The Adsorption of Cd(II) on Manganese Oxide Investigated by Batch and Modeling Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Batch Adsorption
2.3. Characterization
2.4. Surface Complexation Modeling
3. Results and Discussion
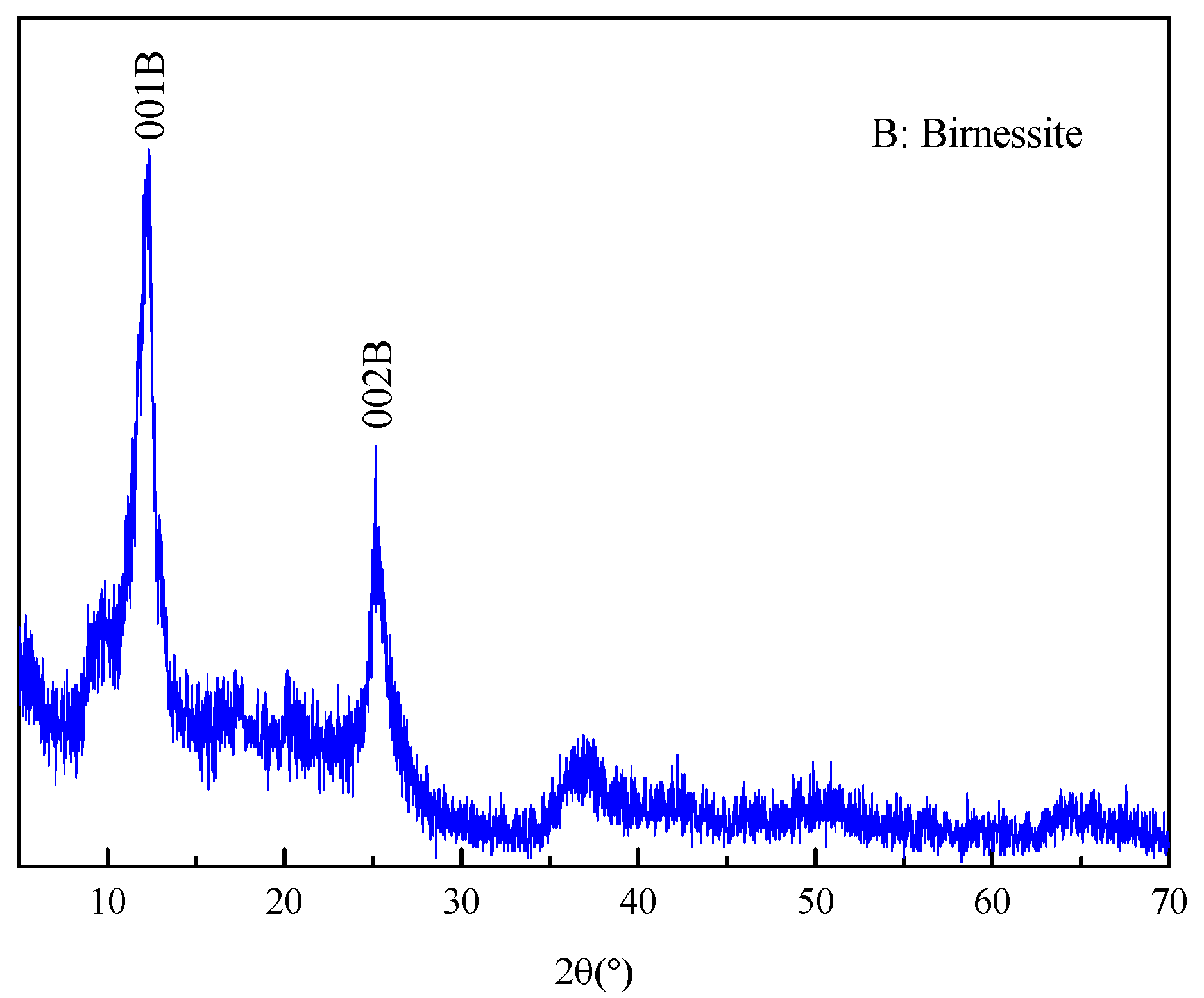
3.1. Characterization
3.2. Adsorption Kinetics
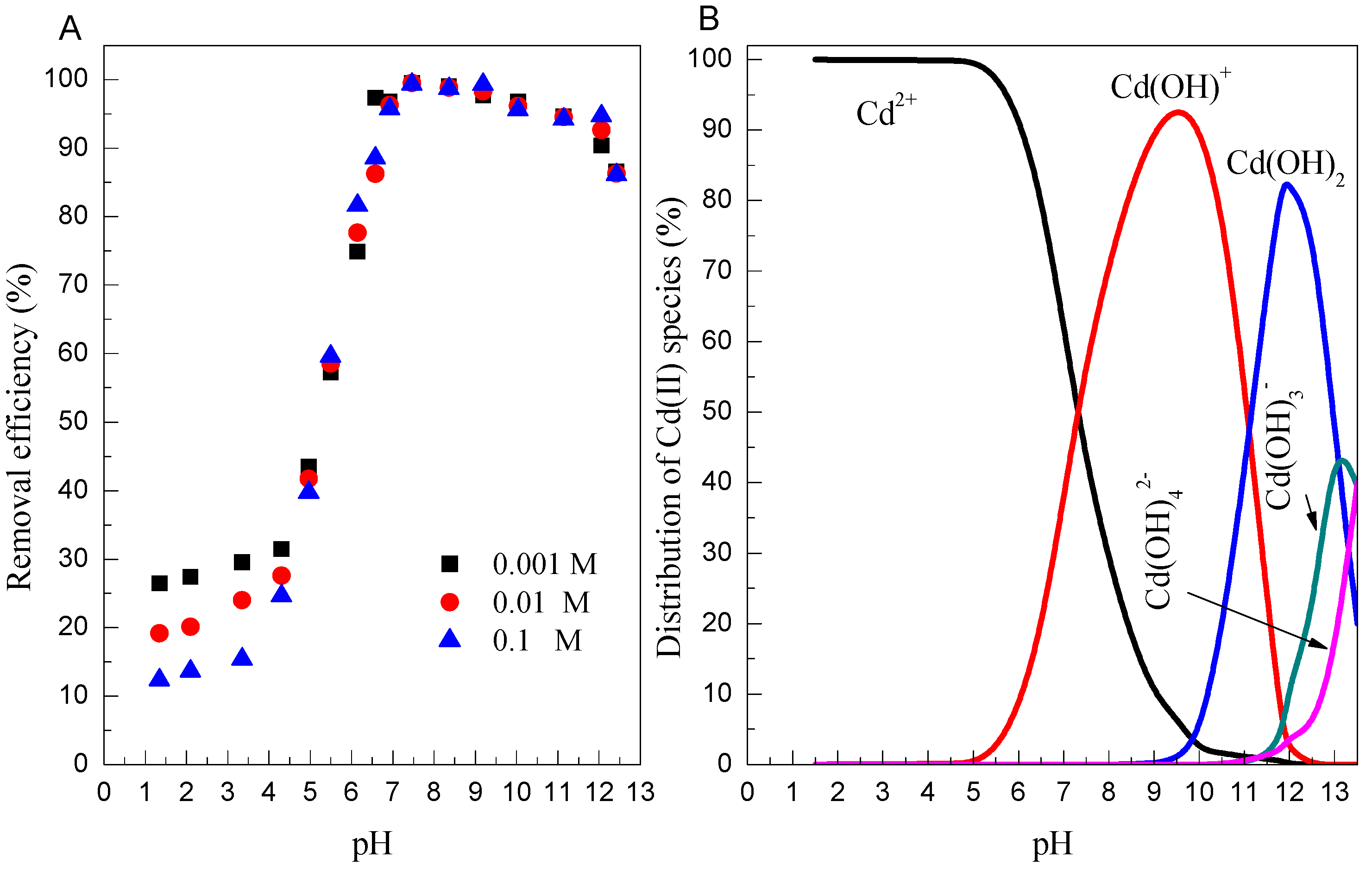
3.3. Effect of pH and Ionic Strength
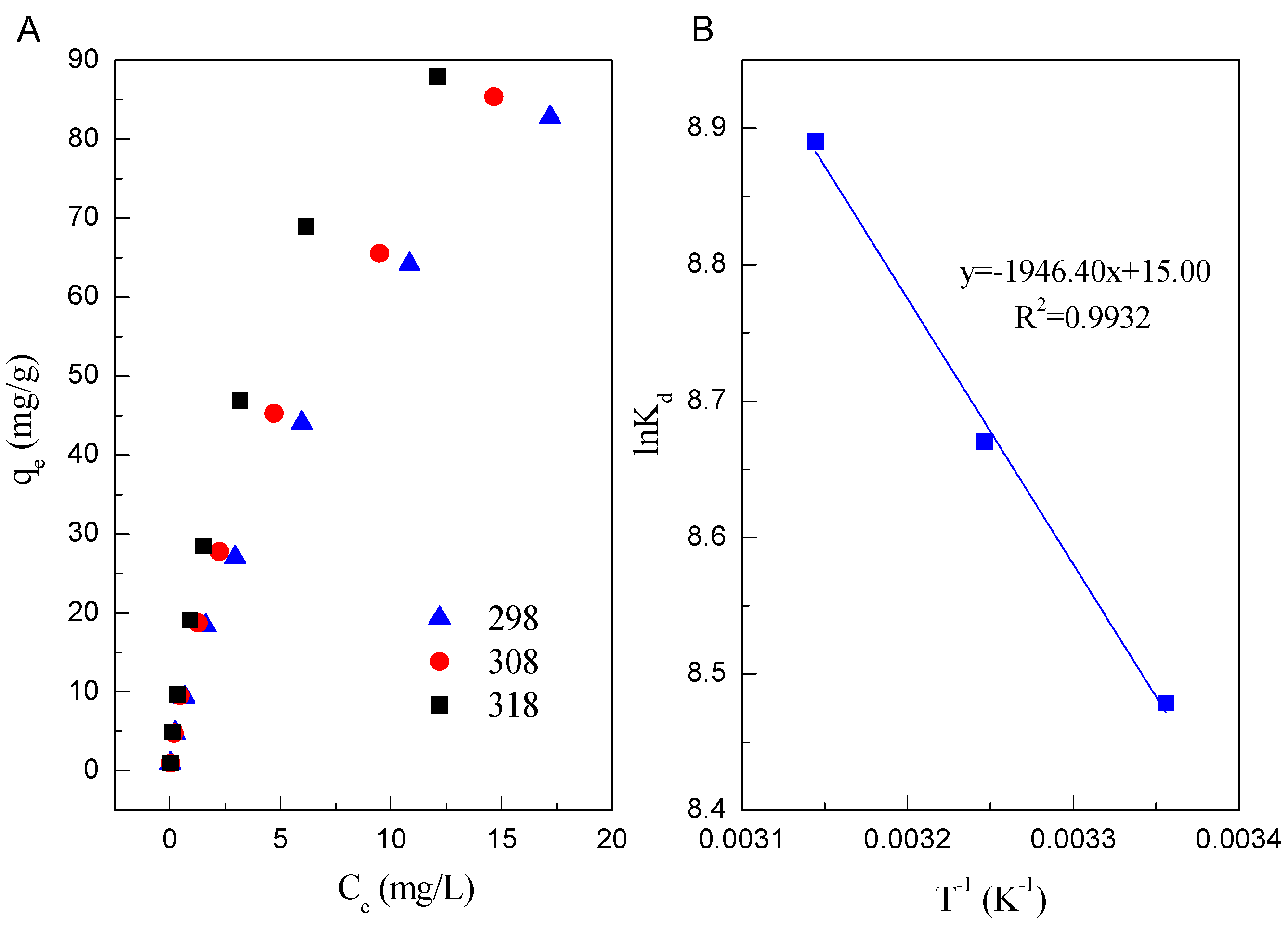
3.4. Adsorption Isotherms
3.5. Thermodynamic Parameters
3.6. Effect of Coexisting Cations
3.7. Surface Complexation Modeling
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giller, K.E.; Witter, E.; Mcgrath, S.P. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soils: A review. Soil Biol. Biochem. 1998, 30, 1389–1414. [Google Scholar] [CrossRef]
- Cheng, Q.M.; Huang, Q.; Khan, S.; Liu, Y.J.; Liao, Z.N.; Li, G.; Ok, Y.S. Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 2016, 87, 240–245. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullah, E.C.; Jayakumar, N.S.; Ganesan, P. Microwave assisted multiwall carbon nanotubes enhancing Cd(II) adsorption capacity in aqueous media. J. Ind. Eng. Chem. 2015, 24, 24–33. [Google Scholar] [CrossRef]
- Wang, F.Y.; Wang, H.; Ma, J.W. Adsorption of cadmium (II) ions from aqueous solution by a new low-cost adsorbent—Bamboo charcoal. J. Hazard. Mater. 2010, 177, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.C. Effects of operating parameters on the removal performance of electrodialysis for treating wastewater containing cadmium. Desalination Water Treat. 2012, 35, 150–157. [Google Scholar] [CrossRef]
- Sun, Y.B.; Yang, S.B.; Chen, Y.; Ding, C.C.; Cheng, W.C.; Wang, X.K. Adsorption and desorption of U(VI) on functionalized graphene oxides: A combined experimental and theoretical study. Environ. Sci. Technol. 2015, 49, 4255–4262. [Google Scholar] [CrossRef] [PubMed]
- Sari, A.; Tuzen, M. Cd(II) adsorption from aqueous solution by raw and modified kaolinite. Appl. Clay Sci. 2014, 88–89, 63–72. [Google Scholar] [CrossRef]
