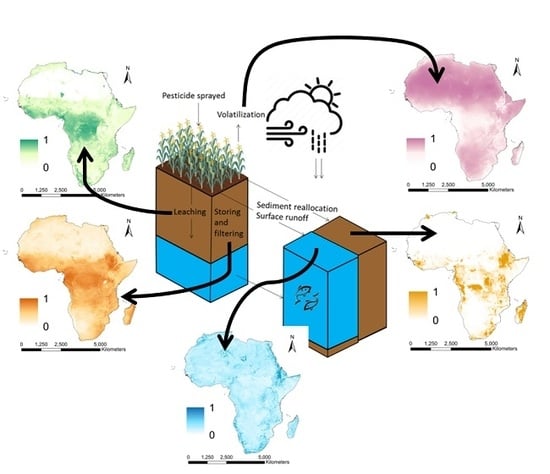
Mapping Geospatial Processes Affecting the Environmental Fate of Agricultural Pesticides in Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Key Processes Identified by Pesticide Fate Models
2.1.1. Identify Pesticide Fate Models
2.1.2. Selecting Key Processes Affecting Pesticide Fate
- -
- Leaching is the process by which rain or irrigation water infiltrates and percolates to deeper groundwater layers.
- -
- Surface runoff is the process by which rain or irrigation water flows overland to other streams or surface water.
- -
- Sedimentation is the process by which soil particles in suspension settle out of fluid, water in this instance, and come to rest.
- -
- Soil storage and filtering capacity indicates the capacity of a soil to store and filter substances (e.g., water or pesticides).
- -
- Volatilization is the process whereby a chemical substance is converted from a liquid or solid state to a gaseous or vapour state.
2.2. Selection of Geospatial Datsets
2.3. Mapping Key Processes Affecting Pesticide Fate
2.3.1. Leaching
2.3.2. Surface Runoff
2.3.3. Sedimentation
2.3.4. Soil Storage and Filtering Capacity
2.3.5. Volatilization
2.4. Testing the Maps Associated with Pesticide Fate
2.4.1. Insecticide Residue Database
2.4.2. Using the Created Maps to Spatially Predict Insecticide Residues
2.4.3. Sensitivity Analysis on Variables and Parameters
3. Results
3.1. Identifying Pesticide Fate Models and Select Key Processes
3.2. Mapping Key Variables Associated with Pesticide Fate
3.2.1. Leaching
3.2.2. Surface Runoff
3.2.3. Sedimentation
3.2.4. Soil Storage and Filtering Capacity
3.2.5. Volatilization
3.2.6. Sensitivity Analysis on Variables and Parameters
4. Potential and Limitations of the Created Maps and Future Perspective
5. Conclusions
Data Availability
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Korrick, S.A.; Chen, C.; Damokosh, A.I.; Ni, J.; Liu, X.; Cho, S.I.; Altshul, L.; Ryan, L.; Xu, X. Association of DDT with spontaneous abortion: A case-control study. Ann. Epidemiol. 2001, 11, 491–496. [Google Scholar] [CrossRef]
- Isenring, R. Pesticides and the Loss of Biodiversity How Intensive Pesticide Use Affects Wildlife Populations and Species Diversity. 2010. Available online: www.pan-europe.info (accessed on 17 September 2019).
- Zaganas, I.; Kapetanaki, S.; Mastorodemos, V.; Kanavouras, K.; Colosio, C.; Wilks, M.F.; Tsatsakis, A.M. Linking pesticide exposure and dementia: What is the evidence? Toxicology 2013, 307, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Moretto, A.; Colosio, C. Biochemical and toxicological evidence of neurological effects of pesticides: The example of Parkinson’s disease. Neurotoxicology 2011, 32, 383–891. [Google Scholar] [CrossRef] [PubMed]
- Djogbénou, L.S.; Assogba, B.; Essandoh, J.; Constant, E.A.; Makoutodé, M.; Akogbéto, M.; Donnelly, M.J.; Weetman, D. Estimation of allele-specific Ace-1 duplication in insecticide-resistant Anopheles mosquitoes from West. Afr. Malar. J. 2015, 14, 507. [Google Scholar]
- Hien, A.S.; Soma, D.D.; Hema, O.; Bayili, B.; Namountougou, M.; Gnankiné, O.; Baldet, T.; Diabaté, A.; Dabiré, K.R. Evidence that agricultural use of pesticides selects pyrethroid resistance within Anopheles gambiae s.l. populations from cotton growing areas in Burkina Faso, West Africa. PLoS ONE 2017, 12, e0173098. [Google Scholar] [CrossRef] [PubMed]
- Sheahan, M.; Barrett, C. Understanding the Agricultural Input Landscape in Sub-Saharan Africa: Recent Plot, Household, and Community-Level Evidence. Policy Research Working Papers; World Bank: Washington, DC, USA, 2014; p. 87. [Google Scholar]
- Sheahan, M.; Barrett, C.B.; Goldvale, C. Human health and pesticide use in Sub-Saharan Africa. Agric. Econ. 2017, 48, 27–41. [Google Scholar] [CrossRef]
- Smart, J.; Snyder, J.; Goeb, J.; Tschirley, D. High Pesticide Use among Smallholders in Africa South of the Sahara Poses Risks for Health, Environment. 2018. Available online: http://www.ifpri.org/blog/high-pesticide-use-among-smallholders-africa-south-sahara-poses-risks-health-environment (accessed on 6 June 2019).
