A Mouse Model for Studying Nutritional Programming: Effects of Early Life Exposure to Soy Isoflavones on Bone and Reproductive Health
Abstract
:1. Introduction
2. Experimental Section
3. Discussion
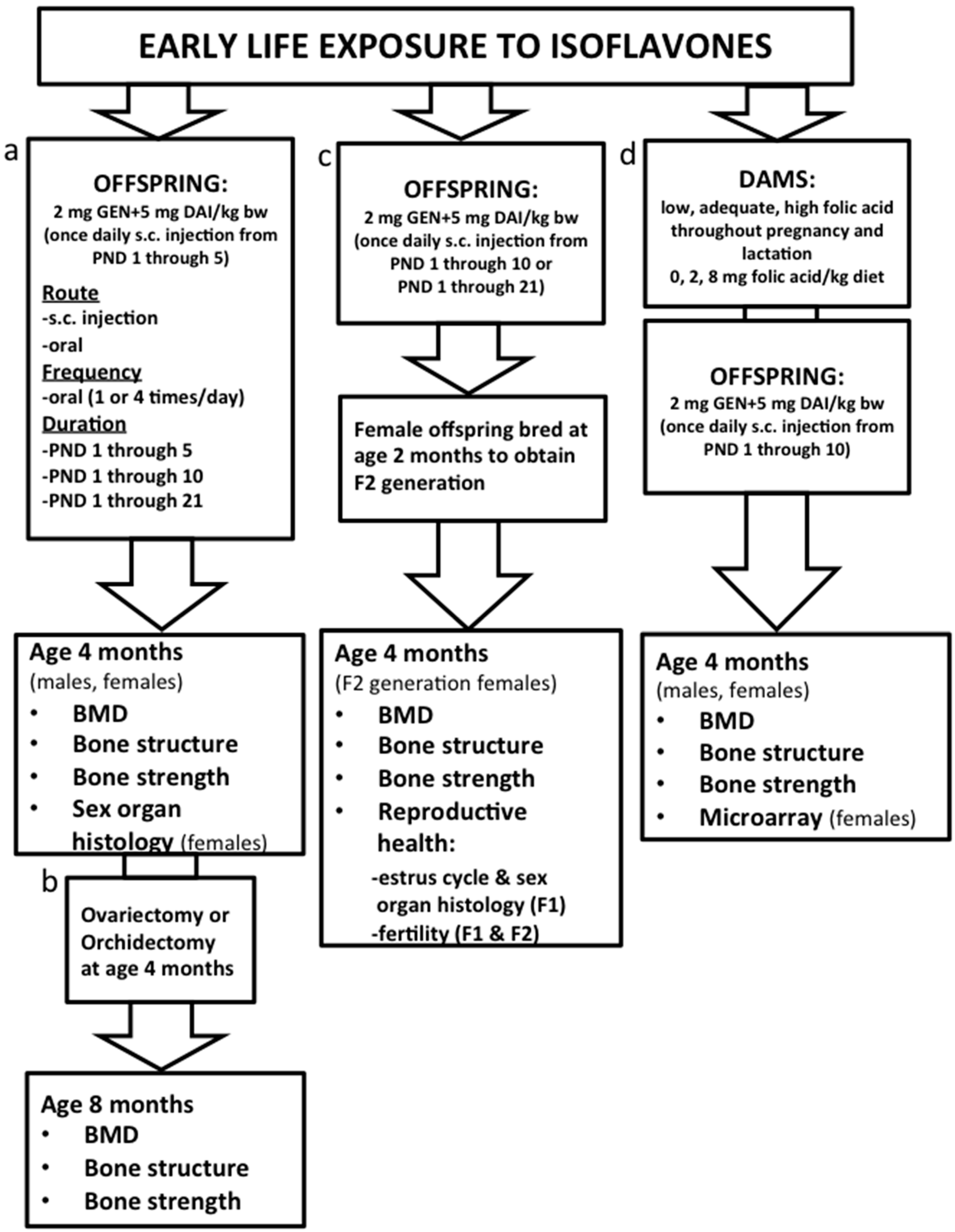
3.1. Characterization of the Mouse Model: Determining Route and Frequency of Administration of ISO
3.2. Effects on Bone Health: Dose and Duration of Isoflavones and Transgenerational Effects
3.3. Mechanisms and Interaction with Supplemental Folic Acid
3.4. Effects on Reproductive Health
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osteoporosis Canada, Osteoporosis Facts and Figures. Available online: http://www.osteoporosis.ca/osteoporosis-and-you/osteoporosis-facts-and-statistics/ (accessed on 30 March 2016).
