Serum Concentrations of Selected Heavy Metals in Patients with Alcoholic Liver Cirrhosis from the Lublin Region in Eastern Poland
Abstract
:1. Introduction
2. Experimental Section
2.1. Patients
2.2. Blood Samples
2.3. Instrumentation and Reagents
2.4. Statistical Analysis
3. Results
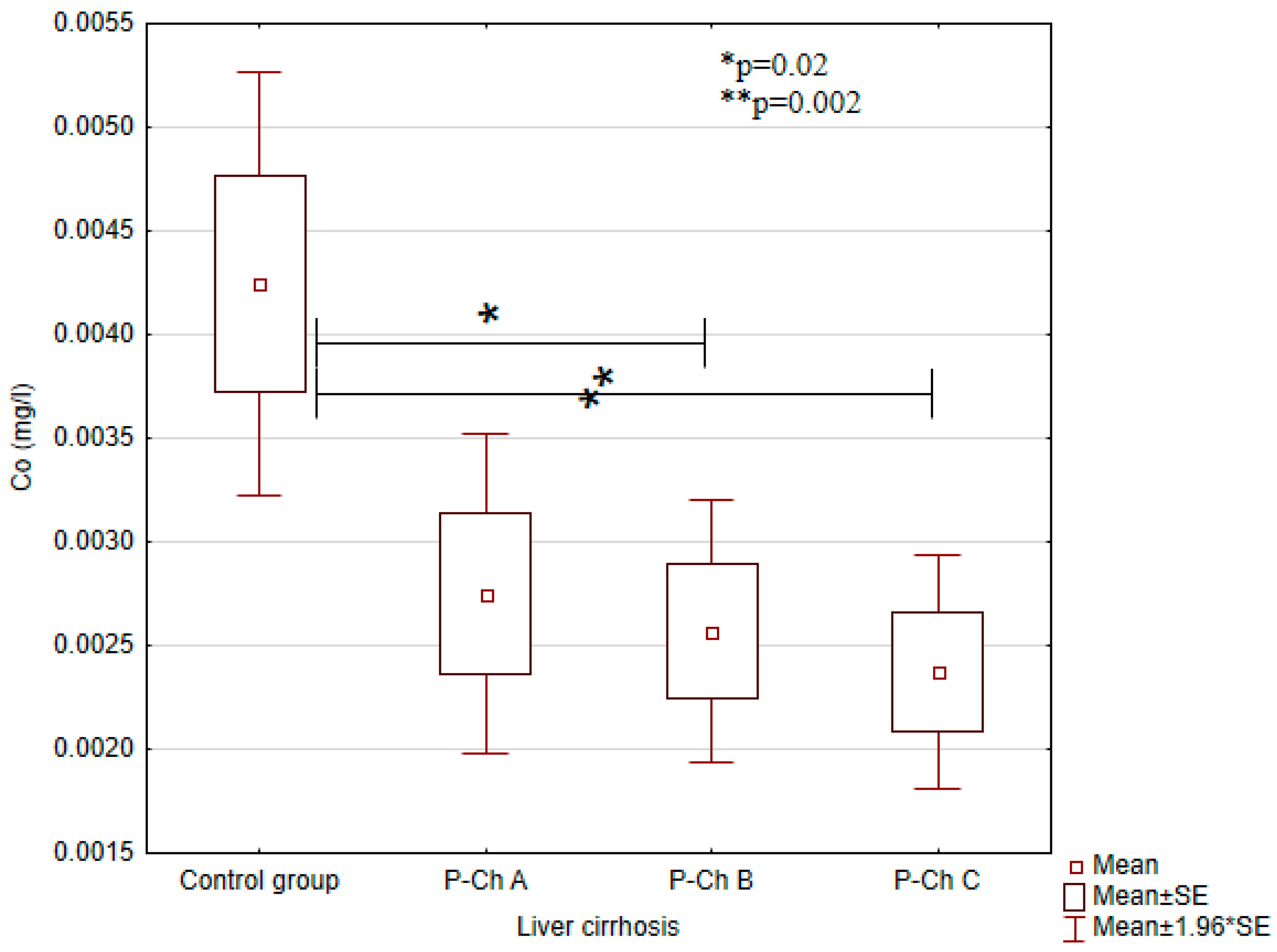
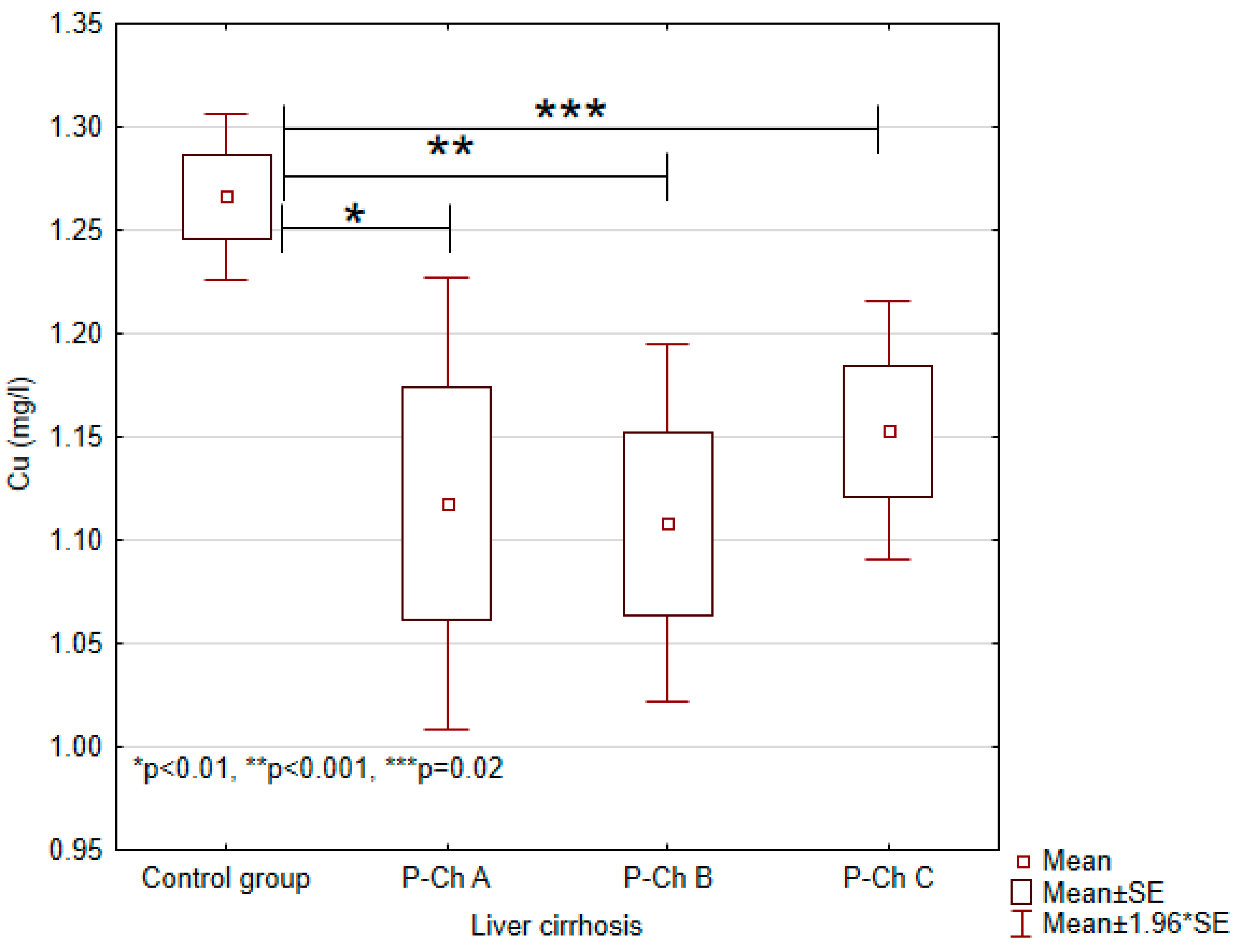
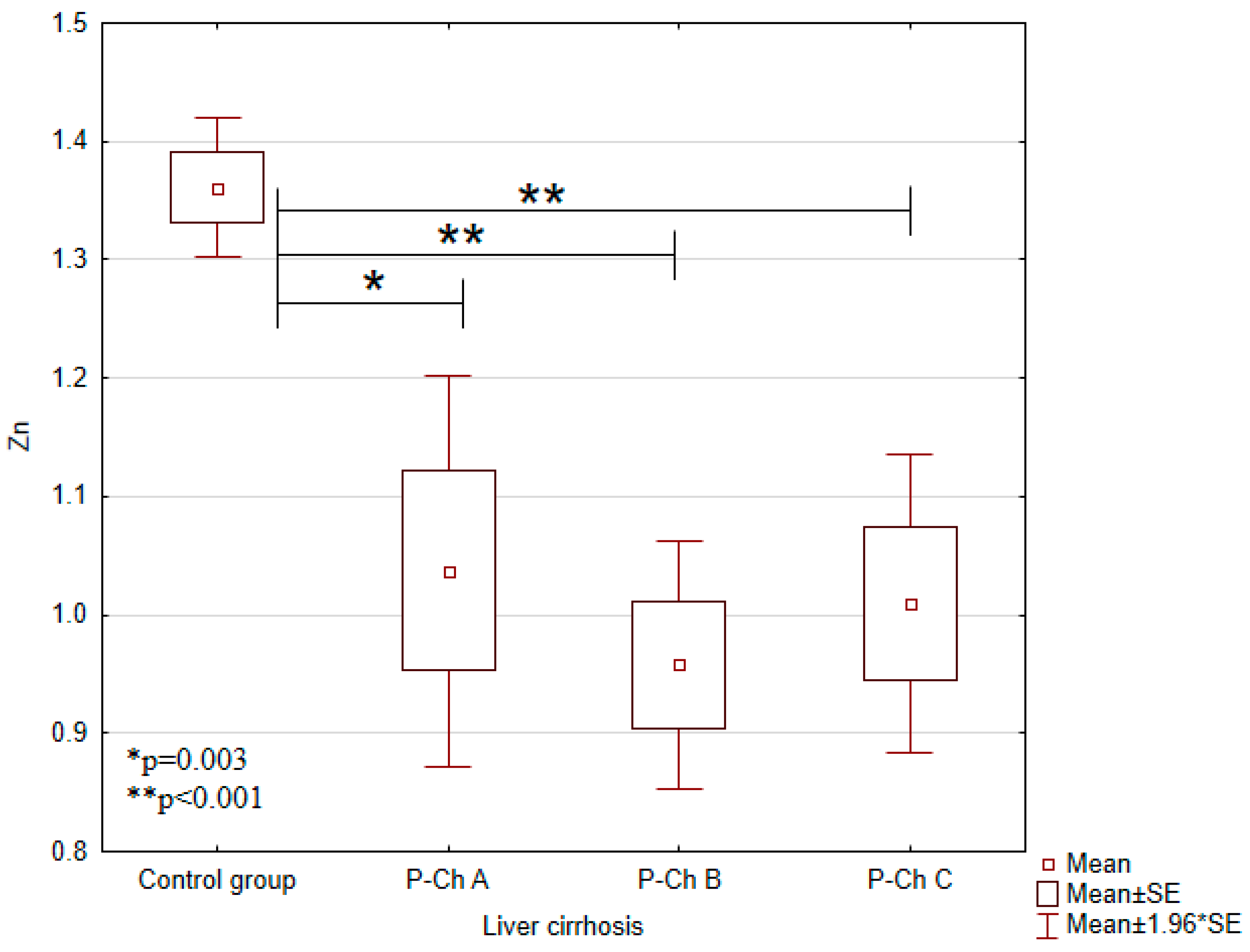
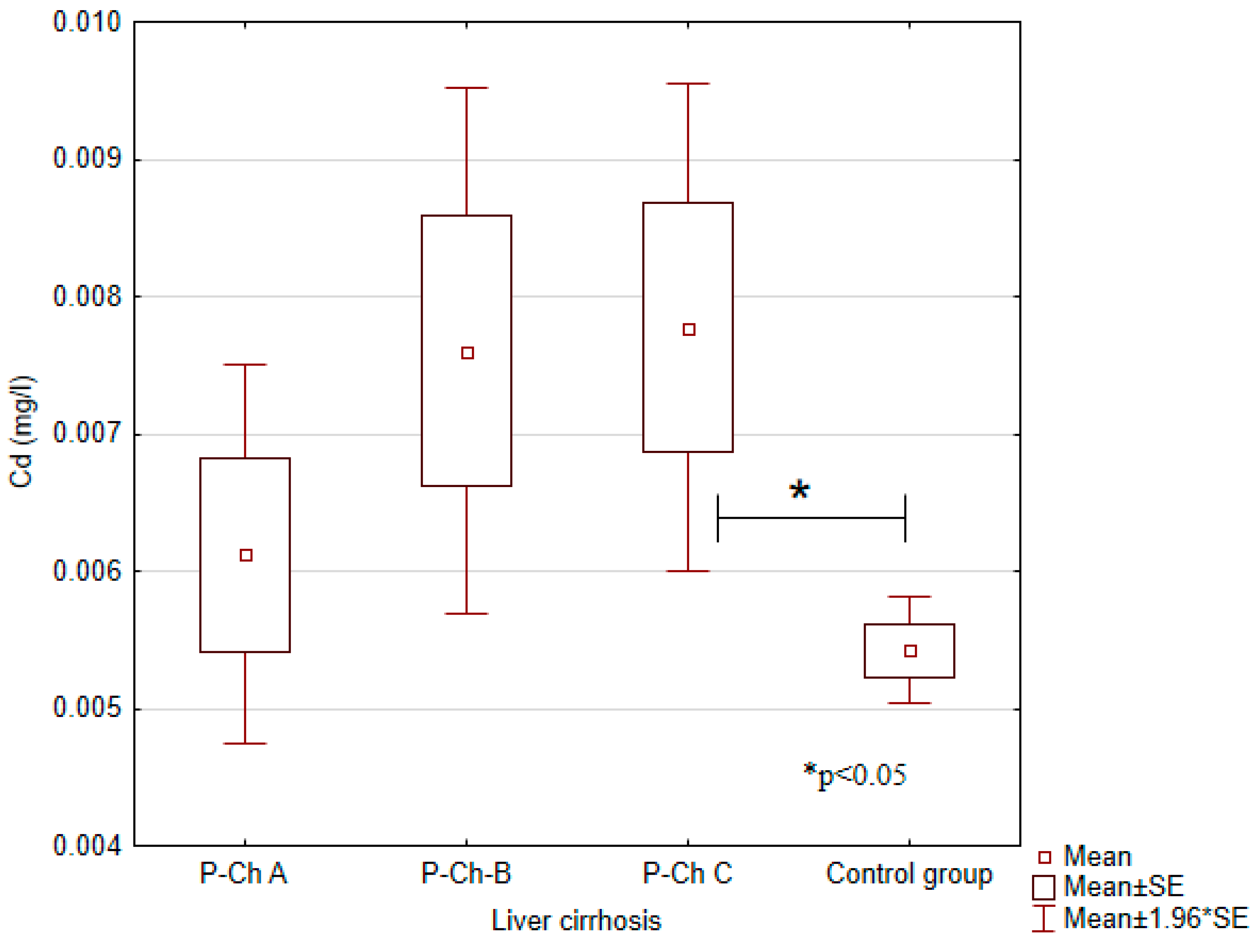
3.1. Concentrations of Heavy Metals in Patients with Alcoholic Liver Cirrhosis Compared to the Control Group
3.2. Correlations between Heavy Metal Concentrations and Age, Gender, and Duration of Alcohol Abuse
3.3. Correlations between Concentrations of Heavy Metals and Laboratory Results
3.4. Concentrations of Heavy Metals according to the Presence of Ascitis, Encephalopathy, and Esophageal Varices
3.5. Correlations among Heavy Metal Concentrations
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Health Risks: Mortality and Burden of Disease Attributable to Selected Major Risks; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz, A.; Orlicz-Szczęsna, G.; Prystupa, A.; Szczęsny, P. Use of ion chromatography for the determination of selected metals in blood serum of patients with type 2 diabetes. J. Trace Elem. Med. Biol. 2010, 24, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Błażewicz, A.; Dolliver, W.; Sivsammye, S.; Deol, A.; Randhawa, R.; Orlicz-Szczęsna, G.; Błażewicz, R. Determination of