- De Bon, H.; Huat, J.; Parrot, L.; Sinzogan, A.; Martin, T.; Malezieux, E.; Vayssieres, J.F. Pesticide risks from fruit and vegetable pest management by small farmers in sub-Saharan Africa. A review. Agron. Sustain. Dev. 2014, 34, 723–736. [Google Scholar] [CrossRef]
- Kookana, R.; Simpson, B.W. Pesticide fate in farming systems: Research and monitoring. Commun. Soil Sci. Plant. Anal. 2000, 31, 1641–1659. [Google Scholar] [CrossRef]
- Lewis, S.E.; Silburn, D.M.; Kookana, R.S.; Shaw, M. Pesticide Behavior, Fate, and Effects in the Tropics: An Overview of the Current State of Knowledge. J. Agric. Food Chem. 2016, 64, 3917–3924. [Google Scholar] [CrossRef]
- Racke, K.D.; Skidmore, M.; Hamilton, D.J.; Unsworth, J.B.; Miyamoto, J.; Cohen, S.Z. Pesticide fate in tropical soils. Pestic. Sci. 1999, 55, 219–220. [Google Scholar] [CrossRef]
- Siimes, K.; Kämäri, J. A review of available pesticide leaching models: Selection of models for simulation of herbicide fate in Finnish sugar beet cultivation. Boreal Environ. Res. 2003, 8, 31–51. [Google Scholar]
- Quilbé, R.; Rousseau, A.N.; Lafrance, P.; Leclerc, J.; Amrani, M. Selecting a pesticide fate model at the watershed scale using a multi-criteria analysis. Water Qual. Res. J. Can. 2006, 41, 283–295. [Google Scholar] [CrossRef]
- Meyers, M.; Albertin, K.; Cocca, P. BASINS 3.0: Modeling tool for improved watershed management. In: Warwick JJ, editor. Water quality monitoring and modeling. Am. Water Resour. 2001, 17–22. [Google Scholar]
- Ter Horst, M.M.S.; Beltman, W.H.J.; Van den Berg, F. The TOXSWA Model Version 3.3 for Pesticide Behaviour in Small Surface Waters: Description of Processes. Statutory Research Tasks Unit for Nature & the Environment; WOt-Technical Report 84; Wageningen University: Wageningen, The Netherlands, 2016; p. 72. [Google Scholar]
- Camenzuli, L.; Scheringer, M.; Gaus, C.; Ng, C.A.; Hungerbühler, K. Describing the environmental fate of diuron in a tropical river catchment. Sci. Total Environ. 2012, 440, 178–185. [Google Scholar] [CrossRef]
- Scheringer, M.; Wegmann, F.; Fenner, K.; Hungerbühler, K. Investigation of the cold condensation of persistent organic pollutants with a global multimedia fate model. Environ. Sci Technol. 2000, 34, 1842–1850. [Google Scholar] [CrossRef]
- Wania, F.; Breivik, K.; Persson, N.J.; McLachlan, M.S. CoZMo-POP 2—A fugacity-based dynamic multi-compartmental mass balance model of the fate of persistent organic pollutants. Environ. Model. Softw. 2006, 21, 868–884. [Google Scholar] [CrossRef]
- Armstrong, A.C.; Matthews, A.M.; Portwood, A.M.; Leeds-Harrison, P.B.; Jarvis, N.J. CRACK-NP: A pesticide leaching model for cracking clay soils. Agric. Water Manag. 2000, 44, 183–199. [Google Scholar] [CrossRef]
- Mendez, A.; Ng, C.A.; Torres, J.P.M.; Bastos, W.; Bogdal, C.; dos Reis, G.A.; Hungerbuehler, K. Modeling the dynamics of DDT in a remote tropical floodplain: Indications of post-ban use? Environ. Sci. Pollut. Res. 2016, 23, 10317–10334. [Google Scholar] [CrossRef]
- Williams, J.R.; Wang, E.; Meinardus, A.; Harman, W.L.; Siemers, M.; Atwood, J.D. EPIC Users Guide v.0509. 2006. Available online: https://agrilifecdn.tamu.edu/epicapex/files/2013/02/epic0509usermanualupdated.pdf (accessed on 16 September 2020).
- Rousseau, A.N.; Mailhot, A.; Turcotte, R.; Duchemin, M.; Blanchette, C. GIBSI—An integrated modelling system prototype for river basin management. Hydrobioloiga 2000, 422, 465–475. [Google Scholar] [CrossRef]
- Leonard, R.A.; Knisel, W.G.; Davis, F.M. Modelling pesticide fate with GLEAMS. Eur. J. Agron. 1995, 4, 485–490. [Google Scholar] [CrossRef]
- Hayter, E.J.; Bergs, M.A.; Gu, R.; McCutcheon, S.C. HSCTM-2D, A Finite Element Model for Depth-Average Hydrodynamics, Sediment and Contaminant Transport; National Exposure Research Laboratory, Office of Research and Development, US EPA: Athens, GA, USA, 1997; p. 220.
