Odorous Compounds from Poultry Manure Induce DNA Damage, Nuclear Changes, and Decrease Cell Membrane Integrity in Chicken Liver Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. LMH Cell Culture
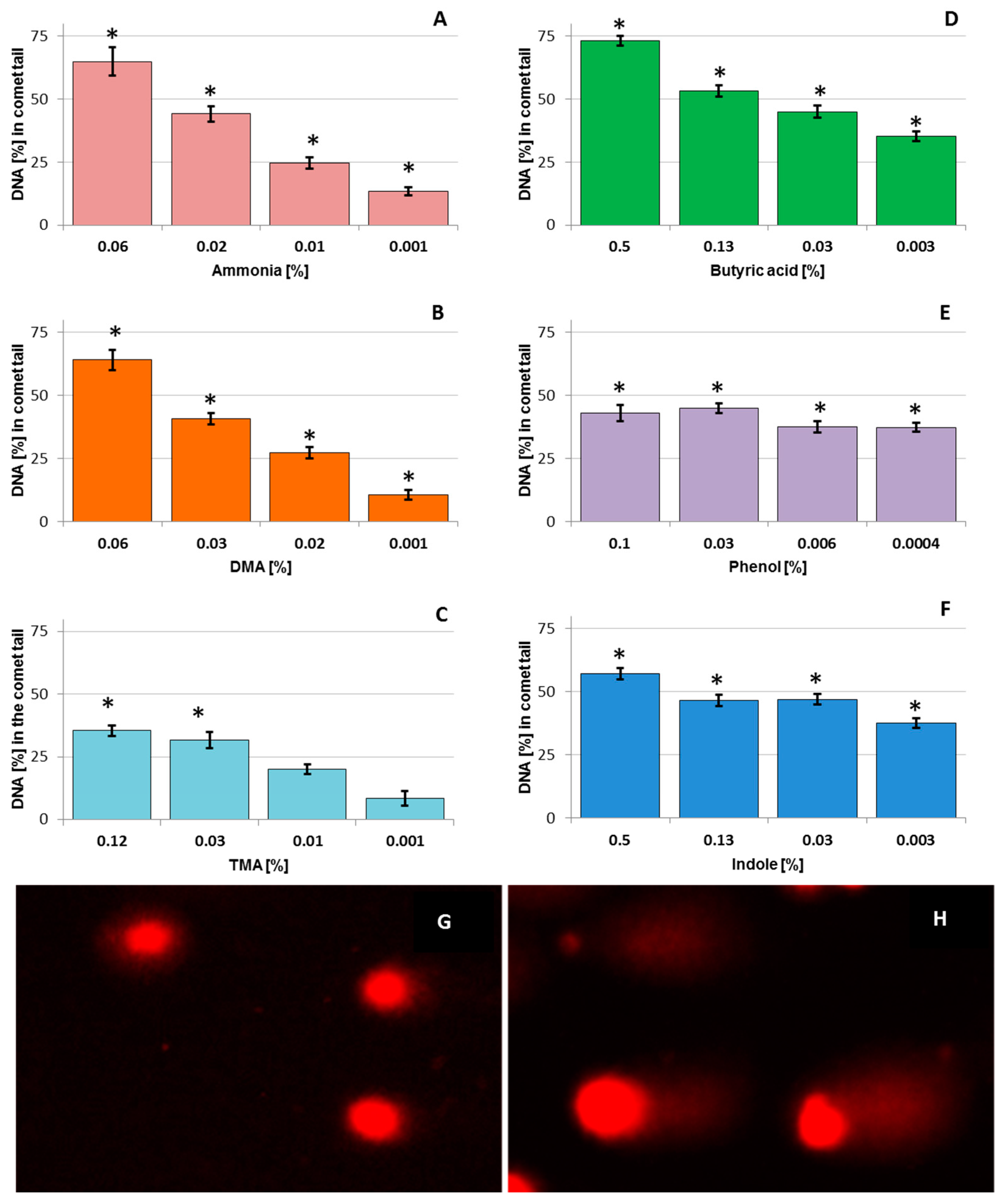
2.3. Comet Assay (SCGE—Single Cell Gel Electrophoresis Assay)
2.4. Lactate Dehydrogenase Activity (LDH) Assay