cadmium, cobalt, copper, iron, manganese and zinc in thyroid glands of patients with diagnosed nodular goitre using ion chromatography. J. Chromatogr. B 2010, 878, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Madden, E.; Fowler, B. Mechanisms of nephrotoxicity from metal combinations: A review. Drug Chem. Toxicol. 2000, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Czeczot, H.; Skrzycki, M. Cadmium—Element completely unnecessary for the organism. Postepy Hig. Med. Dośw. 2010, 64, 38–49. [Google Scholar]
- Kang, M.-Y.; Cho, S.-H.; Lim, Y.-H.; Seo, J.-C.; Hong, Y.-C. Effects of environmental cadmium exposure on liver function in adults. Occup. Environ. Med. 2013, 70, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Türkcan, A.; Scharinger, B.; Grabmann, G.; Keppler, B.; Laufer, G.; Bernhard, D.; Messner, B. Combination of cadmium and high cholesterol levels as a risk factor for heart fibrosis. Toxicol. Sci. 2015, 145, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and cellular mechanisms of cadmium carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Kazi, T.; Kolachi, N.F.; Afridi, H.I.; Kazi, N.G.; Sirajuddin; Naeemullah; Arain, S.S. Effects of mineral supplementation on liver cirrhotic/cancer male patients. Biol. Trace Elem. Res. 2012, 150, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Kolachi, N.F.; Kazi, T.G.; Afridi, H.I.; Kazi, N.G.; Khan, S. Investigation of essential trace and toxic elements in biological