- Hutson, J.L. Leaching Estimation and Chemistry Model, Model Description and User’s Guide; Cornell University: Ithaca, NY, USA, 2003; p. 142. [Google Scholar]
- Jarvis, N.J.; Larsson, M.H. The MACRO Model (Version 4.1): Technical Description; Reports and Dissertations 19; Swedish University of Agricultural Sciences: Uppsala, Sweden, 1998. [Google Scholar]
- Smith, R.E. Opus: An Integrated Simulation Model for Transport of Nonpoint-Source Pollutants and the Field Scale; USDA, ARS, Water Management Research Unit: Washington, DC, USA, 1992; p. 214.
- Tiktak, A.; Van den Berg, F.; Boesten, J.J.T.I.; Leistra, M.; Van der Linden, A.M.A.; Van Kraalingen, D. Pesticide Emission Assessment at Regional and Local Scales: User Manual of Pearl version 1.1. RIVM Report 711401008; Alterra Report 28; Alterra Wageningen UR: Wageningen, The Netherlands, 2000; p. 142. [Google Scholar]
- Klein, M. PELMO: Pesticide Leaching Model, Version 5.00; Fraunhofer-Institute for Molecular Biology and Applied Ecology: Schmallenberg, Germany, 2018; p. 164. [Google Scholar]
- Van den Berg, F.; Boesten, J.J.T.I. Pesticide Leaching and Accumulation Model (PESTLA) Version 3.4. Description and User’s Guide; Technical Document 43; DLO Winand Staring Centre: Wageningen, The Netherlands, 1998; p. 150. [Google Scholar]
- Nicholls, P.H.; Hall, D.G.M. Use of the pesticide leaching model (PLM) to simulate pesticide movement through macroporous soils. In Pesticide Movement to Water, BCPC Monograph 62; Walker, A., Allen, R., Bailey, S.W., Blair, A.M., Brown, C.D., Günther, P., Leake, C.R., Nichollas, P.H., Eds.; British Crop Protection Council: Hampshire, UK, 1995; pp. 187–192. [Google Scholar]
- Peeters, F.M.; Van den Brink, P.J.; Vlaming, J.; Groenwold, J.G.; Beltman, W.H.J.; Boesten, J.J.T.I. PRIMET Version 2.0, Technical Description and Manual: A Decision Support System for Assessing Pesticide RIsks in the Tropics to Man, Environment and Trade; Alterra Rapport 1648; Alterra Wageningen UR: Wageningen, The Netherlands, 2008; p. 77. [Google Scholar]
- Beltman, W.H.J.; Ter Horst, M.M.S.; Adriaanse, P.I.; De Jong, A. Manual of FOCUS_TOXSWA Version 2.2.1; Alterra-Rapport 586; Alterra Wageningen UR: Wageningen, The Netherlands, 2006; p. 198. [Google Scholar]
- Carsel, R.F.; Smith, C.N.; Mulkey, L.A.; Dean, J.D.; Jowise, P. User’s Manual for the Pesticide Root Zone Model (PRZM): Release 1. EPA/600/3-84/109; Environmental Research Laboratory USA: Ann Arbor, MI, USA, 1984.
- Team, R.D.; Hanson, J.D.; Ahuja, L.R.; Shaffer, M.D.; Rojas, K.W.; DeCoursey, D.G.; Farahani, H.; Johnson, K. RZWQM: Simulating the effects of management on water quality and crop production. Agric. Syst. 1998, 57, 161–195. [Google Scholar]
- Hetrick, D.M.; Travis, C.C.; Leonard, S.K.; Kinerson, R.S. Qualitative Validation of Pollutant Transport Components of an Unsaturated Soil Zone Model (SESOIL). United States. 1989. Available online: https://www.osti.gov/servlets/purl/6154212 (accessed on 6 August 2018).
- Aden, K.; Diekkrüger, B. Modeling pesticide dynamics of four different sites using the model system SIMULAT. Agric. Water Manag. 2000, 44, 337–355. [Google Scholar]
- Arnold, J.G.; Kiniry, J.R.; Srinivasan, R.; Williams, J.R.; Haney, E.B.; Neitsch, S.L. Soil & Water Assessment Tool, Input/Output Documentation Version 2012; Texas Water Resources Institute: College Station, TX, USA, 2013. [Google Scholar]
- Lal, R. Soil degradation by erosion. L. Degrad. Dev. 2001, 12, 519–539. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Morgenthal, T.L.; Malherbe, J.; Pretorius, D.J.; Sumner, P.D. Water erosion prediction at a national scale for South Africa. Water SA 2008, 34, 305–314. [Google Scholar] [CrossRef]
- Coleman, M.; Hemingway, J.; Gleave, K.A.; Wiebe, A.; Gething, P.W.; Moyes, C.L. Developing global maps of insecticide resistance risk to improve vector control. Malar. J. 2017, 16, 86. [Google Scholar] [CrossRef]
- Hengl, T.; Heuvelink, G.B.; Kempen, B.; Leenaars, J.G.; Walsh, M.G.; Shepherd, K.D.; Sila, A.; MacMillan, R.A.; de Jesus, J.M.; Tamene, L.; et al. Mapping Soil Properties of Africa at 250 m Resolution: Random Forests Significantly Improve Current Predictions. PLoS ONE 2015, 10, e0125814. [Google Scholar] [CrossRef]
- Fan, Y.; Li, H.; Miguez-Macho, G. Global patterns of groundwater table depth. Science 2013, 339, 940–943. [Google Scholar] [CrossRef]
- Hengl, T.; de Jesus, J.M.; Heuvelink, G.B.; Gonzalez, M.R.; Kilibarda, M.; Blagotić, A.; Shangguan, W.; Wright, M.N.; Geng, X.; Bauer-Marschallinger, B.; et al. SoilGrids250m: Global gridded soil information based on machine learning. PLoS ONE 2017, 12, e0169748. [Google Scholar] [CrossRef]
- Jarvis, A.; Reuter, H.I.; Nelson, A.; Guevara, E. Hole-Filled SRTM for the Globe Version 4. CGIAR-CSI SRTM 90m Database. 2008. Available online: http://srtm.csi.cgiar.org (accessed on 15 March 2019).