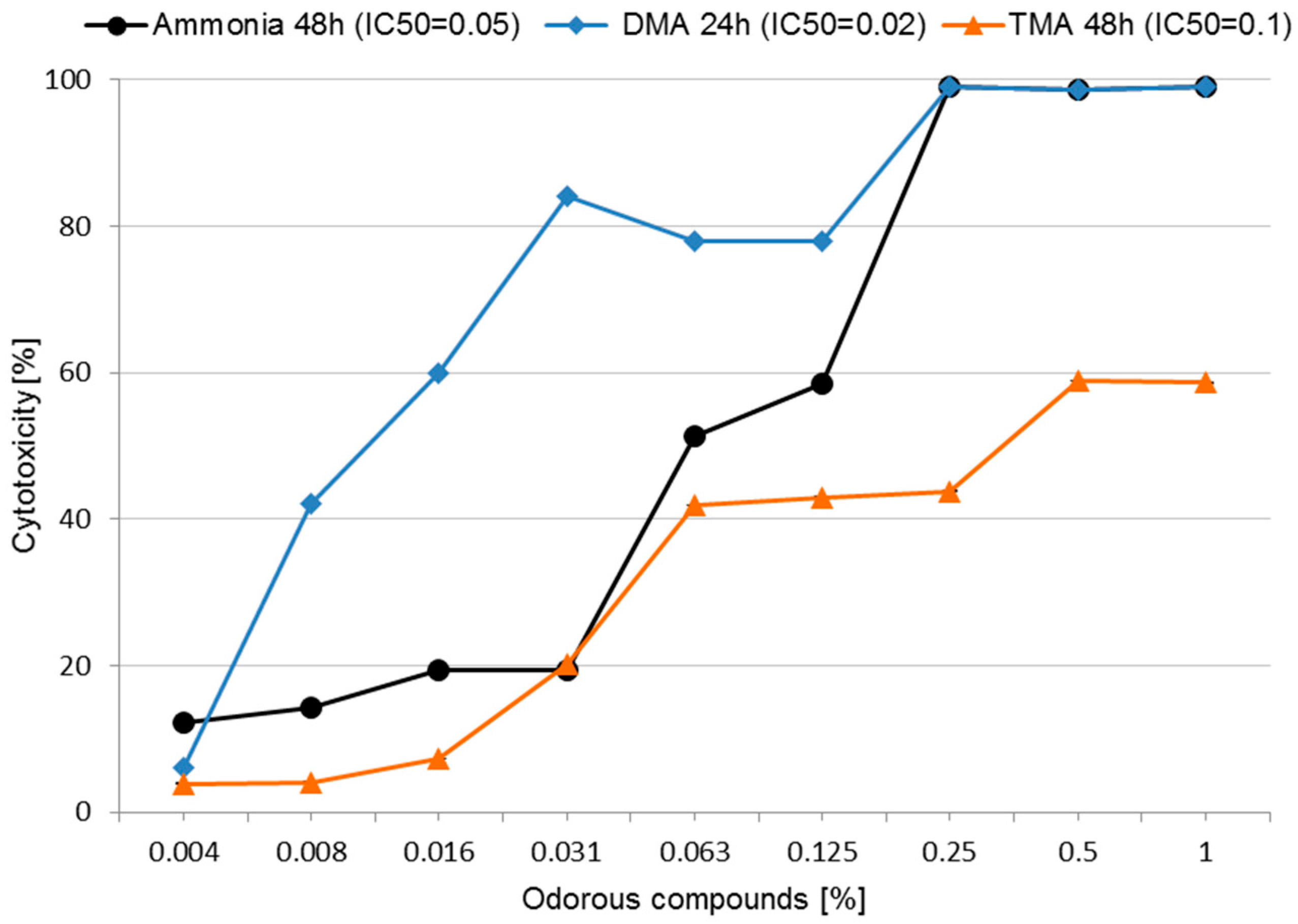
2.5. Calculation of IC50
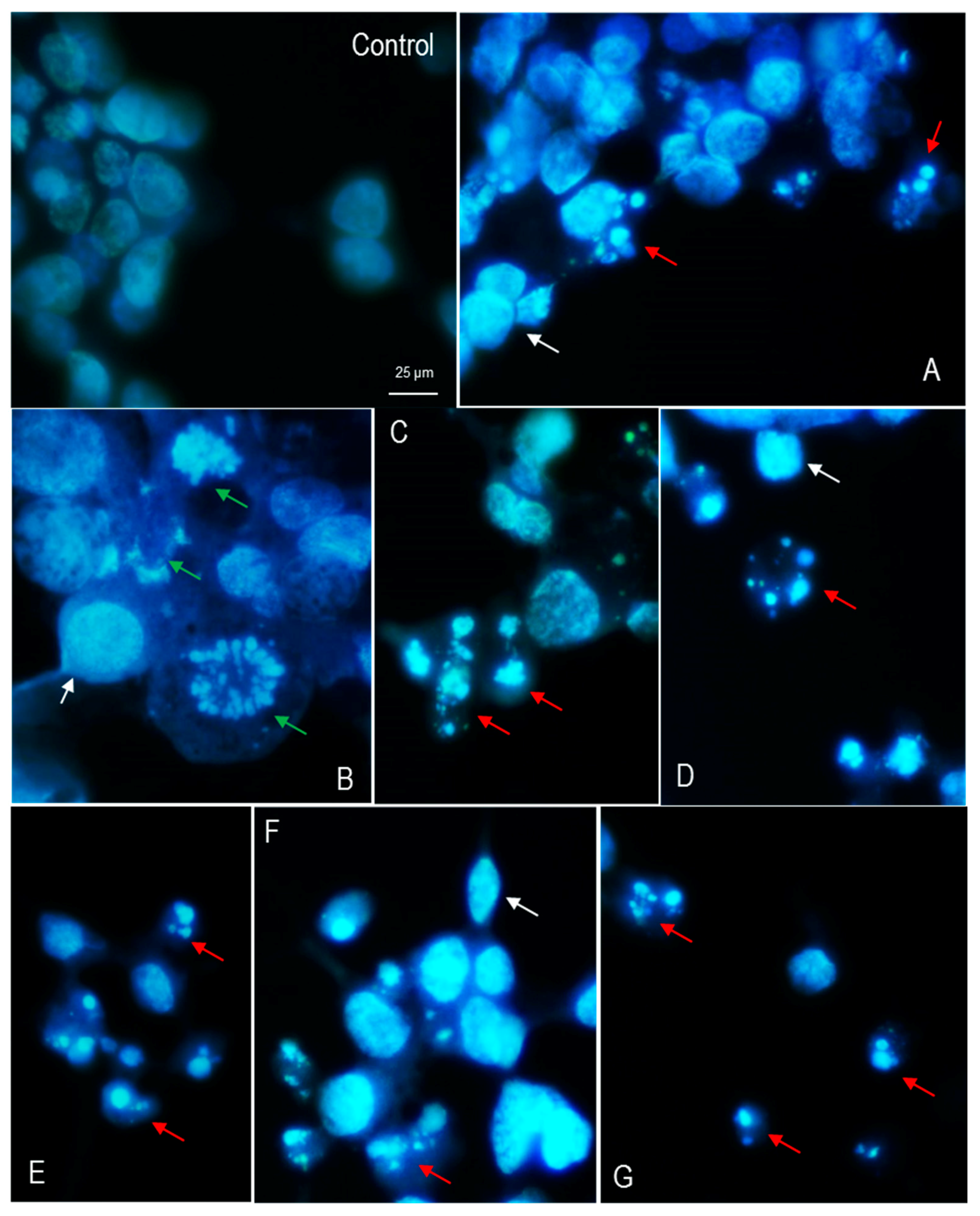
2.6. Fluorescence Microscopic Analysis
2.7. Statistical Analysis
3. Results
3.1. DNA Damage in Chicken Liver Hepatocellular Carcinoma Cells
3.2. Cytotoxicity and Determining IC50
3.3. Nuclear Morphology of LMH Cells
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jacobson, L.D.; Bicudo, J.R.; Schmidt, D.R.; Wood-Gay, S.; Gates, R.S.; Hoff, S.J. Air Emissions from Animal Production Buildings. Available online: http://www.isah-soc.org/userfiles/downloads/proceedings/2003/mainspeakers/18JacobsonUSA.pdf (accessed on 10 April 2017).