samples (blood, serum and scalp hair) of liver cirrhotic/cancer female patients before and after mineral supplementation. Clin. Nutr. 2012, 31, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Czarnek, K.; Terpiłowska, S.; Siwicki, A.-K. Selected aspects of the action of cobalt ions in the human body. Cent. Eur. J. Immunol. 2015, 40, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hunt, A.H.; Parr, R.M.; Taylor, D.M.; Trott, N.-G. Relation between cirrhosis and trace metal content of liver. Brit. Med. J. 1963, 14, 1498–1499. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Sawa, A.; Okita, K. The role of copper and zinc as pathogenic factors in liver disease. In Trace Elements in Clinical Medicine; Tomita, H., Ed.; Springer: Tokyo, Japan, 1990; pp. 89–92. [Google Scholar]
- Rahelić, D.; Kujundzić, M.; Romić, Z.; Brkić, K.; Petrovecki, M. Serum concentration of zinc, copper, manganese and magnesium in patients with liver cirrhosis. Coll. Antropol. 2006, 30, 523–528. [Google Scholar] [PubMed]
- Fields, M.; Lewis, C.G. Alcohol consumption aggravates copper deficiency. Metabolism 1990, 39, 610–613. [Google Scholar] [CrossRef]
- Sidhu, P.; Gorg, M.L.; Morgenstern, P.; Vogt, J.; Butz, T.; Dhawan, D.K. Role of zinc in regulating the levels of hepatic elements following nickel toxicity in rats. Biol. Trace Element. Res. 2004, 102, 161–172. [Google Scholar] [CrossRef]
