1. Introduction
In recent years, industrial emissions, urban construction, and increased vehicle exhausts led to poor air quality in many cities in China [
1,
2]. The adverse health effects of airborne particulate matters (PM) pollution have become a growing concern [
1,
3]. Numerous epidemiological studies have documented a positive correlation between exposure to ambient PM concentrations and increased respiratory and cardiovascular morbidity and mortality [
4,
5,
6]. However, the contribution of specific components of PM to its toxicity is largely unknown, despite the recognition that the chemical composition of PM is clearly important in driving its toxicological effects.
Generally, the size, shape, and chemical composition of PM play crucial roles in the adverse effects on human health. PM can be classified into different size fractions, such as coarse particles (PM
2.5–10, aerodynamic diameter less than 10 µm but larger than 2.5 µm), fine particles (PM
2.5 and PM
1, aerodynamic diameter <2.5 µm and <1 µm), and ultrafine particles (PM
0.1, aerodynamic diameter ≤100 nm) [
7,
8,
9]. The penetration depth and deposition of PM in the lung are particle size dependent [
10]. Particular attention has been paid to respirable fine particles. It has been reported that PM
2.5–10 tend to deposit in the nasopharyngeal compartment, while PM
2.5–1 and PM
0.1 exhibit deposition in the alveolar and tracheobronchial compartments [
11]. These small particles retained in the peripheral lung may be more harmful than larger particles [
12,
13].
On the other hand, PM-induced toxicity is also affected by the chemical composition and source [
14]. PM is a complex mixture of particles with different chemical composition. The coarse fraction is mostly primary particles consisting of crustal elements, sea salt, and organic components [
8]. The fine fraction contains a mixture of carbonaceous material derived from anthropogenic emissions, such as inorganic elements, sulfates, nitrates, chloride, ammonium, element carbon, and organic carbonaceous matter [
15]. In the city of Beijing (China), traffic emissions are the major source of air pollution. Sulfate, nitrate, ammonium, metals, and organic and element carbon have been found to comprise the major chemical composition of PM
2.5 in Beijing [
16,
17]. However, the contribution of individual chemical composition of urban PM to toxicity remains to be elucidated.
Oxidative stress has been recognized as a key molecular mechanism of PM-mediated cytotoxicity [
18,
19]. Basically, PM-induced oxidative stress is a state of redox disequilibrium in which reactive oxygen species (ROS) production overwhelms the antioxidant defenses, thereby leading to adverse biological consequences [
20]. ROS are composed of hydrogen peroxide (H
2O
2), superoxide (O
2−), hydroxyl radicals (OH∙), or other hydroperoxides (ROOH), which are generated by the chemical reaction of specific PM-compositions from anthropogenic sources [
13,
21]. Both
in vivo and
in vitro studies have demonstrated that the chemical composition of PM could induce oxidative injury, inflammation, fibrosis, and cytotoxicity in the lung [
19,
22,
23]. It was found that redox-active transition metals (e.g., Fe, Cu, Cr, Ni, and Zn) undergoing redox cycling directly are associated with ROS generation [
24], whereas organic compounds, such as polyaromatic hydrocarbons (PAH), indirectly affect the production of ROS [
25]. The ability of chemical composition to induce oxidant radical generation may provide evidence to compare the toxic potential of PM.
In the present study, to improve knowledge about the role played by metals coated onto PM in lung cytotoxicity, we investigated the capacity of various size-fractionated PM collected from an urban traffic site (Beijing City) and an industrial site (Anshan City) to induce ROS generation in human bronchial epithelial BEAS-2B cells. Moreover, the effect of PM-induced ROS generation on cell viability was evaluated. The correlation analysis of the chemical compositions of PM and redox activity or cell viability were also performed. Furthermore, we assessed the effect of metal-chelation with desferoxamine (DFO) on ROS generation and cell viability.
2. Materials and Methods
2.1. Materials
Human bronchial epithelial cell line BEAS-2B was obtained from the China Center for Type Culture Collection (Shanghai, China). Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Penicillin and streptomycin were purchased from Gibco (Grand Island, NY, USA). 2’,7’-dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Sigma-Aldrich. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfo-phenyl)-2H-tetra-zolium salt (MTS) was purchased from Promega (Madison, WI, USA). Fetal bovine serum was purchased from PAA (Linz, Austria). Ninety-six-well plates and cell culture dishes were purchased from Costar (Cambridge, MA, USA). A ToxinSensor chromogenic limulus amebocyte lysate endotoxin assay kit was purchased from GenScript (Piscataway Township, NJ, USA). Desferrioxamine (DFO) was purchased from Sigma-Aldrich.
