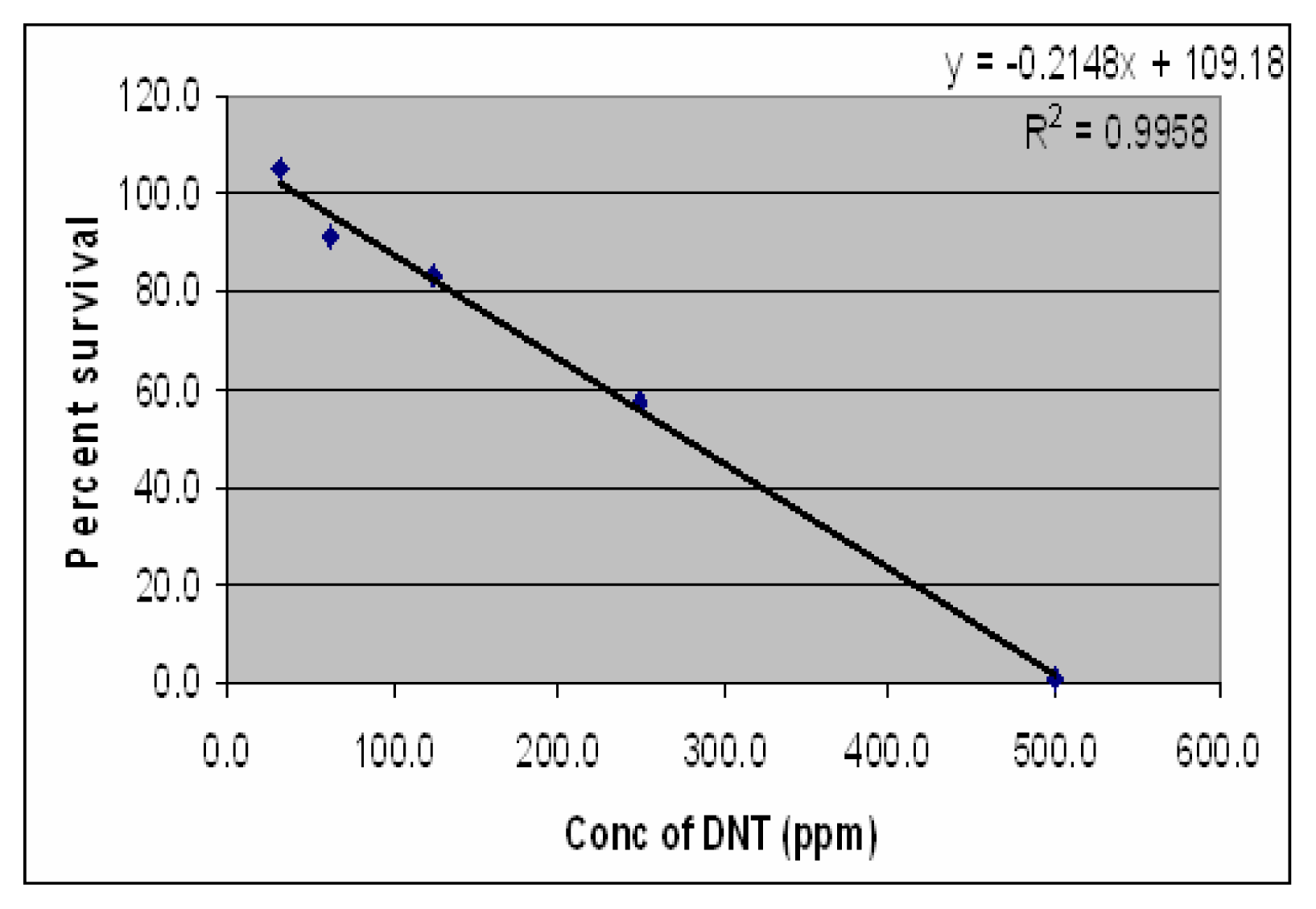
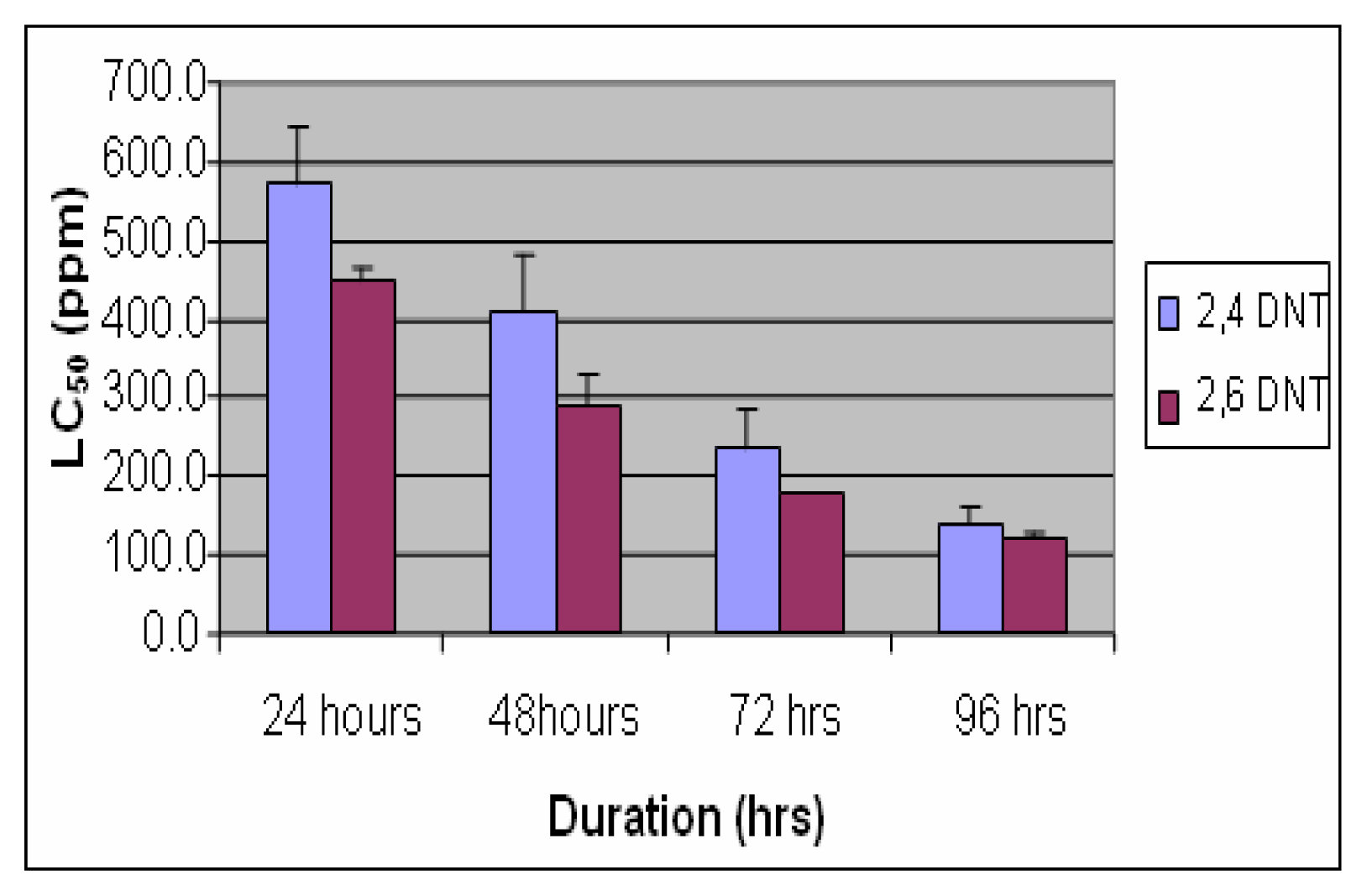
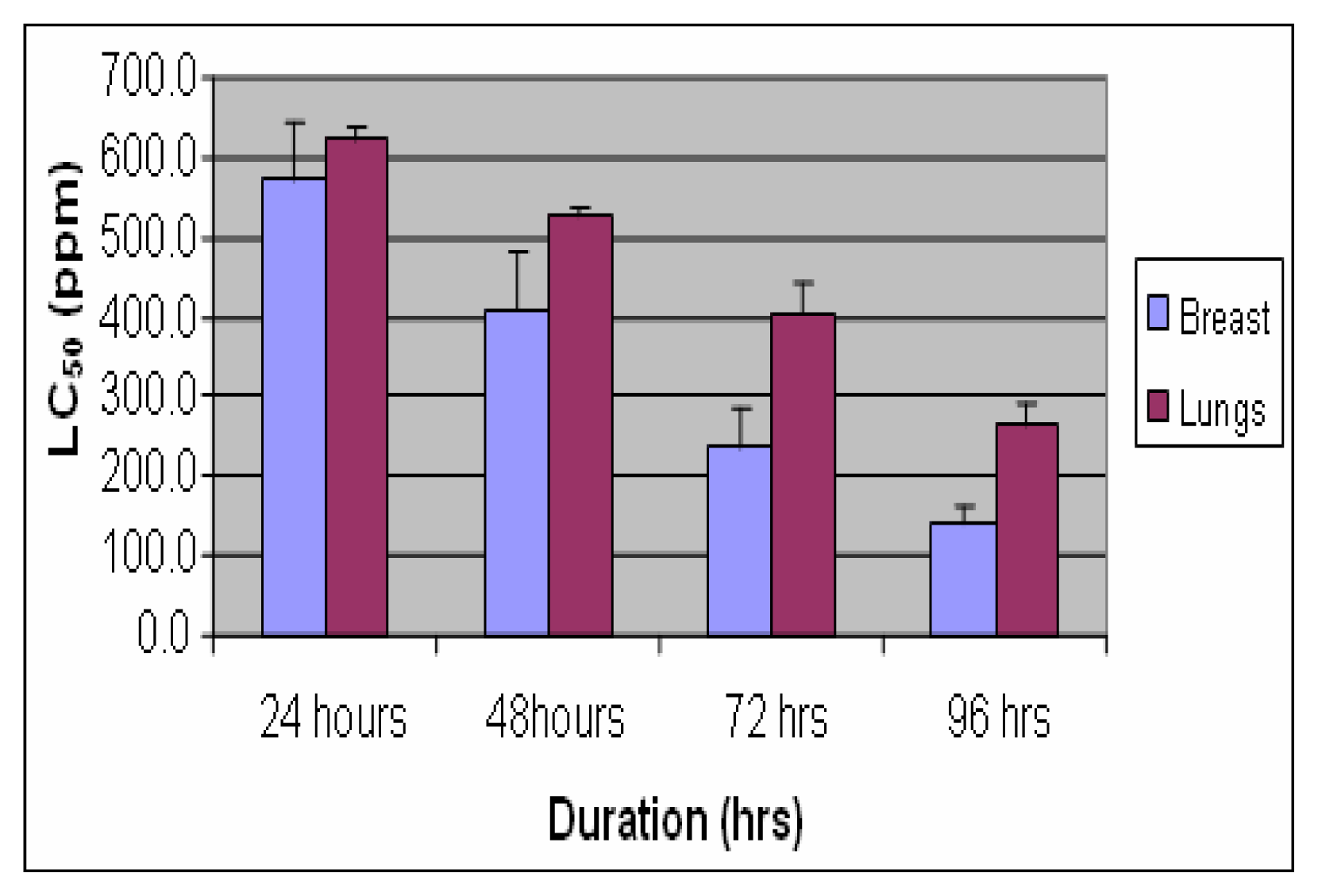
Results
Discussion
Conclusions


Acknowledgments
References
- Tchounwou, P. B.; Newsome, C.; Glass, K.; Centeno, J. A.; Leszczynski, J.; Bryant, J.; Okoh, J.; Ishaque, A. B.; Brow, M. Environmental Toxicology and Health Effects Associated with Dinitrotoluene Exposure. Review on Environ. Health 2003, 18(3), 203–229. [Google Scholar]
- Brower, M. E.; Roberts, W. C.; Hartley, W. R.; Abernathy, C.; Hartley, W. R.; Roberts, W. C.; Commons, B. J. (Eds.) 2, 4- and 2, 6-Dinitrotoluene (DNT) in Drinking Water and Health Advisory: Munitions II; Professional Administrative Services: Washington, DC, 1994; pp. 39–153.
- ATSDR, Toxicological Profile for 2, 4-Dinitrotoluene and 2, 6-Dinitrotoluene; Agency for Toxic Substances and Disease Registry. Centers for Disease Control: Atlanta, GA, 1989.