- Mladenova, I.E.; Bolten, J.D.; Crow, W.T.; Anderson, M.C.; Hain, C.R.; Johnson, D.M.; Mueller, R. Intercomparison of Soil Moisture, Evaporative Stress, and Vegetation Indices for Estimating Corn and Soybean Yields Over the U.S.A. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2017, 10, 1328–1343. [Google Scholar] [CrossRef]
- Stoorvogel, J.J.; Bakkenes, M.; Temme, A.J.A.M.; Batjes, N.H.; Ten Brink, B.J.E. S-World: A Global Soil Map for Environmental Modelling. L Degrad. Dev. 2017, 28, 22–33. [Google Scholar] [CrossRef]
- Channan, S.; Collins, K.; Emanuel, W.R. Global mosaics of the standard MODIS land cover type data. University of Maryland and the Pacific Northwest National Laboratory, USA. 2014. Available online: http://glcf.umd.edu/data/lc/ (accessed on 26 February 2019).
- Panagos, P.; Borrelli, P.; Meusburger, K.; Yu, B.; Klik, A.; Lim, K.J.; Yang, J.E.; Ni, J.; Miao, C.; Chattopadhyay, N.; et al. Global rainfall erosivity assessment based on high-temporal resolution rainfall records. Sci. Rep. 2017, 7, 4175. [Google Scholar] [CrossRef]
- Fischer, G.; Nachtergaele, F.; Prieler, S.; Van Velthuizen, H.T.; Verelst, L.; Wiberg, D. Global Agro-Ecological Zones Assessment for Agriculture (GAEZ 2008). IIASA, FAO. 2008. Available online: http://www.fao.org/soils-portal/soil-survey/soil-maps-and-databases/harmonized-world-soil-database-v12/en/ (accessed on 22 May 2018).
- Weiss, D.J.; Atkinson, P.M.; Bhatt, S.; Mappin, B.; Hay, S.I.; Gething, P.W. An effective approach for gap-filling continental scale remotely sensed time-series. ISPRS J. Photogramm. Remote Sens. 2014, 98, 106–118. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J. Global Aridity Index (Global-Aridity) and Global Potential Evapo-Transpiration (Global-PET) Geospatial Database. CGIAR-CSI GeoPortal. 2009. Available online: https://cgiarcsi.community/data/global-aridity-and-pet-database/ (accessed on 5 January 2019).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Wan, Z.; Hook, S.; Hulley, G. MOD11A1 MODIS/Terra Land Surface Temperature/Emissivity Daily L3 Global 1km SIN Grid V006. NASA EOSDIS LP DAAC. 2015. Available online: https://lpdaac.usgs.gov/node/819 (accessed on 6 August 2018).
- Gilliom, R.J.; Barbash, J.E.; Crawford, C.G.; Hamilton, P.A.; Martin, J.D.; Nakagaki, N.; Nowell, L.H.; Scott, J.C.; Stackelberg, P.E.; Thelin, G.P.; et al. The Quality of Our Nation’s Waters—Pesticides in the Nation’s Streams and Ground Water, 1992–2001; US Geological Survey Circular 1291: Reston, VA, USA, 2006; p. 172.
- Sarmah, A.K.; Müller, K.; Ahmad, R. Fate and behaviour of pesticides in the agroecosystem—A review with a New Zealand perspective. Aus. J. Soil Res. 2004, 42, 125–154. [Google Scholar] [CrossRef]
- FAO. Guidelines: Land Evaluation for Irrigated Agriculture—FAO Soils Bulletin 55; FAO: Rome, Italy, 1985. [Google Scholar]
- Pérez-Lucas, G.; Vela, N.; El Aatik, A.; Navarro, S. Environmental Risk of Groundwater Pollution by Pesticide Leaching through the Soil Profile. In Pesticides—Use and Misuse and Their Impact in the Environment; Larramendy, M., Soloneski, S., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Ahmed, A.A. Using generic and pesticide DRASTIC GIS-based models for vulnerability assessment of the quaternary aquifer at Sohag, Egypt. Hydrogeol. J. 2009, 17, 1203–1217. [Google Scholar] [CrossRef]
- Anane, M.; Abidi, B.; Lachaal, F.; Limam, A.; Jellali, S. GIS-based DRASTIC, pesticide DRASTIC and the susceptibility index (SI): Comparative study for evaluation of pollution potential in the Nabeul-Hammamet shallow aquifer, Tunisia. Hydrogeol. J. 2013, 21, 715–731. [Google Scholar] [CrossRef]
- Saha, D.; Alam, F. Groundwater vulnerability assessment using DRASTIC and Pesticide DRASTIC models in intense agriculture area of the Gangetic plains, India. Environ. Monit. Assess. 2014, 186, 8741–8763. [Google Scholar] [CrossRef]
- Dehotin, J.; Breil, P.; Braud, I.; De Lavenne, A.; Lagouy, M.; Sarrazin, B. Detecting surface runoff location in a small catchment using distributed and simple observation method. J. Hydrol. 2015, 525, 113–129. [Google Scholar] [CrossRef]
- Lagadec, L.R.; Patrice, P.; Braud, I.; Chazelle, B.; Moulin, L.; Dehotin, J.; Hauchard, E.; Breil, P. Description and evaluation of a surface runoff susceptibility mapping method. J. Hydrol. 2016, 541, 495–509. [Google Scholar] [CrossRef]
- Wischmeier, W.H.; Smith, D.D. Predicting Rainfall Erosion Lossess: A Guide to Conservation Planning; Agriculture Handbook No. 537; U.S. Department of Agriculture: Washington, DC, USA, 1978; p. 60.