- International Osteoporosis Foundation. Facts and Statistics. Available online: http://www.iofbonehealth.org/facts-statistics (accessed on 30 March 2016).
- Dinsdale, E.C.; Kaludjerovic, J.; Ward, W.E. Isoflavone exposure throughout suckling results in improved adult bone health in mice. J. Dev. Orig. Health Dis. 2012, 3, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Neonatal exposure to daidzein, genistein, or the combination modulates bone development in female CD-1 mice. J. Nutr. 2009, 139, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Adequate but not supplemental folic acid combined with soy isoflavones during early life improves bone health at adulthood in male mice. J. Nutr. Biochem. 2013, 24, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Bone-specific gene expression patterns and whole bone tissue of female mice are programmed by early life exposure to soy isoflavones and folic acid. J. Nutr. Biochem. 2015, 26, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Piekarz, A.V.; Ward, W.E. Effect of neonatal exposure to genistein on bone metabolism in mice at adulthood. Pediatr. Res. 2007, 61, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, E.C.; Chen, J.; Ward, W.E. Early life exposure to isoflavones adversely affects reproductive health in first but not second generation female CD-1 mice. J. Nutr. 2011, 141, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Chen, J.; Ward, W.E. Early life exposure to genistein and daidzein disrupts structural development of reproductive organs in female mice. J. Toxicol. Environ. Health A 2012, 75, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Franke, A.A.; Ward, W.E. Circulating isoflavonoid levels in cd-1 mice: Effect of oral versus subcutaneous delivery and frequency of administration. J. Nutr. Biochem. 2012, 23, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Kaludjerovic, J.; Ward, W.E. Neonatal administration of isoflavones attenuates deterioration of bone tissue in female but not male mice. J. Nutr. 2010, 140, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.E.; Sacco, S.M.; Dinsdale, E.C.; Kaludjerovic, J. Transgenerational benefits of soy isoflavones to bone structure in the CD-1 mouse moudel. In Proceeding of the 9th International Symposium of Nutritional Aspects of Osteoporosis, Montreal, QC, Canada, 20 June 2015.
- Jefferson, W.; Newbold, R.; Padilla-Banks, E.; Pepling, M. Neonatal genistein treatment alters ovarian differentiation in the mouse: Inhibition of oocyte nest breakdown and increased oocyte survival. Biol. Reprod. 2006, 74, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Couse, J.F.; Padilla-Banks, E.; Korach, K.S.; Newbold, R.R. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: Evidence for erbeta-mediated and nonestrogenic actions. Biol. Reprod. 2002, 67, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Doerge, D.; Padilla-Banks, E.; Woodling, K.A.; Kissling, G.E.; Newbold, R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ. Health Perspect. 2009, 117, 1883–1889. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, W.N.; Padilla-Banks, E.; Newbold, R.R. Disruption of the developing female reproductive system by phytoestrogens: Genistein as an example. Mol. Nutr. Food Res. 2007, 51, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.E.; Piekarz, A.V.; Fonseca, D. Bone mass, bone strength, and their relationship in developing CD-1 mice. Can. J. Physiol. Pharmacol. 2007, 85, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Dolinoy, D.C.; Huang, D.; Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl. Acad. Sci. USA 2007, 104, 13056–13061. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, S.; Newbold, R.R.; Teti, A.; Jefferson, W.J.; Toverud, S.U.; Taranta, A.; Bullock, B.C.; Suggs, C.A.; Spera, G.; Korach, K.S. Transient estrogen exposure of female mice during early development permanently affects osteoclastogenesis in adulthood. Bone 2000, 27, 47–52. [Google Scholar] [CrossRef]
