l-Arginine Enhances Resistance against Oxidative Stress and Heat Stress in Caenorhabditis elegans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Worm Strains and Maintenance
2.3. Stress Resistance Assay
2.4. Intracellular ROS Measurement in C. elegans
2.5. Fluorescence Quantification and Visualization
2.6. Quantitative Real-Time PCR
2.7. Statistical Analyses
3. Results and Discussion
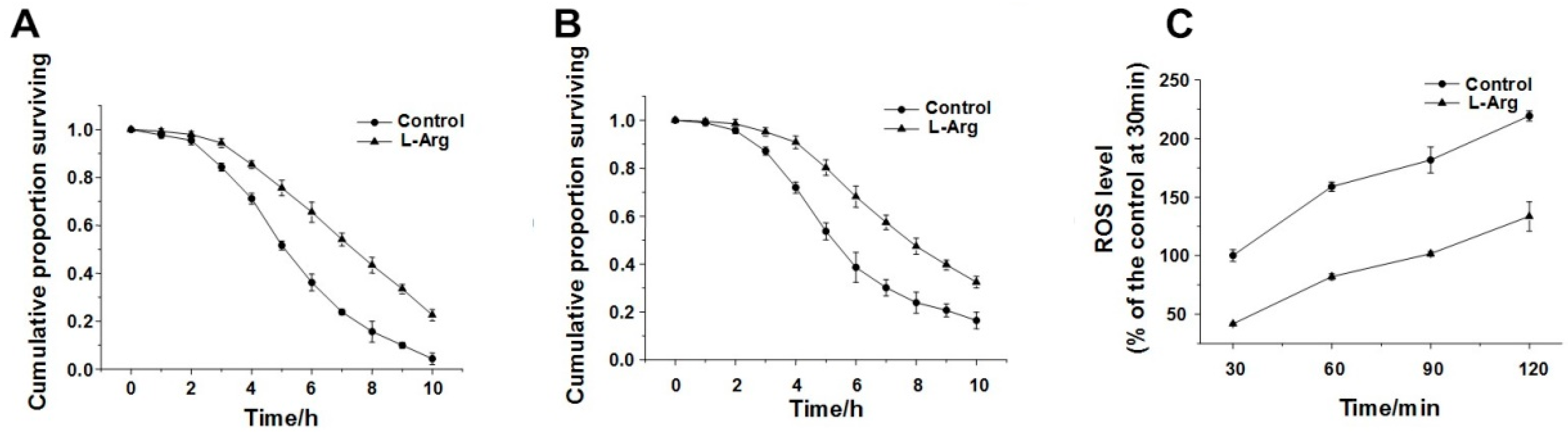
3.1. l-Arg Protect Worms from Environment Stresses
3.2. l-Arg Reduced the Level of Intracellular ROS
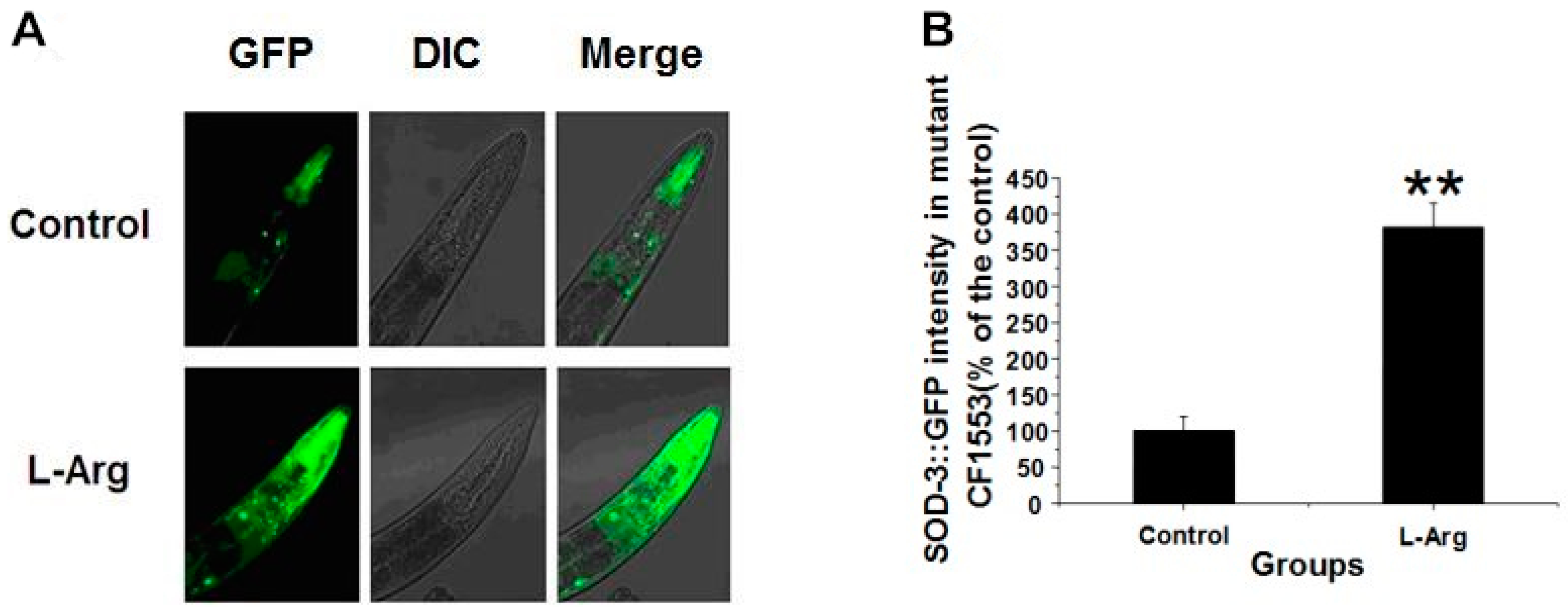
3.3. l-Arg Upregulates SOD-3::GFP in CF1553 Worms
3.4. l-Arg Upregulates HSP-16.2::GFP in CL2070 Worms
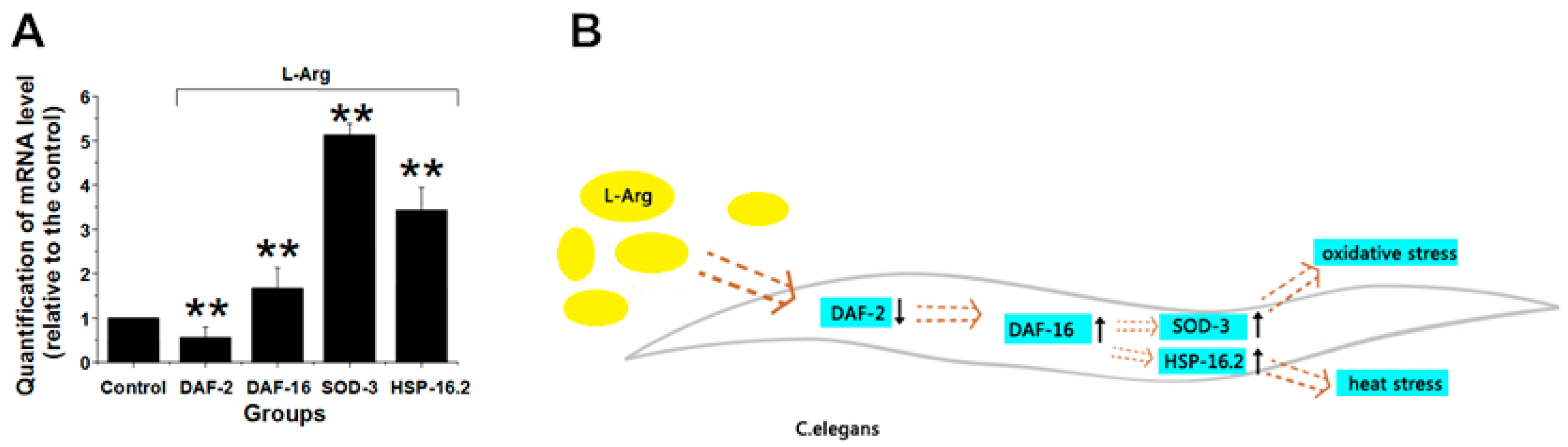
3.5. l-Arg Regulates the Insulin/IGF Signaling Pathway in C. elegans
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| C. elegans | Caenorhabditis elegans |
| ROS | reactive oxygen species |
| l-Arg | l-arginine |
| d-Arg | d-arginine |
| MnSOD | Mn-superoxide dismutase |
| IIS | insulin/IGF signaling |
References
- An, J.H.; Vranas, K.; Lucke, M.; Inoue, H.; Hisamoto, N.; Matsumoto, K.; Blackwell, T.K. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase Kinase-3. Proc. Natl. Acad. Sci. USA 2005, 102, 16275–16280. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhao, Y.; Guo, Y.; Xu, L.; Zhao, B. Significant longevity-extending effects of a tetrapeptide from maize on Caenorhabditis elegans under stress. Food Chem. 2012, 130, 254–260. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.L.; Lustgarten, M.S.; Jang, Y.; Richardson, A.; Van Remmen, H. Trends in oxidative aging theories. Free Radic. Biol. Med. 2007, 43, 477–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chi, Y.J.; Zhao, M.Y.; Lv, L. Purification and identification of antioxidant peptides from egg white protein hydrolysate. Amino Acids 2012, 43, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Power, O.; Jakeman, P.; FitzGerald, R.J. Antioxidative peptides: Enzymatic production, in vitro and in vivo antioxidant activity and potential applications of milk-derived antioxidative peptides. Amino Acids 2013, 44, 797–820. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Hu, Y.; Xue, M.; Dun, Y.; Li, S.; Peng, N.; Liang, Y.; Zhao, S. Purification and identification of antioxidant peptides from enzymatic hydrolysate of spirulina platensis. J. Microbiol. Biotechnol. 2016, 26, 1216–1223. [Google Scholar] [PubMed]
- Chaturvedi, R.; de Sablet, T.; Coburn, L.A.; Gobert, A.P.; Wilson, K.T. Arginine and polyamines in helicobacter pylori-induced immune dysregulation and gastric carcinogenesis. Amino Acids 2012, 42, 627–640. [Google Scholar] [PubMed]