- Guzha, A.C.; Rufino, M.C.; Okoth, S.; Jacobs, S.; Nóbrega, R.L.B. Impacts of land use and land cover change on surface runoff, discharge and low flows: Evidence from East Africa. J. Hydrol. Reg. Stud. 2018, 15, 49–67. [Google Scholar] [CrossRef]
- Horton, R.E. Drainage-basin characteristics. Eos Trans. Am. Geophys. Union 1932, 13, 350–361. [Google Scholar] [CrossRef]
- Lehner, B.; Verdin, K.; Jarvis, A. New global hydrography derived from spaceborne elevation data. Eos Trans. Am. Geophys. Union 2008, 89, 93–94. [Google Scholar] [CrossRef]
- Wischmeier, W.H.; Smith, D.D. Predicting Rainfall Erosion Losses; Agriculture Handbook No. 537; U.S. Department of Agriculture: Washington, DC, USA, 1978; pp. 285–291.
- Hickey, R. Slope Angle and Slope Length Solutions for GIS. Cartography 2000, 29, 1–8. [Google Scholar] [CrossRef]
- Renard, K.G.; Foster, G.R.; Weesies, G.A.; McCool, D.K.; Yoder, D.C. Predicting Soil Erosion by Water: A Guide to Conservation Planning with the Revised Universal Soil Loss Equation (RUSLE); Agricultural Handbook 703; U.S. Government Printing Office: Washington, DC, USA, 1997.
- Feng, Q.; Zhao, W.; Ding, J.; Fang, X.; Zhang, X. Estimation of the cover and management factor based on stratified coverage and remote sensing indices: A case study in the Loess Plateau of China. J. Soils Sediments 2018, 18, 775–790. [Google Scholar] [CrossRef]
- Maidment, D.R.; Olivera, F.; Calver, A.; Eatherall, A.; Fraczek, W. Unit hydrograph derived from a spatially distributed velocity field. Hydrol. Process. 1996, 10, 831–844. [Google Scholar] [CrossRef]
- Makó, A.; Kocsis, M.; Barna, G.; Tóth, G. Mapping the Storing and Filtering Capacity of European Soils; Techincal Report EUR28393; JRC: Ispra, Italy, 2017; p. 62. [Google Scholar]
- Keesstra, S.D.; Geissen, V.; Mosse, K.; Piiranen, S.; Scudiero, E.; Leistra, M.; Van Schaik, L. Soil as a filter for groundwater quality. Curr. Opin. Env. Sust. 2012, 4, 507–516. [Google Scholar] [CrossRef]
- Bedos, C.; Rousseau-Djabri, M.F.; Flura, D.; Masson, S.; Barriuso, E.; Cellier, P. Rate of pesticide volatilization from soil: An experimental approach with a wind tunnel system applied to trifluralin. Atmos. Environ. 2002, 36, 5917–5925. [Google Scholar] [CrossRef]
- Li, Y.; Venkatesh, S.; Li, D. Modeling global emissions and residues of pesticides. Environ. Model. Assess. 2005, 9, 237. [Google Scholar] [CrossRef]
- Peeters, F.M.; van den Brink, P.J.; Vlaming, J.; Groenwold, J.G.; Beltman, W.H.J.; Boesten, J.J.T.I. PRIMET_Registration_Ethiopia_1.1; Alterra-Rapport 2573; Alterra Wageningen UR: Wageningen, The Netherlands, 2014; p. 133. [Google Scholar]
- Arnold, J.G.; Fohrer, N. SWAT2000: Current capabilities and research opportunities in applied watershed modelling. Hydrol Process. 2005, 19, 563–572. [Google Scholar] [CrossRef]
- Arnold, J.G.; Srinivasan, R.; Muttiah, R.S.; Williams, J.R. Large area hydrologic modeling and assessment part I: Model development. JAWRA J. Am. Water Resour. Assoc. 1998, 34, 73–89. [Google Scholar] [CrossRef]
- Carsel, R.F.; Mulkey, L.A.; Lorber, M.N.; Baskin, L.B. The Pesticide Root Zone Model (PRZM): A procedure for evaluating pesticide leaching threats to groundwater. Ecol. Model. 1985, 30, 49–69. [Google Scholar] [CrossRef]
- Teklu, B.M.; Adriaanse, P.I.; Ter Horst, M.M.S.; Deneer, J.W.; Van den Brink, P.J. Surface water risk assessment of pesticides in Ethiopia. Sci. Total Environ. 2015, 508, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Gaiser, T.; De Barros, I.; Sereke, F.; Lange, F.M. Validation and reliability of the EPIC model to simulate maize production in small-holder farming systems in tropical sub-humid West Africa and semi-arid Brazil. Agric. Ecosyst. Environ. 2010, 135, 318–327. [Google Scholar] [CrossRef]