- Volini, F.; de la Huerga, J.; Kent, G. Trace metal studies in the liver disease using atomic absorption spectrometry. In Laboratory Diagnosis of Liver Diseases; Sunderman, F.W., Sunderman, F.W., Jr., Eds.; Warren H. Green Inc.: St. Louis, MO, USA, 1968; p. 199. [Google Scholar]
- McNeely, M.D.; Sunderman, F.W., Jr.; Nechay, M.-W.; Levine, H. Abnormal concentrations of nickel in serum in cases of myocardial infarction, stroke, burns, hepatic cirrhosis, and uremia. Clin. Chem. 1971, 17, 1123–1128. [Google Scholar] [PubMed]
- Sunderman, F.W., Jr. Nickel. In Metals and Their Compounds in the Environment; Merian, E., Anke, M., Ihnat, M., Stoeppler, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1991; pp. 841–867. [Google Scholar]
- Goode, H.F.; Kelleher, J.; Walker, B.E. Relation between zinc status and hepatic functional reserve in patients with liver disease. Gut 1990, 31, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Stewart, A.J.; Sadler, P.J.; Pinheiro, T.J.; Blindauer, C.A. Albumin as a zinc carrier: Properties of its high-affinity zinc-binding site. Biochem. Soc. Trans. 2008, 36, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- McClain, C.; Su, L.C. Zinc deficiency in the alcoholic: A review. Alcohol Clin. Exp. Res. 1983, 7, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.C.; Bradley, C.; Frei, J.V. Hepatic iron and zinc concentrations after portacaval shunting for nonalcoholic cirrhosis. Hepatology 1994, 19, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Keeling, P.W.N.; Feely, J. Tissue zinc