- Kaludjerovic, J.; Ward, W.E. Diethylstilbesterol has gender-specific effects on weight gain and bone development in mice. J. Toxicol. Environ. Health A 2008, 71, 1032–1042. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.A.; Nahin, R.L.; Messina, M.J.; Rader, J.I.; Thompson, L.U.; Badger, T.M.; Dwyer, J.T.; Kim, Y.S.; Pontzer, C.H.; Starke-Reed, P.E.; et al. Guidance from an nih workshop on designing, implementing, and reporting clinical studies of soy interventions. J. Nutr. 2010, 140, 1192s–1204s. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet 1997, 350, 23–27. [Google Scholar] [CrossRef]
- Bloedon, L.T.; Jeffcoat, A.R.; Lopaczynski, W.; Schell, M.J.; Black, T.M.; Dix, K.J.; Thomas, B.F.; Albright, C.; Busby, M.G.; Crowell, J.A.; et al. Safety and pharmacokinetics of purified soy isoflavones: Single-dose administration to postmenopausal women. Am. J. Clin. Nutr. 2002, 76, 1126–1137. [Google Scholar] [PubMed]
- Busby, M.G.; Jeffcoat, A.R.; Bloedon, L.T.; Koch, M.A.; Black, T.; Dix, K.J.; Heizer, W.D.; Thomas, B.F.; Hill, J.M.; Crowell, J.A.; et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: Single-dose administration to healthy men. Am. J. Clin. Nutr. 2002, 75, 126–136. [Google Scholar] [PubMed]
- Axelson, M.; Setchell, K.D. The excretion of lignans in rats—Evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981, 123, 337–342. [Google Scholar] [CrossRef]
- Doerge, D.R.; Twaddle, N.C.; Banks, E.P.; Jefferson, W.N.; Newbold, R.R. Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett. 2002, 184, 21–27. [Google Scholar] [CrossRef]
- Chen, J.R.; Lazarenko, O.P.; Blackburn, M.L.; Badeaux, J.V.; Badger, T.M.; Ronis, M.J. Infant formula promotes bone growth in neonatal piglets by enhancing osteoblastogenesis through bone morphogenic protein signaling. J. Nutr. 2009, 139, 1839–1847. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, E.C.; Ward, W.E. Early exposure to soy isoflavones and effects on reproductive health: A review of human and animal studies. Nutrients 2010, 2, 1156–1187. [Google Scholar] [CrossRef] [PubMed]
- Wiedmeier, J.E.; Joss-Moore, L.A.; Lane, R.H.; Neu, J. Early postnatal nutrition and programming of the preterm neonate. Nutr. Rev. 2011, 69, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Wang, J.F.; Jin, W.F.; Wang, H.F.; Zhang, S.F.; Gao, J.J. Effects of daidzein on steroid receptor coactivator-1 expression in MC3T3-E1 cells and the mechanism. Zhong Xi Yi Jie He Xue Bao 2011, 9, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, H.; Wagner, A.E.; Boesch-Saadatmandi, C.; Kruse, H.P.; Kulling, S.; Rimbach, G. Effect of dietary genistein on phase II and antioxidant enzymes in rat liver. Cancer Genomi. Proteom. 2009, 6, 85–92. [Google Scholar] [PubMed]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344s–1352s. [Google Scholar] [PubMed]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Vanhees, K.; Coort, S.; Ruijters, E.J.; Godschalk, R.W.; van Schooten, F.J.; Barjesteh van Waalwijk van Doorn-Khosrovani, S. Epigenetics: Prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011, 25, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ho, S.M. Epigenetics meets endocrinology. J. Mol. Endocrinol. 2011, 46, R11–R32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, W.; Huang, Y.; Huang, X.; Xu, L.; Lv, Z. Genistein demethylates the promoter of CHD5 and inhibits neuroblastoma growth in vivo. Int. J. Mol. Med. 2012, 30, 1081–1086. [Google Scholar] [PubMed]
- Sato, K.; Fukata, H.; Kogo, Y.; Ohgane, J.; Shiota, K.; Mori, C. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr. J. 2009, 56, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liang, J.; Li, X.; Yu, H.; Li, X.; Xiao, R. Folic acid and soybean isoflavone combined supplementation protects the post-neural tube closure defects of rodents induced by cyclophosphamide in vivo and in vitro. Neurotoxicology 2010, 31, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Jiang, H.; Grange, R.W. Genistein activates the 3′,5′-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology 2005, 146, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Si, H.; Yu, J.; Jiang, H.; Lum, H.; Liu, D. Phytoestrogen genistein up-regulates endothelial nitric oxide synthase expression via activation of camp response element-binding protein in human aortic endothelial cells. Endocrinology 2012, 153, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Krum, S.A.; Miranda-Carboni, G.A.; Hauschka, P.V.; Carroll, J.S.; Lane, T.F.; Freedman, L.P.; Brown, M. Estrogen protects bone by inducing fas ligand in osteoblasts to regulate osteoclast survival. EMBO J. 2008, 27, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Han, L.; Chen, J.R.; Almeida, M.; Plotkin, L.I.; Bellido, T.; Manolagas, S.C. