Polycyclic Aromatic Hydrocarbon Residues in Serum Samples of Autopsied Individuals from Tennessee
Abstract
:1. Introduction
2. Methods
2.1. Sample Processing
2.2. Extraction
2.3. Internal Standards and Calibration
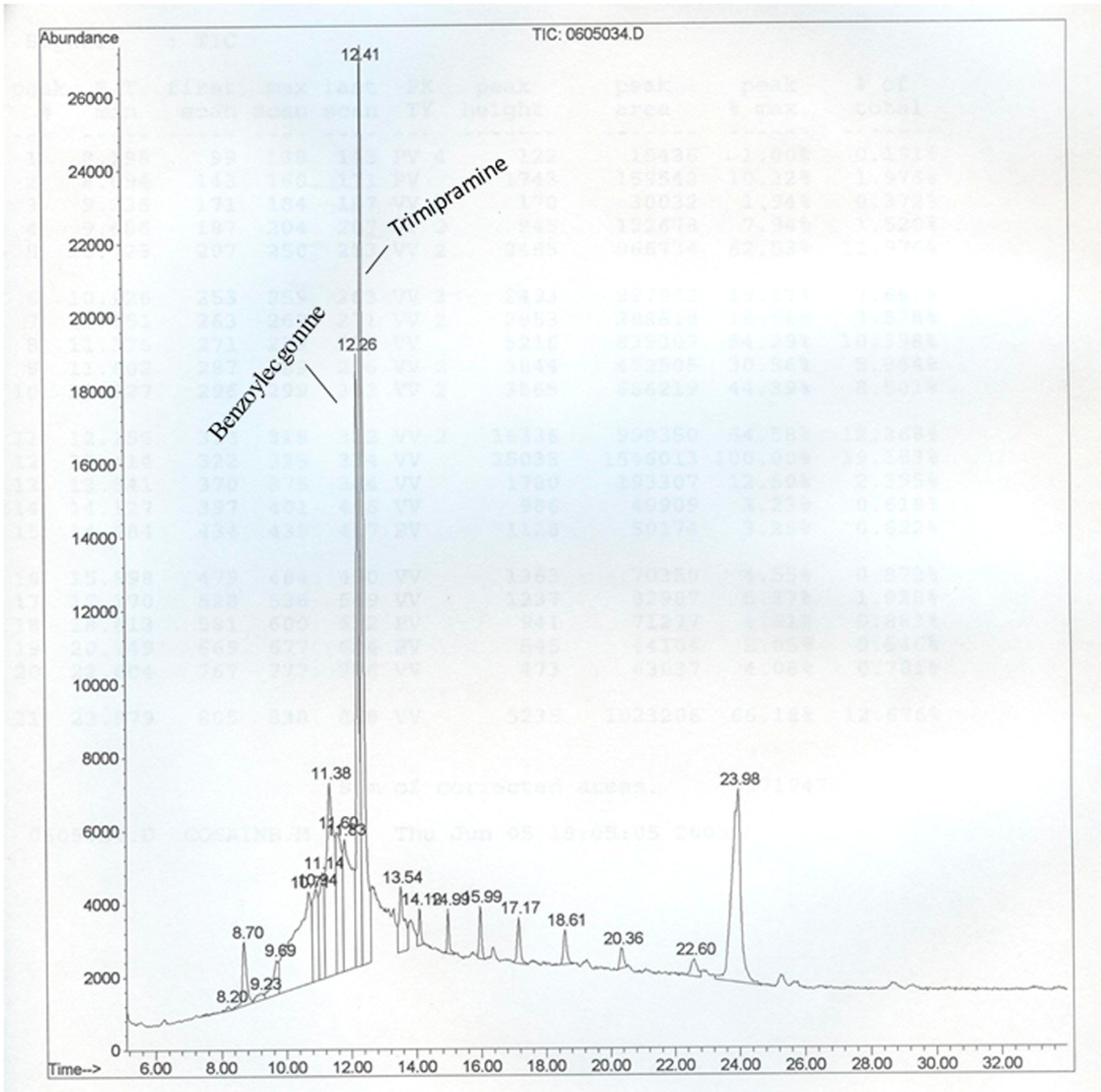
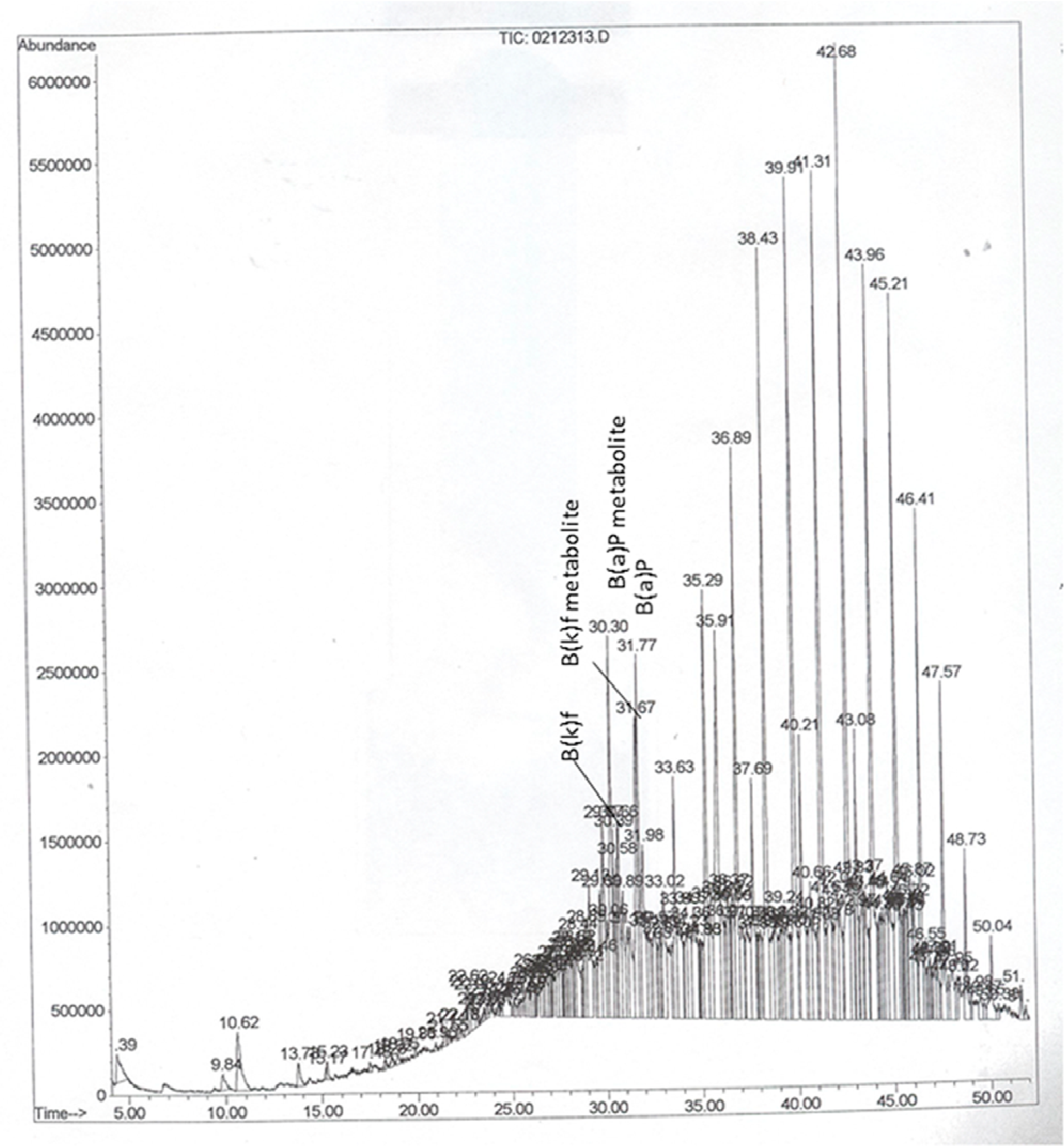
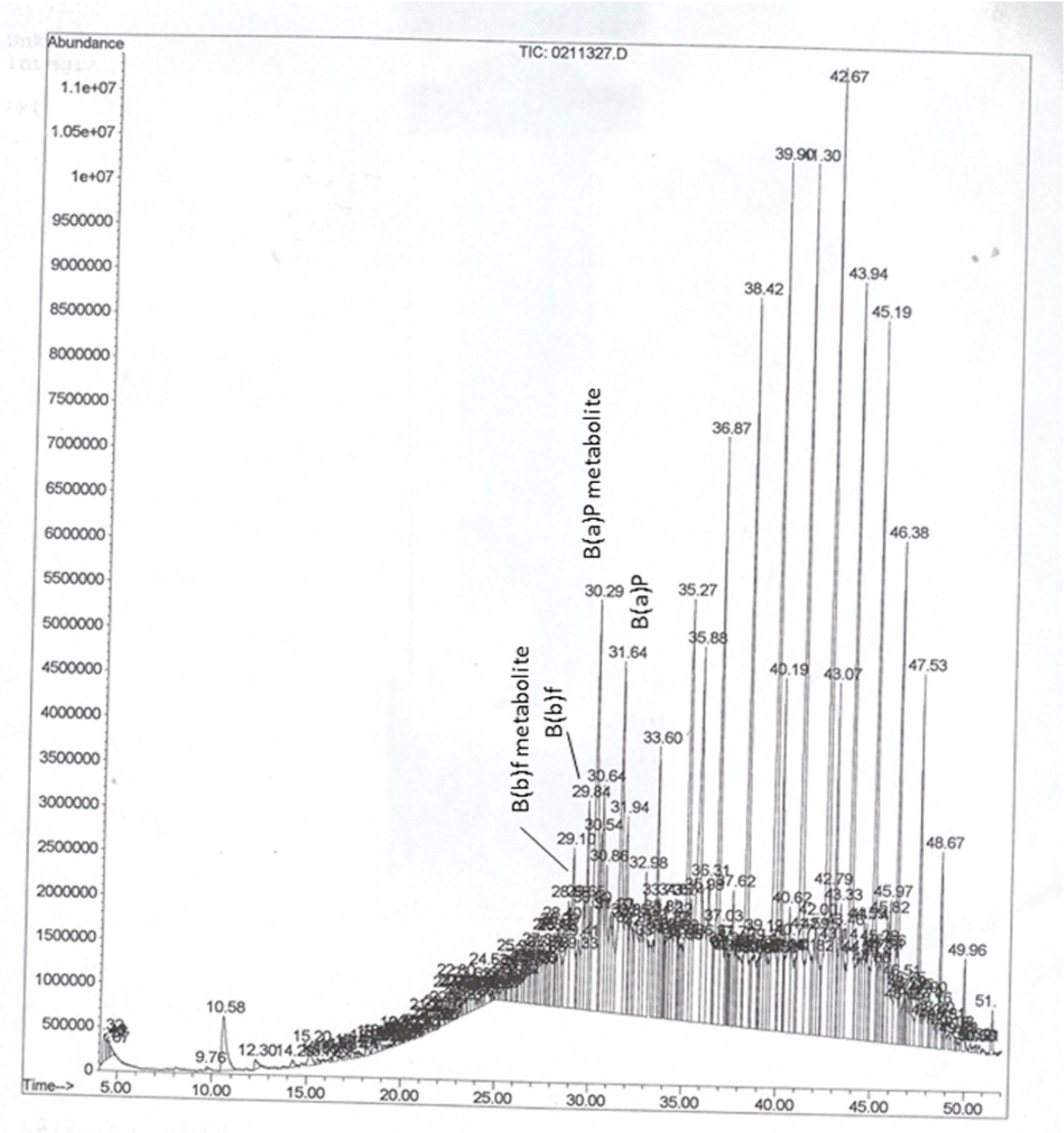
2.4. Instrumentation and Chromatographic Conditions
3. Results and Discussion
| Racial Groups | Children & Teens | Twenties & Thirties | Forties & Fifties Male | Sixties & above Female |
|---|---|---|---|---|
| Caucasian male | 5.2 ± 1.5 (3) | 4.5 ± 0.76 (25) | 4.4 ± 0.55 (39) | 3.4 ± 0.96 (14) |
| Caucasian female | 5.2 ± 1.2 (9) | 4.4 ± 1.3 (14) | 6.2 ± 2.0 (23) | 3.9 ± 1.7 (5) |
| African American male | 6.8 ± 2.1 (9) | 4.5 ± 0.39 (54) | 5.2 ± 0.98 (28) | 5.0 ± 1.3 (8) |