- Ma, M.H.; Gao, H.Y.; Sun, Y.B.; Huang, M.S. The adsorption and desorption of Ni(II) on Al substituted goethite. J. Mol. Liq. 2015, 201, 30–35. [Google Scholar] [CrossRef]
- Sun, Y.B.; Wang, Q.; Chen, C.L.; Tan, X.L.; Wang, X.K. Interaction between Eu(III) and graphene oxide nanosheets investigated by batch and extended X-ray absorption fine structure spectroscopy and by modeling techniques. Environ. Sci. Technol. 2012, 46, 6020–6027. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.M.; Pan, M. The highly efficient adsorption of Pb(II) on graphene oxides: A process combined by batch experiments and modeling techniques. J. Mol. Liq. 2016, 215, 410–416. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Effects of Humic Acid and Suspended Solids on the Removal of Heavy Metals from Water by Adsorption onto Granular Activated Carbon. Int. J. Environ. Res. Public Health 2015, 12, 10475–10489. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, Z.G.; Zeng, G.M.; Huang, B.B.; Dong, H.R.; Huang, J.H.; Yang, Z.Z.; Wei, J.J.; Hu, L.; Zhang, Q. Phase transformation of crystalline iron oxides and their adsorption abilities for Pb and Cd. Chem. Eng. J. 2016, 284, 247–259. [Google Scholar] [CrossRef]
- Lee, S.M.; Laldawngliana, C.; Tiwari, D. Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem. Eng. J. 2012, 195, 103–111. [Google Scholar] [CrossRef]
- Tani, Y.; Miyata, N.; Ohashi, M.; Ohnuki, T.; Seyama, H.; Iwahori, K.; Soma, M. Interaction of inorganic arsenic with biogenic manganese oxide produced by a Mn-oxidizing fungus, strain KR21–2. Environ. Sci. Technol. 2004, 38, 6618–6624. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, B.J.; Ginder-Vogel, M.; Sparks, D.L. Arsenite oxidation by a poorly-crystalline manganese oxide. 3. Arsenic and manganese desorption. Environ. Sci. Technol. 2011, 45, 9218–9223. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, Y.L.; Gu, J.D. Oxidation of As(III) by MnO2 in the absence and presence of Fe(II) under acidic conditions. Geochim. Cosmochim. Acta 2011, 75, 368–379. [Google Scholar] [CrossRef]