2.2. Sampling and Particle Preparation
PM of three aerodynamic diameter ranges (<1, 1–2.5 and 2.5–10 µm) were collected at two different sites: (i) An urban ambient site (u) was chosen on a rooftop (about 13 m aboveground) in the Yuquan campus of the University of Chinese Academy Sciences (UCAS), close to the Western Fifth-ring road of Beijing City, in March–July 2013. There is high traffic flow and a high density of population in the daytime. The UCAS campus is surrounded by some institutes and residential areas. Large industrial and thermoelectric plants were absent; the distance of the sampler from the main road was 10 m; (ii) The steel factory ambient site (s) is located in the industrial area of Anshan City, China (about 670 km away from Beijing). Air sampling was performed in November–December 2014, with sunny weather. PM was collected on 90 mm Teflon filters (diameter = 90 mm, Whatman, Piscataway, NJ, USA) by medium-volume PM samplers (Wuhan Tianhong Intelligence Instrumentation Facility, model TH-150D II, flow rate: 100 L/min). Before and after the sampling, the Teflon filters were equilibrated in conditions of 30% relative humidity and 25 °C room temperature for over 48 h and then weighted on a high-precision microbalance (Mettler Toledo, OH, USA) to measure the collected PM. All sampled filters were stored in the dark at −20 °C before further chemical and physical characterization. Unexposed filters (blank samples) were prepared using the same method except for sampling and were used as a control in all experiments.
Particles on Teflon filters were extracted according to the method of Imrich
et al. [
26]. Briefly, PM samples were extracted from the sampled filters by immersing them in deionized water (18.2 MΩ/cm) and then sonicating them for 30 min in a water-bath sonicator (KQ-700V, 700W). PM samples were then stored at −80 °C until use. Blank samples were processed simultaneously with the PM samples and used as a control in all experiments. To adjust the concentration of PM preparations, 100 µL aliquots of PM samples were placed on filters and air-dried. The samples and filters were weighed on a microbalance (Mettler Toledo, Switzerland). PM samples were prepared in deionized water at 5 mg/mL and sonicated for 1 min prior to use.
2.3. PM Physical and Chemical Characterization
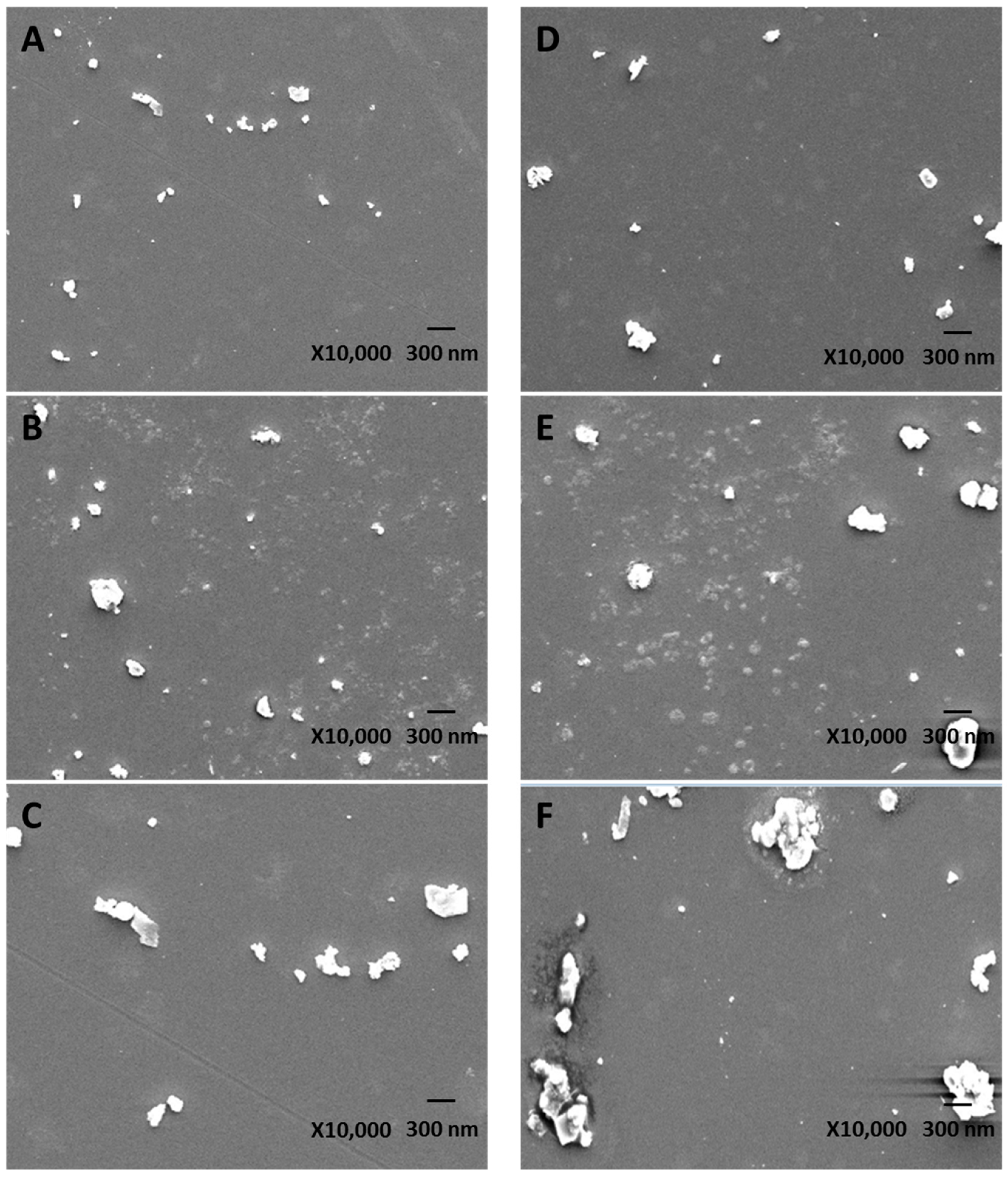
The size distribution of various uPM and sPM was measured using scanning electron microscopy (SEM, JEOL JSM-6700F, Tokyo, Japan) as described by Deng
et al. [
19]. Prior to analysis, PM was suspended in an n-hexane solution with a assistance of ultrasonic treatment, and the suspended particles was then filtered through a nucleopore filter to obtain well distribution and dispersed PM, without agglomerates. The filter was then carbon coated and measured using automatic mode.