- Shunthirasingham, C.; Mmereki, B.T.; Masamba, W.; Oyiliagu, C.E.; Lei, Y.D.; Wania, F. Fate of Pesticides in the Arid Subtropics, Botswana, Southern Africa. Environ. Sci. Technol. 2010, 44, 8082–8088. [Google Scholar] [CrossRef] [PubMed]
- Scorza Júnior, R.P.; Boesten, J.J.T.I. Simulation of pesticide leaching in a cracking clay soil with the PEARL model. Pest. Manag. Sci. 2005, 61, 432–448. [Google Scholar] [CrossRef] [PubMed]
- Tebebu, T.Y.; Abiy, A.Z.; Zegeye, A.D.; Dahlke, H.E.; Easton, Z.M.; Tilahun, S.A.; Collick, A.S.; Kidnau, S.; Moges, S.; Dadgari, F.; et al. Surface and subsurface flow effect on permanent gully formation and upland erosion near Lake Tana in the northern highlands of Ethiopia. Hydrol. Earth Syst. Sci. 2010, 14, 2207–2217. [Google Scholar] [CrossRef]
- Tibebe, D.; Bewket, W. Surface runoff and soil erosion estimation using the SWAT model in the Keleta Watershed, Ethiopia. L. Degrad. Dev. 2011, 22, 551–564. [Google Scholar] [CrossRef]
- Randrianarijaona, P. The Erosion of Madagascar. Ambio 1983, 12, 308–311. [Google Scholar]
- Ali, Y.S.A.; Crosato, A.; Mohamed, Y.A.; Abdalla, S.H.; Wright, N.G. Sediment balances in the Blue Nile River Basin. Int. J. Sediment. Res. 2014, 29, 316–328. [Google Scholar] [CrossRef]
- Ayele, G.T.; Teshale, E.Z.; Yu, B.; Rutherfurd, I.D.; Jeong, J. Streamflow and sediment yield prediction for watershed prioritization in the upper Blue Nile river basin, Ethiopia. Water 2017, 9, 782. [Google Scholar] [CrossRef]
- Beernaert, F.R. Development of a Soil and Terrain Map/Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- Bagalwa, M.; Karume, K.; Bayongwa, C.; Ndahama, N.; Ndegeyi, K. Land use effects on Cirhanyobowa river water quality in D.R. Congo. Greener J. Biol. Sci. 2013, 3, 21–30. [Google Scholar] [CrossRef]
- Ludwig, M.; Wilmes, P.; Schrader, S. Measuring soil sustainability via soil resilience. Sci. Total Environ. 2018, 626, 1484–1493. [Google Scholar] [CrossRef]
- Laabs, V.; Amelung, W. Sorption and aging of corn and soybean pesticides in tropical soils of Brazil. J. Agric. Food Chem. 2005, 53, 7184–7192. [Google Scholar] [CrossRef]
- Oliver, D.P.; Baldock, J.A.; Kookana, R.S.; Grocke, S. The effect of landuse on soil organic carbon chemistry and sorption of pesticides and metabolites. Chemosphere 2005, 60, 531–541. [Google Scholar] [CrossRef]
- Zheng, S.-Q.; Cooper, J.-F. Adsorption, desorption, and degradation of three pesticides in different soils. Arch. Environ. Contam Toxicol. 1996, 30, 15–20. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Han, I.-K.; Lee, S.-Y.; Yeo, I.-H.; Lee, S.-R. Drift and Volatilization of Some Pesticides Sprayed on Chinese Cabbages. Korean J. Environ. Agric. 1997, 16, 373–381. [Google Scholar]
- Zivan, O.; Bohbot-Raviv, Y.; Dubowski, Y. Primary and secondary pesticide drift profiles from a peach orchard. Chemosphere 2017, 177, 303–310. [Google Scholar] [CrossRef]
- Hogarh, J.N.; Seike, N.; Kobara, Y.; Ofosu-Budu, G.K.; Carboo, D.; Masunaga, S. Atmospheric burden of organochlorine pesticides in Ghana. Chemosphere 2014, 102, 1–5. [Google Scholar] [CrossRef]
- Trinh, T.; van den Akker, B.; Coleman, H.M.; Stuetz, R.M.; Drewes, J.E.; Le-Clech, P.; Khan, S.J. Seasonal variations in fate and removal of trace organic chemical contaminants while operating a full-scale membrane bioreactor. Sci. Total Environ. 2016, 550, 176–183. [Google Scholar] [CrossRef]
- Ouedraogo, I.; Defourny, P.; Vanclooster, M. Mapping the groundwater vulnerability for pollution at the pan African scale. Sci. Total Environ. 2016, 544, 939–953. [Google Scholar] [CrossRef]
- Borrelli, P.; Robinson, D.A.; Fleischer, L.R.; Lugato, E.; Ballabio, C.; Alewell, C.; Meusburger, K.; Modugno, S.; Schütt, B.; Ferro, V.; et al. An assessment of the global impact of 21st century land use change on soil erosion. Nat. Commun. 2017, 8, 2013. [Google Scholar] [CrossRef]
- Vrieling, A.; Hoedjes, J.C.B.; Van der Velde, M. Towards large-scale monitoring of soil erosion in Africa: Accounting for the dynamics of rainfall erosivity. Glob. Planet. Change 2014, 115, 33–43. [Google Scholar] [CrossRef]