- Villalobos, M.; Escobar-Quiroz, I.N.; Salazar-Camacho, C. The influence of particle size and structure on the sorption and oxidation behavior of birnessite: I. Adsorption of As(V) and oxidation of As(III). Geochim. Cosmochim. Acta 2014, 125, 564–581. [Google Scholar] [CrossRef]
- Kennedy, C.; Smith, D.S.; Warren, L.A. Surface chemistry and relative Ni sorptive capacities of synthetic hydrous Mnoxyhydroxides under variable wetting and drying regimes. Geochim. Cosmochim. Acta 2004, 68, 443–454. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Sorption of Ni by birnessite: Equilibrium controls on Ni in seawater. Chem. Geol. 2007, 238, 94–106. [Google Scholar] [CrossRef]
- Villalobos, M.; Bargar, J.; Sposito, G. Mechanisms of Pb(II) sorption on a biogenic manganese oxide. Environ. Sci. Technol. 2005, 39, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Tan, W.F.; Zheng, L.R.; Cui, H.J.; Qiu, F.H.; Liu, F.; Feng, X.H. Characterization of Ni-rich hexagonal birnessite and its geochemical effects on aqueous Pb2+/Zn2+ and As(III). Geochim. Cosmochim. Acta 2012, 93, 47–62. [Google Scholar] [CrossRef]
- Beak, D.G.; Basta, N.T.; Scheckel, K.G.; Traina, S.J. Linking solid phase speciation of Pb sequestered to birnessite to oral Pbbioaccessibility: Implications for soil remediation. Environ. Sci. Technol. 2008, 42, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sreekanth, P.M.; Smirniotis, P.G.; Thiel, S.W.; Pinto, N.G. Manganese oxide/titania materials for removal of NOx and elemental mercury from flue gas. Energy Fuels 2008, 22, 2299–2306. [Google Scholar]
- Liang, P.; Li, Y.C.; Zhang, C.; Wu, S.C.; Cui, H.J.; Yu, S.; Wong, M.H. Effects of salinity and humic acid on the sorption of Hg on Fe and Mn hydroxides. J. Hazard. Mater. 2013, 244–245, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, T.K.; Wan, J.M.; Lanzirotti, A.; Sutton, S.R.; Newville, M.; Rao, W. Long-term stability of organic carbon-stimulated chromate reduction in contaminated soils and its relation to manganese redox status. Environ. Sci. Technol. 2007, 41, 4326–4331. [Google Scholar] [CrossRef] [PubMed]