The size distribution of different PM in suspension were analyzed using Nano-Zetasizer (1000 HS, Malvern Instruments Ltd., Malvern, UK) based on the dynamic light scattering measurement technique. Before analysis, particles were first suspended in a serum-free culture medium and sonicated with an ultrasonic processor (VCX130, Sonics, Newtown, CT, USA) for 30 s at 40 W in a bath to disperse evenly.
PM samples from both sampling sites were chemically characterized for elemental carbon (EC) and organic carbon (OC), water soluble inorganic ions, and inorganic elements. All chemical components were analyzed for each single sample and the pooled sample. For metal analysis, the analytical procedure for inorganic element determination comprised the acidic total digestion (HNO
3:HF = 7:3) of collected PM samples (1 mg) and the subsequent analysis of 20 elements by inductively coupled plasma-mass spectrometry (ICP-MS, Elemental X7, Thermo, Waltham, MA, USA). Element stock solutions were prepared, containing 1 µg/mL of each analyte (Ti, V, Cr, Mn, Co, Ni, Cu, As, Sr, Cd, Pb, Zn, Fe, Ca, K, Mg, and Na). The multi-element working standard solution was prepared (using the reagent blank solution as diluent) from the abovementioned stock solution to span the concentration range from 10 to 100 ng/mL. At these concentration levels, all elements could be mixed together. In (20 ng/mL) and Bi (20 ng/mL) were used as the internal standards. The detection limit for each element in solution was calculated as three times the standard deviation of the ion counts obtained for the sample blank (measured for 10 replicate determinations), divided by the sensitivity of 10 ng/mL multi-element standard solutions. For Co, V, Cr, Ni, Ti, Cu, Zn, Sr, Mo, Cs, Ba, and Pb the detection limits ranged from 0.01 to 0.2 ng/mL. The water-soluble inorganic components (e.g., SO
42−, NO
3−, NH
4+, and Cl
−) were extracted in ultra-pure water for 30 min using an ultrasonic bath, filtered (0.45 mm PTFE filters), and identified by ionic chromatography (Dionex-600, Sunnyvale, CA, USA). The detection limits in µg/mL were 0.01 for Cl
−, 0.03 for NO
3−, 0.02 for SO
42−, 0.02 for NH
4+, and 0.01 for Ca
2+, Mg
2+, Na
+, and K
+. Elemental and organic carbon in PM samples were measured on-filter using a thermal-optical analyzer (Sunset Laboratory, Inc., Hillsborough, NC, USA) according to the method of Zhang
et al. [
27]. Endotoxin levels in PM were determined by Limulus amebocyte lysate assay kits following the manufacturer’s instructions.