- Clemmensen, C.; Madsen, A.N.; Smajilovic, S.; Holst, B.; Brauner-Osborne, H. l-arginine improves multiple physiological parameters in mice exposed to diet-induced metabolic disturbances. Amino Acids 2012, 43, 1265–1275. [Google Scholar] [PubMed]
- Popolo, A.; Adesso, S.; Pinto, A.; Autore, G.; Marzocco, S. l-arginine and its metabolites in kidney and cardiovascular disease. Amino Acids 2014, 46, 2271–2286. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S. Clinical use of amino acids as dietary supplement: Pros and cons. J. Caxhexia Sarcopenia Muscle 2011, 2, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S. To Give or Not to Give? Lessons from the Arginine Paradox. J. Nutrigenet Nutrigenom 2011, 4, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, F.S. Wasting and the substrate-to-energy controlled pathway: A role for insulin resistance and amino acids. Am. J. Cardiol. 2004, 93, 6A–12A. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, T.D.; Poyares, L.L.; Machado, U.F.; Nunes, M.T. Chronic oral administration of arginine induces GH gene expression and insulin resistance. Life Sci. 2006, 79, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Jiang, W.; Wu, L.Y. Dietary l-arginine supplement alleviates hepatic heat stress and improves feed conversion ratio of Pekin ducks exposed to high environmental temperature. J. Anim. Physiol. Anim. Nutr. 2014, 98, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Xiao, L.; Liu, G.M.; Fang, T.T.; Wu, X.J.; Jia, G.; Zhao, H.; Chen, X.L.; Wu, C.M.; Cai, J.Y.; et al. Dietary arginine and N-carbamylglutamate supplementation enhances the antioxidant statuses of the liver and plasma against oxidative stress in rats. Food Funct. 2016, 7, 2303–2311. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ren, M.; Habte-Tsion, H.-M.; Ge, X.; Xie, J.; Mi, H.; Xi, B.; Miao, L.; Liu, B.; Zhou, Q.; et al. Dietary arginine affects growth performance, plasma amino acid contents and gene expressions of the TOR signaling pathway in juvenile blunt snout bream, Megalobrama amblycephala. Aquaculture 2016, 461, 1–8. [Google Scholar] [CrossRef]
- Goud, A.P.; Goud, P.T.; Diamond, M.P.; Abu-Soud, H.M. Nitric oxide delays oocyte aging. Biochemistry 2005, 44, 11361–11368. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Wang, T.; Li, W.; Tang, W.; Zhang, D.; Dong, H. Exogenous nitric oxide delays salt-induced leaf senescence in cotton (Gossypium hirsutum L.). Acta Physiol. Plant. 2016, 38. [Google Scholar] [CrossRef]