- Auerswald, K.; Kainz, M.; Fiener, P. Soil erosion potential of organic versus conventional farming evaluated by USLE modelling of cropping statistics for agricultural districts in Bavaria. Soil Use Manag. 2003, 19, 305–311. [Google Scholar] [CrossRef]
- Kawamura, K.; Akiyama, T.; Yokota, H.; Tsutsumi, M.; Yasuda, T.; Watanabe, O.; Wang, S. Comparing MODIS vegetation indices with AVHRR NDVI for monitoring the forage quantity and quality in Inner Mongolia grassland, China. Grassl. Sci. 2005, 51, 33–40. [Google Scholar] [CrossRef]
- Christiaensen, L. Agriculture in Africa—Telling myths from facts: A synthesis. Food Policy 2017, 67, 1–11. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, F.; Ou, J. Global pesticide consumption and pollution: With China as a focus. Proc. Int. Ac. Ecol. Environ. Sci. 2011, 1, 125–144. [Google Scholar]
- Geospatial Layers on Processes Affecting the Environmental Fate of Agricultural Pesticides in Africa. Available online: https://doi.org/10.6084/m9.figshare.7923455.v2 (accessed on 1 April 2019).
- Insecticide residue database for Africa. Available online: https://doi.org/10.6084/m9.figshare.7932485.v3 (accessed on 1 April 2019).







| Number | Model Name | Country | Source |
|---|---|---|---|
| 1 | BASINS | USA | [16] |
| 2 | CASCADE-TOXSWA | The Netherlands | [17] |
| 3 | Chemical fate model | Australia | [18] |
| 4 | CliMoChem | Global | [19] |
| 5 | CoZMo-POP-2 | USA | [20] |
| 6 | CRACK-NP | United Kingdom | [21] |
| 7 | Dynamic multimedia environmental fate model | Brazil | [22] |
| 8 | EPIC | USA | [23] |
| 9 | GIBSI | Canada | [24] |
| 10 | GLEAMS | USA | [25] |
| 11 | HSCTM-2D | USA | [26] |
| 12 | LEACHM | USA | [27] |
| 13 | MACRO | Sweden | [28] |
| 14 | OPUS | USA | [29] |
| 15 | PEARL | The Netherlands | [30] |
| 16 | PELMO | Germany | [31] |
| 17 | PESTLA | The Netherlands | [32] |
| 18 | PLM | United Kingdom | [33] |
| 19 | PRIMET | Southeast Asia | [34] |
| 20 | PRZM | USA | [35,36] |
| 21 | RZWQM | USA | [37] |
| 22 | SESOIL | USA | [38] |
| 23 | SIMULAT | Germany | [39] |
| 24 | SWAT | USA | [40] |
| Pesticide Fate Process | Required Variables | Selected Geospatial Dataset | Source of Geospatial Dataset |
|---|---|---|---|
| Leaching | Soil drainage rate | Soil drainage class | [44] |
| Groundwater depth | Groundwater depth | [45] | |
| Depth to bedrock | Depth to bedrock | [46] | |
| Type of bedrock | Soil drainage class | [46] | |
| Slope | Slope | [47] | |
| Soil moisture | Soil moisture | [48] | |
| Surface runoff—Generation | Soil drainage rate | Soil drainage class | [46] |
| Soil thickness | Soil thickness | [49] | |
| Soil erodibility | Soil erodibility factor | NA | |
| Topography | Slope | [47] | |
| Flow accumulation | [47] | ||
| Land use | Land use class | [50] | |
| Surface runoff—Transfer | Surface runoff—Generation | Surface runoff—Generation | NA |
| Slope | Slope | [47] | |
| Break of slope | -- | -- | |
| Catchment capacity | Watershed area | [47] | |
| Stream length | [47] | ||
| Artificial linear axes | -- | -- | |
| Surface runoff—Accumulation | Surface runoff—Generation | Surface runoff—Generation | NA |
| Slope | Slope | [47] | |
| Break of slope | -- | -- | |
| Topographic index | Elevation | [47] | |
| Flow accumulation | Flow accumulation | [47] | |
| Sedimentation | Rainfall erosivity factor | Rainfall erosivity | [51] |
| Soil erodibility factor | Silt content | [46] | |
| Sand content | [46] | ||
| Clay content | [46] | ||
| Soil organic matter content | [46] | ||
| Soil structure class | [52] | ||
| Cover-management factor | Enhanced Vegetation Index | [53] | |
| Slope length and slope steepness factor | Slope | [47] | |
| Support practice factor | -- | -- | |
| Erosion | Erosion | NA | |