- Dunlop, M.W.; Blackall, P.J.; Stuetz, R.M. Odour emissions from poultry litter—A review litter properties, odour formation and odorant emissions from porous materials. J. Environ. Manag. 2016, 177, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xin, H.; Burns, R.T.; Jacobson, L.D.; Noll, S.; Hoff, S.J.; Harmon, J.D.; Koziel, J.A.; Hetchler, I. Air Emissions from Tom and Hen Turkey Houses in the U.S. Midwest. Available online: http://www.abe.iastate.edu/adl/files/2011/10/Turkey-Emissions1.pdf (accessed on 24 April 2017).
- Rabadheera, C.S.; Mcconchie, R.M.; Phan-Thien, K.; Bell, T. Strategies for eliminating chicken odour in horticultural applications. World Poult. Sci. J. 2017, 73, 365–378. [Google Scholar] [CrossRef]
- Gutarowska, B.; Borowski, S.; Durka, K.; Korczyński, M.; Kołacz, R. Screening of microorganisms capable to remove odorous compounds from poultry manure. Przem. Chem. 2009, 88, 440–445. [Google Scholar]
- Borowski, S.; Matusiak, K.; Powałowski, S.; Pielech-Przybylska, K.; Makowski, K.; Nowak, A.; Rosowski, M.; Komorowski, P.; Gutarowska, B. A novel microbial-mineral preparation for the removal of offensive odors from poultry manure. Int. Biodeter. Biodeg. 2017, 119, 299–308. [Google Scholar] [CrossRef]
- McGahan, E. Strategies to reduce odour emissions from meat chicken farms. Proc. Poult. Inf. Exch. 2002, 27–39. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.582.29&rep=rep1&type=pdf (accessed on 3 April 2017).
- Dinh, H.H.T. Analysis of Ammonia and Volatile Organic Amine Emissions in a Confined Poultry Facility. Available online: http://digitalcommons.usu.edu/etd/598/ (accessed on 3 April 2017).
- Gutarowska, B.; Matusiak, K.; Borowski, S.; Rajkowska, A.; Brycki, B. Removal of odorous compounds from poultry manure by microorganisms on perlite-bentonite carrier. J. Environ. Manag. 2014, 141, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Matusiak, K.; Borowski, S.; Bakuła, T.; Opaliński, S.; Kołacz, R.; Gutarowska, B. Cytotoxicity of odorous compounds from poultry manure. Int. J. Environ. Res. Public Health 2016, 13, 1046. [Google Scholar] [CrossRef] [PubMed]
- Błasiak, J.; Kowalik, J. A comparison of the in vitro genotoxicity of tri- and hexavalent chromium. Mutat. Res. 2000, 469, 135–145. [Google Scholar] [CrossRef]
- Nowak, A.; Śliżewska, K. β-Glucuronidase and β-glucosidase activity and human fecal water genotoxicity in the presence of probiotic lactobacilli and the heterocyclic aromatic amine IQ in vitro. Environ. Toxicol. Pharmacol. 2014, 37, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.; Moriwaki, K.; De Rosa, M.J. Detection of necrosis by release of lactate dehydrogenase (LDH) activity methods. Mol. Biol. 2013, 979, 65–70. [Google Scholar]
- OECD Guidelines for the Testing of Chemicals, Section 4. Test No. 442D: In Vitro Skin Sensitisation are-nrf2 Luciferase Test Method. Available online: https://ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecd-tg442d-508.pdf (accessed on 20 March 2017).