- Landrot, G.; Ginder-Vogel, M.; Sparks, D.L. Kinetics of chromium(III) oxidation by manganese(IV) oxides using quick scanning X-ray absorption fine structure spectroscopy (Q-XAFS). Environ. Sci. Technol. 2010, 44, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Landrot, G.; Ginder-Vogel, M.; Livi, K.; Fitts, J.P.; Sparks, D.L. Chromium(III) oxidation by three poorly crystalline manganese(IV) oxides. 2. Solid phase analyses. Environ. Sci. Technol. 2012, 46, 11601–11609. [Google Scholar] [CrossRef] [PubMed]
- Suib, S.L. Porous manganese oxide octahedral molecular sieves and octahedral layered materials. Acc. Chem. Res. 2008, 41, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.A.; Manceau, A.; Kersten, M. Mn, Fe, Zn and as speciation in a fast-growing ferromanganese marine nodule. Geochim. Cosmochim. Acta 2004, 68, 3125–3136. [Google Scholar] [CrossRef]
- Dong, D.M.; Hua, X.Y.; Li, Y.; Zhang, J.J.; Yan, D.X. Cd adsorption properties of components in different freshwater surface coatings: The important role of ferromanganese oxides. Environ. Sci. Technol. 2003, 37, 4106–4112. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Le Roux, S.M.; Millward, G.E. Adsorption of cadmium to iron and manganese oxides during estuarine mixing. Mar. Chem. 2008, 108, 77–84. [Google Scholar] [CrossRef]
- Meng, Y.T.; Zheng, Y.M.; Zhang, L.M.; He, J.Z. Biogenic Mn oxides for effective adsorption of Cd from aquatic environment. Environ. Pollut. 2009, 157, 2577–2583. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.L.; Zhao, X.; Lv, L.; Su, Q.; Gu, H.N.; Pan, B.C.; Zhang, W.M.; Lin, Z.W.; Luan, J.F. Selective adsorption of Cd(II) and Zn(II) ions by nano-hydrous manganese dioxide (HMO)-encapsulated cation exchanger. Ind. Eng. Chem. Res. 2010, 49, 7574–7579. [Google Scholar] [CrossRef]
- Luo, J.; Huang, A.M.; Park, S.H.; Suib, S.L.; O’Young, C.L. Crystallization of sodium-birnessite and accompanied phase transformation. Chem. Mater. 1998, 10, 1561–1568. [Google Scholar] [CrossRef]