status and drug elimination in patients with chronic liver disease. Clin. Sci. 1990, 78, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Reding, P.; Duchateau, J.; Bataille, C. Oral zinc supplementation improves hepatic encephalopathy. Results of a randomised controlled trial. Lancet 1984, 8401, 493–495. [Google Scholar] [CrossRef]
- Moscarella, S.; Duchini, A.; Buzzelli, G. Lipoperoxidation, trace elements and vitamin E in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 1994, 6, 633–636. [Google Scholar] [CrossRef]
- Krieger, D.; Krieger, S.; Jansen, O.; Gass, P.; Theilmann, L.; Lichtnecker, H. Manganese and chronic hepatic encephalopathy. Manganese and chronic hepatic encephalopathy. Lancet 1995, 8970, 270–274. [Google Scholar] [CrossRef]
- Jurczyk, K.; Wawrzynowicz-Syczewska, M.; Boroń-Kaczmarska, A.; Sych, Z. Serum iron parameters in patients with alcoholic and chronic cirrhosis and hepatitis. Med. Sci. Monit. 2001, 7, 962–965. [Google Scholar] [PubMed]
- Kowala-Piaskowska, A.; Tomaszewska, A.; Adamek, A.; Bura, M.; Mozer-Lisiewska, I. Malnutrition in liver cirrhosis. Hepatology 2014, 14, 90–93. [Google Scholar]

| Variables | Control Group (n = 18) | Study Group (n = 62) |
|---|---|---|
| Age (years) | 43.7 ± 14.6 | 54.9 ± 10.4 |
| Percentage of males (%) | 67% | 74% |
| Alcohol abuse (years) | - | 14.05 ± 5.17 |
| Body Wright (kg) | 67.5 ± 8.8 | 68.7 ± 13.7 |
| Total bilirubin (mg/dL) | 0.59 ± 0.29 | 5.33 ± 7.07 |