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miymae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Collins, A.R. The comet assay for DNA damage and repair. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Kaushik, V.K. Genotoxic effect of ammonia exposure on workers in a fertility factory. Indian J. Exp. Biol. 1997, 35, 487–4920. [Google Scholar] [PubMed]
- Mouillé, B.; Delpal, S.; Mayeur, C.; Blachier, F. Inhibition of human colon carcinoma cell growth by ammonia: A non-cytotoxic process associated with polyamine synthesis reduction. Biochim. Biophys. Acta 2003, 1624, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.L.; Madsen, I.J.; Bolton, R.P.; Graves, L.; Sistrunk, T. Ammonia/ammonium toxicity root symptoms induced by inorganic and organic fertilizers and placement. Agron. J. 2016, 108, 2485–2492. [Google Scholar] [CrossRef]
- Gupta, G.; Borowiec, J.; Okoh, J. Toxicity identification of poultry litter aqueous leachate. Poult. Sci. 1997, 76, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Galitskaya, P.; Selivanovskaya, S. Co-composting as a method to decrease toxicity of chicken manure. Int. J. Adv. Biotechnol. Res. 2016, 7, 1276–1282. [Google Scholar]
- Delgado, M.; Martin, J.V.; De Imperial, R.M.; León-Cófreces, C.; Cruz García, M. Phytotoxicity of uncomposted and composted poultry manure. Afr. J. Plant Sci. 2010, 4, 154–162. [Google Scholar]
- Gupta, G.; Kelly, P. Poultry litter toxicity comparison from various bioassays. J. Environ. Sci. Health. Part A Environ. Sci. Eng. Toxicol. 1992, 27, 1083–1093. [Google Scholar] [CrossRef]
- Slivac, I.; Blajić, V.; Radošević, K.; Kniewald, Z.; Gaurina Srček, V. Influence of different ammonium, lactate and glutamine concentrations on CCO cell growth. Cytotechnology 2010, 62, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Tae, K.-H.; Lee, G.M. Glutamine substitution: The role it can play to enhance therapeutic protein production. Pharm. Bioprocess. 2015, 3, 249–261. [Google Scholar]
- Mirabet, M.; Navarro, A.; Lopez, A.; Canela, E.I.; Mallol, J.; Lluis, C.; Franco, R. Ammonium toxicity in different cell lines. Biotechnol. Bioeng. 1997, 56, 530–537. [Google Scholar] [CrossRef]
- Galli, A.; Paolini, M.; Lattanzi, G.; Cantelli-Forti, G.; Bronzetti, G. Genotoxic and biochemical effects of dimethylamine. Mutagenesis 1993, 8, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Pool, B.L.; Brendler, S.Y.; Liegibel, U.M.; Tompa, A.; Schmezer, P. Employment of adult mammalian primary in toxicology: In vivo and in vitro genotoxic effects of environmentally significant N-nitrosodialkylamines in cells of the liver, lung, and kidney. Environ. Molec. Mutagen. 1990, 15, 24–35. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency, Acute Exposure Guideline Levels (AEGLs) for Dimethylamine. Available online: https://www.epa.gov/sites/production/files/2014-08/documents/dimethylamine_tsd_interim_version_106_2008.pdf (accessed on 4 April 2017).
- Mortelmans, K.; Haworth, S.; Lawlor, T.; Speck, W.; Tainer, B.; Zeiger, E. Salmonella mutagenicity tests. 2. Results from the testing of 270 chemicals. Environ. Mutagen. 1986, 8, 1–119. [Google Scholar] [CrossRef] [PubMed]
- MHLW (Japanese Ministry of Health, Labour and Welfare). Trimethylamine; MHLW: Tokyo, Japan, 2003.
- MHLW (Japanese Ministry of Health, Labour and Welfare). In Vitro Chromosomal Aberration Test of Trimethylamine on Cultured Chinese Hamster Cells; MHLW: Tokyo, Japan, 2003.