- Liu, R.P.; Liu, F.; Hu, C.Z.; He, Z.; Liu, H.J.; Qu, J.J. Simultaneous removal of Cd(II) and Sb(V) by Fe–Mn binary oxide: Positive effects of Cd(II) on Sb(V) adsorption. J. Hazard. Mater. 2015, 300, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Pan, B.C.; Wan, S.L.; Zhang, W.M.; Lv, L. Use of hydrous manganese dioxide as a potential sorbent for selective removal of lead, cadmium, and zinc ions from water. J. Colloid Interface Sci. 2010, 349, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Berber-Mendoza, M.S.; Leyva-Ramos, R.; Alonso-davila, P.; Mendoza-Barron, J.; Diaz-Flores, P.E. Effect of pH and temperature on the ion-exchange isotherm of Cd(II) on Pb(II) on clinoptilolite. J. Chem. Technol. Biotechnol. 2006, 81, 966–973. [Google Scholar] [CrossRef]
- Feng, X.H. Syntheses, Transformations, and Surface Chemistry Characteristics of the Several Common Manganese Oxide Minerals. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2003. [Google Scholar]
- Kim, E.J.; Lee, C.S.; Chang, Y.Y.; Chang, Y.S. Hierarchically structured manganese oxide-coated magnetic nanocomposites for the efficient removal of heavy metal ions from aqueous systems. ACS Appl. Mater. Interfaces 2013, 5, 9628–9634. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Wang, X.K. Influence of pH, soil humicfulvic acid, ionic strength and foreign ions on sorption of thorium(IV) onto γ-Al2O3. Appl. Geochem. 2007, 22, 436–445. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Pan, M.; Lin, X.M.; Xie, J.J.; Huang, X.M. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption on aluminum hydroxide modified palygorskitenano-composites. RSC Adv. 2017, 7, 4492–4500. [Google Scholar] [CrossRef]
- Peng, L.; Zeng, Q.R.; Tie, B.Q.; Lei, M.; Yang, Y.; Luo, S.; Song, Z.G. Manganese Dioxide nanosheet suspension: A novel absorbent for Cadmium(II) contamination in waterbody. J. Colloid Interface Sci. 2015, 456, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.D.; Wang, Q.Q.; Fu, D.F.; Ma, J. An efficient approach for Pb(II) and Cd(II) removal using manganese dioxide formed in situ. Chem. Eng. J. 2011, 172, 68–74. [Google Scholar] [CrossRef]
- Westall, J.C.; Zachary, J.L.; Morel, F. MINEQL: A Computer Program for the Calculation of Chemical Equilibrium Composition of Aqueous Systems; Technical Note 18; Department of Civil Engineering, Massachusetts Institute of Technology: Cambridge, MA, USA, 1976. [Google Scholar]