2.4. Cell Culture and PM Treatment
Human bronchial epithelial BEAS-2B cells were maintained in high glucose DMEM supplemented with 10% (v/v) FBS, 100 IU/mL penicillin and 100 µg/mL streptomycin in a 5% CO2 humidified atmosphere at 37 °C. All cell exposure experiments were performed at 80%–90% of cell confluence, with viability ≥90% determined by trypan blue staining.
The cells were then harvested using 0.25% trypsin and were sub-cultured into 24-well plates or 96-well plates according to selection of experiments. Cells were allowed to attach to the surface in DMEM supplemented 10% FBS for 24 h prior to treatment. Then, the culture medium was replaced with a serum-free medium. The cells were exposed to freshly dispersed PM preparations at a final concentration of 100 µg/mL for 24 h [
19,
28].
2.5. MTS Assay
MTS assay was carried out to assess the cell viability after exposure to PM according to the method of Malich
et al. [
29]. Briefly, BEAS-2B cells were plated into 96-well plates at a density of 1.0 × 10
4 cells per well in 100 µL medium and cultured for 24 h. After incubation, BEAS-2B cells were treated with 0 or 100 µg/mL PM for 24 h. After exposure, 10 µL of MTS reagent was added directly to the wells and cell plates were incubated at for 1 h. The absorbance was quantified by a microplate spectrophotometer (Thermo MK3, MA, USA) at a wavelength of 492 nm. The viability of the treated cells was presented as a percentage of untreated cells, which was assumed to be 100%.
2.6. Reactive Oxygen Species Assay
The level of intracellular reactive oxygen species (ROS) in BEAS-2B cells was determined by measuring the oxidative conversion of DCFH-DA to fluorescent compound dichlorofluorescein (DCF). DCFH-DA, dissolved in ethanol, was added to cell culture at a final concentration of 40 µM for 30 min at 37 °C. BEAS-2B cells (2 × 105 cells) were washed with PBS and then exposed to 0 or 100 µg/mL of PM for 3 h, respectively. After exposure, the cells were lysed with 400 mM of NaOH. The total green fluorescence intensity was detected in a fluorescence multi-well plate reader (TriStar LB 941, Berthold, Bad Wildbad, Germany) with excitation and emission wavelengths of 485 nm and 535 nm. Results were measured as the fluorescence intensity change of untreated cells.
2.7. DFO Administration in MTS and ROS Assay
To further investigate the effects of metal elements on the cell viability and ROS generation, the chelator desferoxamine (DFO) was used to chelate the metal ions and to inhibit metal-mediated secondary oxidant generation. DFO is a metal chelator with an affinity for iron several logs of magnitude stronger than other metals’ [
30]. Complexation with DFO was carried out according to the method of Shafer
et al. [
30]. The PM suspensions were processed by adding 50 µL of 1000 µM DFO solution to 950 µL of PM sample (concentration of DFO is 50 µM). DFO was allowed to equilibrate for a minimum of 30 min in the samples and then the samples were immediately prepared for the ROS assay and MTS assay.
2.8. Statistical Analysis
Data were expressed as mean ± standard deviation (SD) of three independent experiments. Data were analyzed using one-way analysis of variance (ANOVA) followed by post hoc comparisons using the Tukey’s multiple paired comparison test. Statistical significance was set at p < 0.05. The correlation analysis was performed using Pearson’s test to assess associations between ROS generation/cell viability and chemical components of PM. All individual measurements for data of all particle sizes were combined and reflected the pooled association. GraphPad Prism 5.0 was used for all the statistical analyses.