- Li, Y.; Qu, M.; Sun, L.; Wu, Y.; Chen, Y.; Chen, H.; Kong, Z.; Liu, Z. Genotoxicity study of phenol and o-cresol using the micronucleus test and the comet assay. Toxicol. Environ. Chem. 2005, 87, 365–372. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, J.; Zhang, Q.; Cao, X. Studies on the utilization of a plant SCE test in detecting potential mutagenic agents. Mutat. Res. 1991, 261, 69–73. [Google Scholar] [CrossRef]
- Kolachana, P.; Subrahmanyam, V.V.; Meyer, K.B.; Zhang, L.; Smith, M.T. Benzene and its phenolic metabolites produce oxidative damage in HL60 cells in vitro and in the bone marrow in vivo. Cancer Res. 1993, 53, 1023–1026. [Google Scholar] [PubMed]
- Morimoto, K.; Wolff, S.; Koizumi, A. Induction of sister-chromatid exchanges in human lymphocytes by microsomal activation of benzene metabolites. Mutat. Res. 1983, 119, 355–360. [Google Scholar] [CrossRef]
- Erexson, G.L.; Wilmer, J.L.; Kligerman, A.D. Sister chromatid exchanges induction in human lymphocytes exposed to benzene and its metabolites in vitro. Cancer Res. 1985, 45, 2471–2477. [Google Scholar] [PubMed]
- Ciranni, R.; Barale, R.; Marrazzini, A.; Loprieno, N. Benzene and the genotoxicity of its metabolites: I. Transplacental activity in mouse fetuses and in their dams. Mutat. Res. 1988, 208, 61–67. [Google Scholar] [CrossRef]
- Chen, H.; Eastmond, D.A. Synergistic increase in chromosomal breakage within the euchromatin induced by an interaction of the benzene metabolites phenol and hydroquinone on mice. Carcinogenesis 1995, 16, 1963–1969. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.V.; Storer, R.D.; Laws, G.M.; Armstrong, M.J.; Barnum, J.E.; Gara, J.P.; McKnight, C.G.; Skopek, T.R.; Sina, J.F.; DeLuca, J.G.; et al. Genotoxicity of naturally occurring indole compounds: Correlation between covalent DNA binding and other genotoxicity tests. Environ. Mol. Mutagen. 2002, 40, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Ochiai, T.; Hashizume, T.; Yonezawa, H.; Ochiai, K.; Yamamoto, M. Characterization of the effects of butyric acid on cell proliferation, cell cycle distribution and apoptosis. FEMS Immunol. Med. Microbiol. 2006, 47, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Watkins, S.M.; Carter, L.C.; Mak, J.; Tsau, J.; Yamamoto, S.; German, J.B. Butyric acid and tributyrin induce apoptosis in human hepatic tumour cells. J. Dairy Res. 1999, 66, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Ochiai, T.; Ochiai, K.; Fukushima, K. Butyric Acid-induced T-cell apoptosis is mediated by caspase-8 and -9 activation in a fas-independent manner. Clin. Diagn. Lab. Immunol. 2001, 8, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Kurita-Ochiai, T.; Seto, S.; Suzuki, N.; Yamamoto, M.; Otsuka, K.; Abe, K.; Ochiai, K. Butyric acid induces apoptosis in inflamed fibroblasts. J. Dent. Res. 2008, 87, 51–55. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Bakuła, T.; Matusiak, K.; Gałęcki, R.; Borowski, S.; Gutarowska, B. Odorous Compounds from Poultry Manure Induce DNA Damage, Nuclear Changes, and Decrease Cell Membrane Integrity in Chicken Liver Hepatocellular Carcinoma Cells. Int. J. Environ. Res. Public Health 2017, 14, 933. https://doi.org/10.3390/ijerph14080933
Nowak A, Bakuła T, Matusiak K, Gałęcki R, Borowski S, Gutarowska B. Odorous Compounds from Poultry Manure Induce DNA Damage, Nuclear Changes, and Decrease Cell Membrane Integrity in Chicken Liver Hepatocellular Carcinoma Cells. International Journal of Environmental Research and Public Health. 2017; 14(8):933. https://doi.org/10.3390/ijerph14080933
Chicago/Turabian StyleNowak, Adriana, Tadeusz Bakuła, Katarzyna Matusiak, Remigiusz Gałęcki, Sebastian Borowski, and Beata Gutarowska. 2017. "Odorous Compounds from Poultry Manure Induce DNA Damage, Nuclear Changes, and Decrease Cell Membrane Integrity in Chicken Liver Hepatocellular Carcinoma Cells" International Journal of Environmental Research and Public Health 14, no. 8: 933. https://doi.org/10.3390/ijerph14080933