| Metal | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (g/(mg·min)) | R2 | qe (mg/g) | k2 (g/(mg·min)) | R2 | |
| Cd(II) | 80.2 | 0.0205 | 0.9941 | 104.2 | 0.00032 | 0.9991 |
| T (K) | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| qm (mg/g) | k (L/mg) | R2 | Kf (mg/g) | 1/n | R2 | |
| 298 K | 104.17 | 0.1627 | 0.8889 | 12.95 | 0.665 | 0.9994 |
| 308 K | 107.53 | 0.1958 | 0.9446 | 14.44 | 0.714 | 0.9930 |
| 318 K | 109.89 | 0.2826 | 0.9610 | 19.28 | 0.702 | 0.9898 |
| T (K) | ΔG0 (kJ/mol) | ΔS0 (kJ/mol/K) | ΔH0 (kJ/mol) |
|---|---|---|---|
| 298 | −21.006 | ||
| 308 | −22.202 | 0.125 | 16.182 |
| 318 | −23.503 |
| Heavy Metal | Coexisting Cations | Adsorption Capacity at Different Concentrations of Coexisting Cations (mg/g) | ||
|---|---|---|---|---|
| 0 mmol/L | 1 mmol/L | 10 mmol/L | ||
| Cd(II) | Ca2+ | 82.79 | 79.56 | 81.22 |
| Mg2+ | 83.13 | 81.37 | ||
| Equations | Log K |
|---|---|
| Protonation and deprotonation | |
| SOH + H+ = SOH2+ | 5.21 |
| SOH = SO− + H+ | −8.94 |
| Surface complexation modeling | |
| 2XH + Cd2+ = X2Cd + 2H+ | 4.64 |
| SOH + Cd2+ = SOCd+ + H+ | 5.72 |
| 2SOH + Cd2+ + H2O = (SO)2CdOH− + 3H+ | −10.63 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Chen, T.; Zou, X.; Zhu, M.; Chen, D.; Pan, M. The Adsorption of Cd(II) on Manganese Oxide Investigated by Batch and Modeling Techniques. Int. J. Environ. Res. Public Health 2017, 14, 1145. https://doi.org/10.3390/ijerph14101145
Huang X, Chen T, Zou X, Zhu M, Chen D, Pan M. The Adsorption of Cd(II) on Manganese Oxide Investigated by Batch and Modeling Techniques. International Journal of Environmental Research and Public Health. 2017; 14(10):1145. https://doi.org/10.3390/ijerph14101145
Chicago/Turabian StyleHuang, Xiaoming, Tianhu Chen, Xuehua Zou, Mulan Zhu, Dong Chen, and Min Pan. 2017. "The Adsorption of Cd(II) on Manganese Oxide Investigated by Batch and Modeling Techniques" International Journal of Environmental Research and Public Health 14, no. 10: 1145. https://doi.org/10.3390/ijerph14101145