| Albumin (g/dL) | 5.24 ± 0.55 | 2.63 ± 0.66 |
| INR | 1.24 ± 0.15 | 1.52 ± 0.35 |
| Total protein (g/dL) | 6.2 ± 0.31 | 5.6 ± 0.84 |
| ALT (U/L) | 17.9 ± 5.9 | 64.3 ± 123.8 |
| AST (U/L) | 18.2 ± 7.1 | 115.5 ± 166.5 |
| PLT (G/L) | 231.3 ± 29.8 | 133.7 ± 73.9 |
| MCV (fL) | 84.7 ± 3.4 | 93.7 ± 9.3 |
| Urea (mg/dL) | 24.5 ± 10.1 | 32.4 ± 26.1 |
| Sodium (mmol/L) | 140 ± 3.2 | 134.3 ± 5.4 |
| Potassium (mmol/L) | 4.3 ± 0.39 | 3.8 ± 0.65 |
| CRP (mg/L) | 2.51 ± 2.29 | 21.07 ± 10.96 |
| Complications | ||
| Oesophageal varices (%) | - | 53% |
| Ascitis (%) | - | 42% |
| Encephalopathy (%) | - | 64% |
| Heavy Metals | Controls | P-Ch A (n = 19) | P-Ch B (n = 20) | P-Ch C (n = 23) | p * |
|---|---|---|---|---|---|
| Cadmium (Cd) (mg/L) | 0.0054 ± 0.0007 | 0.0061 ± 0.0028 | 0.0076 ± 0.0045 | 0.0078 ± 0.0044 | <0.05 |
| Cobalt(Co) (mg/L) | 0.0042 ± 0.0020 | 0.0028 ± 0.0016 | 0.0026 ± 0.0015 | 0.0024 ± 0.0014 | <0.01 |
| Copper (Cu) | 1.2663 ± 0.0771 | 1.1178 ± 0.2240 | 1.1080 ± 0.2024 | 1.1529 ± 0.1559 | <0.001 |
| Manganese (Mg) (mg/L) | 0.0089 ± 0.0006 | 0.0126 ± 0.021 | 0.0144 ± 0.0214 | 0.0154 ± 0.0158 | NS |
| Zinc (Zn) (mg/L) | 1.3607 ± 0.1123 | 1.0371 ± 0.3373 | 0.9579 ± 0.2441 | 1.0095 ± 0.3164 | <0.001 |
| Nickel(Ni) (mg/L) | 0.0019 ± 0.001 | 0.0007 ± 0.001 | 0.0005 ± 0.001 | 0.0003 ± 0.0011 | 0.0001 |
| Lead(Pb) (mg/L) | 0.0384 ± 0.0076 | 0.0447 ± 0.0135 | 0.0439 ± 0.0127 | 0.0478 ± 0.0176 | NS |