- Gusarov, I.; Shatalin, K.; Starodubtseva, M.; Nudler, E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 2009, 325, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
- Liochev, S.I. Free radical paradoxes. Free Radic. Biol. Med. 2013, 65, 232–233. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Honda, S. The DAF-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999, 13, 1385–1393. [Google Scholar] [PubMed]
- Hansen, M.; Hsu, A.L.; Dillin, A.; Kenyon, C. New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet. 2005, 1, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lithgow, G.J.; White, T.M.; Melov, S.; Johnson, T.E. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc. Natl. Acad. Sci. USA 1995, 92, 7540–7544. [Google Scholar] [CrossRef] [PubMed]
- Rea, S.; Wu, D., Jr.; Vaupel, J.; Johnson, T. A stress-sensitive reporter predicts longevity in isogenic populations of Caenorhabditis elegans. Nat. Genet. 2005, 37, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(t)(-delta delta c) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, C.; Luersen, K.; Heinick, A.; Hussein, A.; Domagalski, M.; Walter, R.D.; Liebau, E. Oxidative stress in Caenorhabditis elegans: Protective effects of the omega class glutathione transferase (GSTO-1). FASEB J. 2008, 22, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kampkotter, A.; Gombitang Nkwonkam, C.; Zurawski, R.F.; Timpel, C.; Chovolou, Y.; Watjen, W.; Kahl, R. Effects of the flavonoids kaempferol and fisetin on thermotolerance, oxidative stress and foxo transcription factor daf-16 in the model organism Caenorhabditis elegans. Arch. Toxicol. 2007, 81, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Antebi, A. Genetics of aging in Caenorhabditis elegans. PLoS Genet. 2007, 3, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C. The plasticity of aging: Insights from long-lived mutants. Cell 2005, 120, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Gems, D.; Partridge, L. Insulin/IGF signalling and ageing: Seeing the bigger picture. Curr. Opin. Genet. Dev. 2001, 11, 287–292. [Google Scholar] [CrossRef]
- Kenyon, C.; Chang, J.; Gensch, E.; Rudner, A.; Tabtiang, R. A C. elegans mutant that lives twice as long as wild type. Nature 1993, 366, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Kenyon, C. DAF-16: An HNF-3/Forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science 1997, 278, 1319–1322. [Google Scholar] [CrossRef] [PubMed]
- Ogg, S.; Paradis, S.; Gottlieb, S.; Patterson, G.I.; Lee, L.; Tissenbaum, H.A.; Ruvkun, G. The fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 1997, 389, 994–999. [Google Scholar] [PubMed]