4. Discussion
In this study, we used the human bronchial epithelial cell line BEAS-2B as a model to investigate the effects of the chemical composition of various size-fractionated PM on cytotoxicity and intracellular oxidant activity. Previous studies have reported that the chemical composition of PM may be related to the size-fractions and sources [
32,
33]. It has been demonstrated that the coarse fraction of PM is richer in crustal elements, whereas the fine fraction of PM is composed mainly of combustion components [
8,
15]. In the present study, various size-fractioned PM (PM
1.0, PM
1.0–2.5, and PM
2.5–10) from an urban site and a steel-factory site were collected and chemically characterized. Differences in chemical composition between urban and steel-factory PMs were related to differences in the size fractions of uPM and sPM. The concentrations of SO
42−, NO
3−, NH
4+, OC, and EC in the uPM were higher than those in sPM. This may reflect its multiple sources: coal combustion, motor vehicle exhaust, and biomass burning in Beijing [
19]. Among 12 detected inorganic elements including natural sourced elements (Ca, Mg, K, Na, Ti,
etc.) and conventionally anthropogenic inorganic elements (Fe, Mn, Ni, Cr, Pb, Zn, Cu,
etc.), relatively higher levels of Fe, Cu, Mn, Pb, and Zn were determined in the PM
1.0 and PM
1.0–2.5 than in the PM
2.5–10. This observation is in agreement with the previous report that metals are more abundant in the fine fraction than in the coarse fraction [
34]. In addition, we observed that the concentrations of Ca, Fe, K, Na, Cr, Mn, Ni, Cu, As, Cd, Cs, Pb, and Zn were higher in sPM
1.0 than those in uPM
1.0. In terms of concentration values, the concentrations of Mn, Cu, Fe, Pb and Zn in sPM
1.0 were 24, 14, 11, 9, 7, and 4 times higher than those in uPM
1.0. It has been reported that these metals are often associated with industrial emissions [
35]. Among the trace elements, the most abundant element in uPM and sPM was Fe, which may be derived from car exhaust and other urban combustion sources as well as industrial activities [
36]. Moreover, we found that endotoxins generally increased as particle size increased and were enriched in coarse particles, which is similar to the results reported in the literature [