- Small, M.J.; Rosenblatt, D.H. Munitions Production Products of Potential Concern as Waterborne Pollutants; Phase II. Technical report No. 7404. Contract No. AD- 9191 031; U.S. Army Medical Bioengineering Research and Development Laboratory: Aberdeen Proving Ground, MD, 1974. [Google Scholar]
- Gordon, L.; Hartley, W. R. 2, 4, 6–trinitrotoluene. In Drinking Water and Health Advisory: Munitions; Roberts, W. C., Hartley, W. R., Eds.; Lewis Publishers: Boca Raton, FL, 1992; pp. 327–398. [Google Scholar]
- Zakhari, A.; Villaume, J. E. A Literature Review: Problem Definition Studies on Selected Chemicals: Occupational Health and Safety Aspects of 2, 4, 6–trinitrotoluene (TNT). Final Report Contract No. DAMD 17- 77- C–7020. 1978, 3. [Google Scholar]
- Etnier, E. L.; Water, Quality. Criteria for 2, 4- dinitrotoluene and 2, 6- dinitrotoluene. In Final Report; U.S. Army Medical Bioengineering Research and Development Laboratory: Fort Detrick, MD, 1987. [Google Scholar]
- IARC, IARC Monographs on the Evaluation of Carcinogenic Risk to humans: 2,4-Dinitrotoluene and 2,6-Dinitrotoluene; Lyon, France; WHO, IARC, 1996; Volume 65.
- Ellis, H. V., III.; Hagensen, J. H.; Hodgson, J. R.; Minor, J. L.; Hong, C. B.; Ellis, E. R.; Girvin, J. D.; Helton, D. O.; Herndon, B. L.; Lee, C. C. Mammalian Toxicity of Munitions Compounds. Phase III: Effects of Lifetime Exposure. Part 1. 2, 4- dinitroutoluene. In Final Report; No. 7. Order No. ADA077692; U.S. Army Medical Bioengineering Research and Development Laboratory: Fort Detrick, MD, 1979. [Google Scholar]
- Levine, B. S.; Furedi, E. M.; Gordon, D. E.; Lish, P. M.; Barkley, J. J. Toxicology 1984, 32, 253–259.
- EPA, Drinking water regulations and health advisories, Office of Water. In EPA 822-R-96-001; Washington, D. C, 1996.
- Freshney, R. I. Culture of Animal Cells: A manual of Basic Technique, Fourth Edition ed; John Wiley and Sons Inc. Publisher: New York, NY, 2000; p. 577. [Google Scholar]
- Tchouwnou, P. B.; Wilson, B. A.; Ishaque, A. B.; Schneida, J. Transcriptional activation of stress genes, and cytotoxicity in human liver carcinoma cells (HepG2) exposed to 2,4,6- Trinitrotoluene, 2,4-Dinitrotoluene and 2,6- Dinitrotoluene. Environ. Toxicol 2001, 16, 209–216. [Google Scholar]
- Vernot, E. H.; McEwen, J. D.; Haun, C. C.; Kinkead, E. R. Acute Toxicity and Skin corrosion data for some organic and inorganic compounds and aqueous solutions. Toxicol. Appl. Pharmacol 1977, 42, 417–423. [Google Scholar]
- Levine, J. R.; Turner, M. J.; Crume, Y. S.; Dale, M. E.; Starr, T. B.; Rickert, D. E. Assessing exposure to dinitrotoluene using a biological monitor. J. Occup. Med 1985, 27, 627–638. [Google Scholar]
© 2005 MDPI. All rights reserved.
Share and Cite
Ishaque, A.B.; Timmons, C.; Ballard, F.V.; Hupke, C.; Dulal, K.; Johnson, L.R.; Gerald, T.M.; Boucaud, D.; Tchounwou, P.B. Cytotoxicity of Dinitrotoluenes (2,4-DNT, 2,6-DNT ) to MCF-7 and MRC-5 Cells. Int. J. Environ. Res. Public Health 2005, 2, 304-307. https://doi.org/10.3390/ijerph2005020015
Ishaque AB, Timmons C, Ballard FV, Hupke C, Dulal K, Johnson LR, Gerald TM, Boucaud D, Tchounwou PB. Cytotoxicity of Dinitrotoluenes (2,4-DNT, 2,6-DNT ) to MCF-7 and MRC-5 Cells. International Journal of Environmental Research and Public Health. 2005; 2(2):304-307. https://doi.org/10.3390/ijerph2005020015
Chicago/Turabian StyleIshaque, Ali B., Christine Timmons, Frederick V. Ballard, Carine Hupke, Kalpana Dulal, Linda R. Johnson, Tonya M. Gerald, Dwayne Boucaud, and Paul B. Tchounwou. 2005. "Cytotoxicity of Dinitrotoluenes (2,4-DNT, 2,6-DNT ) to MCF-7 and MRC-5 Cells" International Journal of Environmental Research and Public Health 2, no. 2: 304-307. https://doi.org/10.3390/ijerph2005020015