- Dorman, J.B.; Albinder, B.; Shroyer, T.; Kenyon, C. The age-1 and DAF-2 genes function in a common pathway to control the lifespan of Caenorhabditis elegans. Genetics 1995, 141, 1399–1406. [Google Scholar] [PubMed]
- Henderson, S.T.; Johnson, T.E. Daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr. Biol. 2001, 11, 1975–1980. [Google Scholar] [CrossRef]
- Hertweck, M.; Gobel, C.; Baumeister, R. C. elegans SGK-1 is the critical component in the Akt/PKB kinase complex to control stress response and life span. Dev. Cell 2004, 6, 577–588. [Google Scholar] [CrossRef]
- Lin, K.; Hsin, H.; Libina, N.; Kenyon, C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 2001, 28, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.T.; Folk, D.; Bradley, T.J.; Tower, J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult drosophila melanogaster. Genetics 2002, 161, 661–672. [Google Scholar] [PubMed]
- Sun, J.T.; Tower, J. FLP recombinase-mediated induction of Cu/Zn-superoxide dismutase transgene expression can extend the life span of adult drosophila melanogaster flies. Mol. Cell. Biol. 1999, 19, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Braeckman, B.P.; Vanfleteren, J.R. Genetic control of longevity in C. elegans. Exp. Gerontol. 2007, 42, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Lithgow, G.J. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2003, 2, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Kazemi-Esfarjani, P.; Benzer, S. Multiple-stress analysis for isolation of drosophila longevity genes. Proc. Natl. Acad. Sci. USA 2004, 101, 12610–12615. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Ma, Y.; Zhang, Z.; Zhao, Z.; Lin, R.; Zhu, J.; Guo, Y.; Xu, L. l-Arginine Enhances Resistance against Oxidative Stress and Heat Stress in Caenorhabditis elegans. Int. J. Environ. Res. Public Health 2016, 13, 969. https://doi.org/10.3390/ijerph13100969
Ma H, Ma Y, Zhang Z, Zhao Z, Lin R, Zhu J, Guo Y, Xu L. l-Arginine Enhances Resistance against Oxidative Stress and Heat Stress in Caenorhabditis elegans. International Journal of Environmental Research and Public Health. 2016; 13(10):969. https://doi.org/10.3390/ijerph13100969
Chicago/Turabian StyleMa, Heran, Yudan Ma, Zhixian Zhang, Ziyuan Zhao, Ran Lin, Jinming Zhu, Yi Guo, and Li Xu. 2016. "l-Arginine Enhances Resistance against Oxidative Stress and Heat Stress in Caenorhabditis elegans" International Journal of Environmental Research and Public Health 13, no. 10: 969. https://doi.org/10.3390/ijerph13100969