37]. The concentrations of endotoxin in the uPM
2.5–10 (133.25 ± 3.57 EU/mg) and sPM
2.5–10 (134.86 ± 2.64 EU/mg) were much higher than those in PM
10 (20.1–49.3 EU/mg) collected from Mexico City [
38].
Several studies have demonstrated that PM toxicity is dependent on both size and composition [
37,
38]. Fine PM have higher cytotoxicity when compared with coarse PM [
7,
37]. However, coarse PM is found to be more toxic than fine PM due to high levels of endotoxin and transition metals [
38,
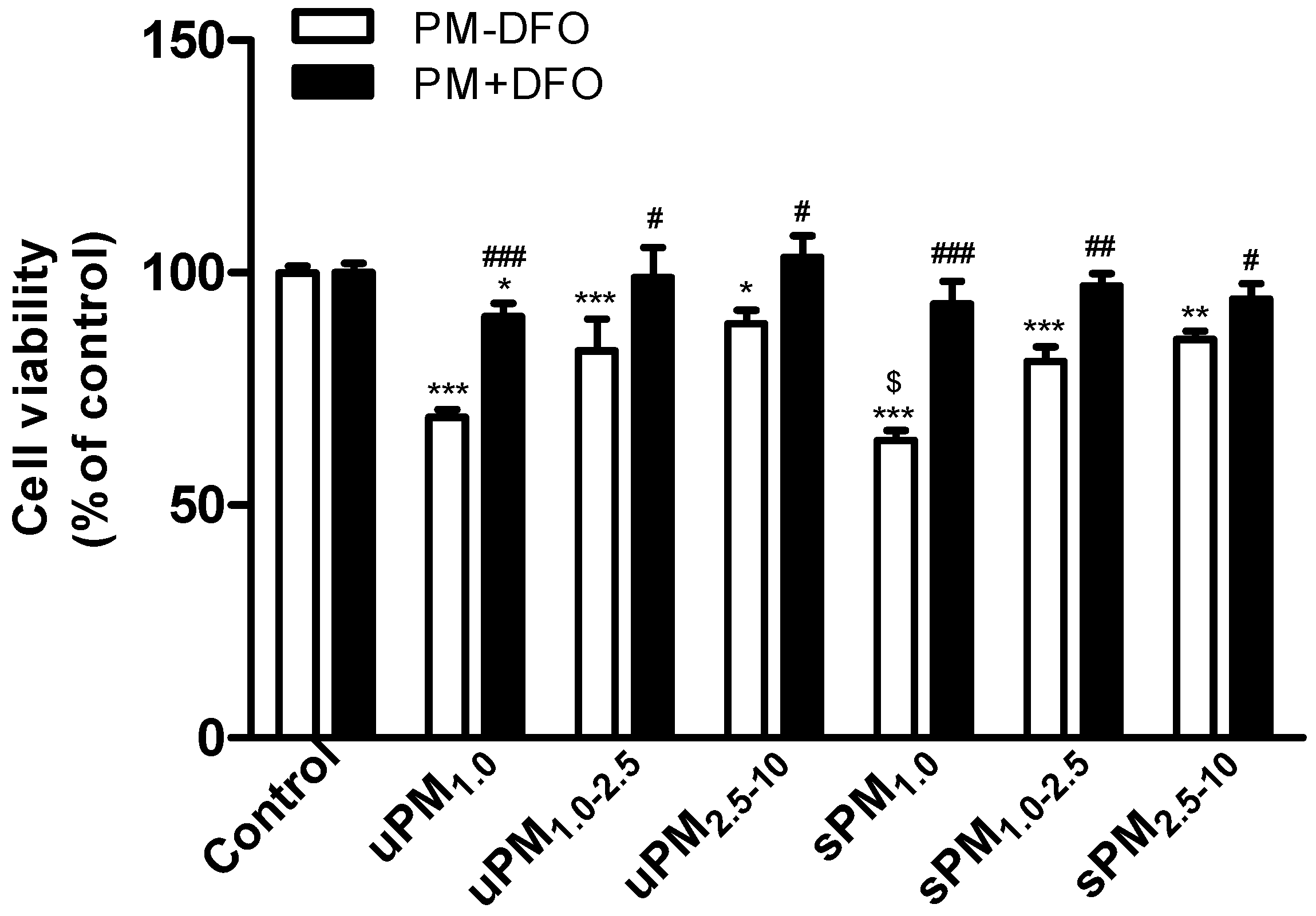
39]. In the present study, we found that different size-fractioned uPM and sPM caused a significant decrease in cell viability. No significant difference between PM
1.0–2.5 and PM
2.5–10 was observed (
Figure 2), although PM
1.0 was more toxic than PM
1.0–2.5 and PM
2.5–10, especially sPM
1.0. These results are in agreement with the report that the toxicity difference between fine and coarse PM depended on sampling location [
40]. In addition, we found that in uPM, metal concentrations including Fe increased as the particle size increased. However, cell viability was the lowest in uPM
1.0. Significant difference among the size fractions can be seen for all metals (
Table 2). The highest concentrations of Cu, As, Cd, Pb, and Zn occurred in the uPM
1.0, similar to the results reported in the literature [
9]. Alfaro-Moreno
et al. found that Ni and Zn are responsible for the loss of viability induced by PM [
36]. Al, Fe, Zn, Ba, and Mn of fine PM decrease cell viability in human lung epithelial A549 cells [
37]. However, it was found that Zn and Cu were more toxic to cells than Ni, Fe, Pb, or V [
41]. Further analysis showed that the cell viability was negatively correlated with the EC, SO
42−, NH
4+, Cl
−, Al, Ti, As, Sr, Cd, and Zn content of the PM samples. This observation is consistent with a previous report of a negative correlation between cell viability reduction and the elements As, Zn, Cr, Cu, and Mn [
42]. A significant correlation was found between Al, As, Cr, Cu, and Zn of fine particulate matter (PM
1 and PM
2.5) and the biological response in human lung epithelial cells (A549) [
42]. This suggests that the cytotoxic effects of the chemical composition of various PM size fractions differ greatly [
40].