| Surface runoff—Accumulation | Surface runoff—Accumulation | ||
| Watershed area | Watershed area | [47] | |
| Soil storage and filtering capacity | Soil organic matter content | Soil organic matter content | [46] |
| Clay content | Clay content | [46] | |
| Soil pH | Soil pH in H2O | [46] | |
| Cation Exchange Capacity | Cation Exchange Capacity | [47] | |
| Volatilization | Evapotranspiration | Potential evapotranspiration | [54] |
| Wind velocity | Wind velocity | [55] | |
| Temperature | Land surface temperature | [56] | |
| Relative humidity | Relative humidity | [56] | |
| Solar radiation | Solar radiation | [55] |
| Forest | 0 |
| Grass/scrub/woodland | 0.2 |
| Barren/very sparsely vegetated land | 0.6 |
| Irrigated and rain-fed cultivated land | 0.8 |
| Built-up land | 1 |
| Process | Variables | −5% | +5% |
|---|---|---|---|
| Leaching | Drainage class | 2.4 (0.4) | 2.8 (0.5) |
| Groundwater depth | 6.2 (1.1) | 3.2 (0.6) | |
| Depth to bedrock | 2.4 (0.4) | 4.1 (0.7) | |
| Slope | 1.2 (0.2) | 1.8 (0.3) | |
| Soil moisture | 5.8 (1.0) | 2.4 (0.4) | |
| Surface runoff—generation | Soil drainage | 1.2 (0.4) | 1.2 (0.4) |
| Soil thickness | 2.1 (0.4) | 2.1 (0.4) | |
| Erodibility | 0.3 (0.3) | 0.3 (0.3) | |
| Topography | 0.3 (0.1) | 0.3 (0.1) | |
| Land use | 1.1 (0.5) | 1.1 (0.5) | |
| Surface runoff—transfer | Surface runoff—generation | 3.7 (0.9) | 3.7 (0.9) |
| Slope | 1.0 (0.7) | 1.0 (0.7) | |
| Catchment capacity | 0.3 (0.7) | 0.3 (0.7) | |
| Surface runoff—accumulation | Surface runoff—generation | 1.7 (1.3) | 1.7 (1.3) |
| Slope | 0.6 (0.6) | 0.6 (0.6) | |
| Elevation | 0.6 (0.6) | 0.6 (0.6) | |
| Flow accumulation | 2.1 (2.1) | 2.1 (2.1) | |
| Sedimentation | Rainfall erosivity | 0.3 (1.0) | 0.2 (0.6) |
| Soil erodibility | 0.6 (1.9) | 0.7 (2.4) | |
| Cropping factor | 0.3 (0.9) | 0.4 (1.3) | |
| Slope | 0.0 (0.0) | 0.1 (0.4) | |
| Flow velocity | 0.0 (0.0) | 0.1 (0.3) | |
| Soil storage and filtering capacity | Organic carbon | 0.3 (0.4) | 0.3 (0.4) |
| Clay content | 1.2 (0.6) | 1.2 (0.6) | |
| soil pH | 4.3 (3.6) | 4.3 (3.6) | |
| Cation Exchange Capacity | 0.7 (0.4) | 0.7 (0.4) | |
| Volatilization | Wind speed | 0.5 (0.2) | 0.5 (0.2) |
| Solar radiation | 1.0 (0.1) | 1.0 (0.1) | |
| Temperature | 1.2 (0.2) | 1.2 (0.2) | |
| Potential Evapotranspiration | 1.3 (0.2) | 1.3 (0.2) | |
| Relative Humidity | 0.7 (0.6) | 0.7 (0.6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendriks, C.M.J.; Gibson, H.S.; Trett, A.; Python, A.; Weiss, D.J.; Vrieling, A.; Coleman, M.; Gething, P.W.; Hancock, P.A.; Moyes, C.L. Mapping Geospatial Processes Affecting the Environmental Fate of Agricultural Pesticides in Africa. Int. J. Environ. Res. Public Health 2019, 16, 3523. https://doi.org/10.3390/ijerph16193523
Hendriks CMJ, Gibson HS, Trett A, Python A, Weiss DJ, Vrieling A, Coleman M, Gething PW, Hancock PA, Moyes CL. Mapping Geospatial Processes Affecting the Environmental Fate of Agricultural Pesticides in Africa. International Journal of Environmental Research and Public Health. 2019; 16(19):3523. https://doi.org/10.3390/ijerph16193523
Chicago/Turabian StyleHendriks, Chantal M. J., Harry S. Gibson, Anna Trett, André Python, Daniel J. Weiss, Anton Vrieling, Michael Coleman, Peter W. Gething, Penny A. Hancock, and Catherine L. Moyes. 2019. "Mapping Geospatial Processes Affecting the Environmental Fate of Agricultural Pesticides in Africa" International Journal of Environmental Research and Public Health 16, no. 19: 3523. https://doi.org/10.3390/ijerph16193523
APA StyleHendriks, C. M. J., Gibson, H. S., Trett, A., Python, A., Weiss, D. J., Vrieling, A., Coleman, M., Gething, P. W., Hancock, P. A., & Moyes, C. L. (2019). Mapping Geospatial Processes Affecting the Environmental Fate of Agricultural Pesticides in Africa. International Journal of Environmental Research and Public Health, 16(19), 3523. https://doi.org/10.3390/ijerph16193523