| Iron (Fe) (mg/L) | 1.3682 ± 0.1452 | 1.2288 ± 0.2272 | 1.3540 ± 0.3264 | 1.3170 ± 0.2687 | NS |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prystupa, A.; Błażewicz, A.; Kiciński, P.; Sak, J.J.; Niedziałek, J.; Załuska, W. Serum Concentrations of Selected Heavy Metals in Patients with Alcoholic Liver Cirrhosis from the Lublin Region in Eastern Poland. Int. J. Environ. Res. Public Health 2016, 13, 582. https://doi.org/10.3390/ijerph13060582
Prystupa A, Błażewicz A, Kiciński P, Sak JJ, Niedziałek J, Załuska W. Serum Concentrations of Selected Heavy Metals in Patients with Alcoholic Liver Cirrhosis from the Lublin Region in Eastern Poland. International Journal of Environmental Research and Public Health. 2016; 13(6):582. https://doi.org/10.3390/ijerph13060582
Chicago/Turabian StylePrystupa, Andrzej, Anna Błażewicz, Paweł Kiciński, Jarosław J. Sak, Jarosław Niedziałek, and Wojciech Załuska. 2016. "Serum Concentrations of Selected Heavy Metals in Patients with Alcoholic Liver Cirrhosis from the Lublin Region in Eastern Poland" International Journal of Environmental Research and Public Health 13, no. 6: 582. https://doi.org/10.3390/ijerph13060582
APA StylePrystupa, A., Błażewicz, A., Kiciński, P., Sak, J. J., Niedziałek, J., & Załuska, W. (2016). Serum Concentrations of Selected Heavy Metals in Patients with Alcoholic Liver Cirrhosis from the Lublin Region in Eastern Poland. International Journal of Environmental Research and Public Health, 13(6), 582. https://doi.org/10.3390/ijerph13060582