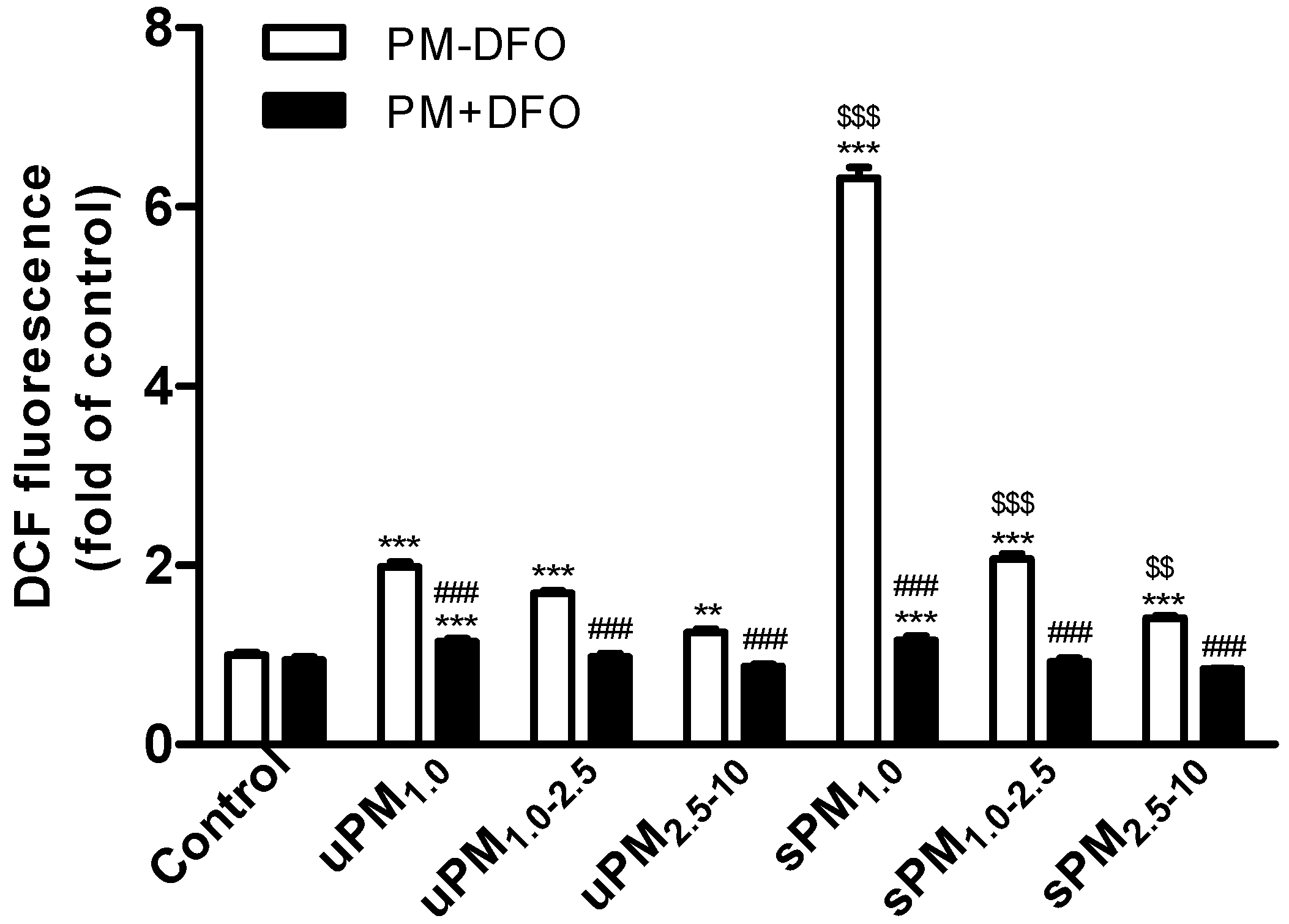
It has been identified that PM could provoke intracellular ROS generation [
43,
44]. ROS overproduction by redox-active transition metals, water soluble inorganic ions, carbonaceous fractions, and polycyclic aromatic hydrocarbons of PM
2.5 have been described [
45,
46,
47,
48,
49]. In the present study, we found that exposure to size-fractioned uPM and sPM led to a significant increase in intracellular ROS generation. Among all PM samples, sPM
1.0 was the strongest ROS inducer. We found that the increase of ROS in sPM
1.0-treated cells was triple that in uPM
1.0-treated cells. Intracellular ROS generation in this study was positively correlated with Fe, Cr, Mn, Ni, Cu, As, Sr, Cd, Cs, Ba, Pb, and Zn. Among these trace elements, transition metals are responsible for the oxidant activity of PM and result in ROS generation via Fenton-type reactions [
24]. Cd, Pb, and Zn can induce oxidative stress via depletion of cellular antioxidant pools (e.g., glutathione), and increase lipid peroxidation [
30]. Moreover, pretreatment with DFO significantly decreased uPM- and sPM-induced intracellular ROS generation, suggesting that Fe accounts for a large majority of PM-induced ROS activity.