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J. Clin. Investig. 2003, 111, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Kousteni, S.; Bellido, T.; Plotkin, L.I.; O’Brien, C.A.; Bodenner, D.L.; Han, L.; Han, K.; DiGregorio, G.B.; Katzenellenbogen, J.A.; Katzenellenbogen, B.S.; et al. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell 2001, 104, 719–730. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 2006, 147, S11–S17. [Google Scholar] [CrossRef] [PubMed]
- Newbold, R.R.; Jefferson, W.N.; Padilla-Banks, E.; Haseman, J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: Low versus high dose effects. Reprod. Toxicol. 2004, 18, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Bernbaum, J.C.; Umbach, D.M.; Ragan, N.B.; Ballard, J.L.; Archer, J.I.; Schmidt-Davis, H.; Rogan, W.J. Pilot studies of estrogen-related physical findings in infants. Environ. Health Perspect. 2008, 116, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Zung, A.; Glaser, T.; Kerem, Z.; Zadik, Z. Breast development in the first 2 years of life: An association with soy-based infant formulas. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Andres, A.; Moore, M.B.; Linam, L.E.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Compared with feeding infants breast milk or cow-milk formula, soy formula feeding does not affect subsequent reproductive organ size at 5 years of age. J. Nutr. 2015, 145, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Strom, B.L.; Schinnar, R.; Ziegler, E.E.; Barnhart, K.T.; Sammel, M.D.; Macones, G.A.; Stallings, V.A.; Drulis, J.M.; Nelson, S.E.; Hanson, S.A. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001, 286, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Harmon, Q.E.; Baird, D.D. Soy-based infant formula feeding and ultrasound-detected uterine fibroids among young African-American women with no prior clinical diagnosis of fibroids. Environ. Health Perspect. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Adgent, M.A.; Daniels, J.L.; Rogan, W.J.; Adair, L.; Edwards, L.J.; Westreich, D.; Maisonet, M.; Marcus, M. Early-life soy exposure and age at menarche. Paediatr. Perinat. Epidemiol. 2012, 26, 163–175. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ward, W.E.; Kaludjerovic, J.; Dinsdale, E.C. A Mouse Model for Studying Nutritional Programming: Effects of Early Life Exposure to Soy Isoflavones on Bone and Reproductive Health. Int. J. Environ. Res. Public Health 2016, 13, 488. https://doi.org/10.3390/ijerph13050488
Ward WE, Kaludjerovic J, Dinsdale EC. A Mouse Model for Studying Nutritional Programming: Effects of Early Life Exposure to Soy Isoflavones on Bone and Reproductive Health. International Journal of Environmental Research and Public Health. 2016; 13(5):488. https://doi.org/10.3390/ijerph13050488
Chicago/Turabian StyleWard, Wendy E., Jovana Kaludjerovic, and Elsa C. Dinsdale. 2016. "A Mouse Model for Studying Nutritional Programming: Effects of Early Life Exposure to Soy Isoflavones on Bone and Reproductive Health" International Journal of Environmental Research and Public Health 13, no. 5: 488. https://doi.org/10.3390/ijerph13050488
APA StyleWard, W. E., Kaludjerovic, J., & Dinsdale, E. C. (2016). A Mouse Model for Studying Nutritional Programming: Effects of Early Life Exposure to Soy Isoflavones on Bone and Reproductive Health. International Journal of Environmental Research and Public Health, 13(5), 488. https://doi.org/10.3390/ijerph13050488