| African American female | 5.3 ± 2.5 (7) | 6.1 ± 0.87 (39) | 9.0 ± 1.84 (13) | 6.8 (2) |
| Hispanic male | ND | 3.4 ± 0.78 (5) | 7.5 (1) | ND |
| Hispanic female | ND | ND | ND | ND |
| Asian male | 12.8 (1) | 9.1 (1) | ND | ND |
| Asian female | ND | ND | ND | ND |
| Racial Groups | Children & Teens | Twenties & Thirties | Forties & Fifties | Sixties & Above | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B(a)P | B(b)f | Others & B(k)f | B(a)P | B(b)f | Others & B(k)f | B(a)P | B(b)f | Others & B(k)f | B(a)P | B(b)f | Others & B(k)f | |
| Caucasian male | 60 | 40 | 0 | 60 | 35 | 5 | 60 | 35 | 5 | 60 | 35 | 5 |
| Caucasian female | 50 | 45 | 5 | 50 | 45 | 5 | 50 | 45 | 5 | 50 | 50 | 0 |
| African American male | 60 | 40 | 0 | 60 | 40 | 0 | 60 | 40 | 0 | 60 | 40 | 0 |
| African American female | 50 | 50 | 0 | 50 | 47 | 3 | 50 | 45 | 5 | 50 | 45 | 5 |
| Hispanic male | ND | ND | ND | 55 | 45 | 0 | 55 | 45 | 0 | ND | ND | ND |
| Hispanic female | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| Asian male | 50 | 47 | 3 | 50 | 45 | 5 | ND | ND | ND | ND | ND | ND |
| Asian female | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO. IARC Monographs on the evaluation of carcinogenic risks to humans. In Some Non-Heterocyclic Polycyclic Aromatic Hydrocarbons and Some Related Exposures; WHO: Lyon, France, 2010; Volume 92, p. 853. [Google Scholar]
- Choi, H.; Spengler, J. Source attribution of personal exposure to airborne polycyclic aromatic hydrocarbon mixture using concurrent personal, indoor, and outdoor measurements. Environ. Int. 2014, 63, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Walker, S.A.; Hood, D.B.; Guillen, M.D.; Schneider, H.; Weyand, E.H. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 2004, 23, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Archibong, A.E.; Hood, D.B.; Guo, Z.; Loganathan, B.G. Global environmental distribution and human health effects of polycyclic aromatic hydrocarbons. In Global Contamination Trends of Persistent Organic Chemicals; Loganathan, B.G., Lam, P.K.-S., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 95–124. [Google Scholar]
- Aquilina, N.J.; Delgado-Saborit, J.M.; Meddings, C.; Baker, S.; Harrison, R.M.; Jacob, P., 3rd; Wilson, M.; Yu, L.; Duan, M.; Benowitz, N.L. Environmental and biological monitoring of exposures to PAHs and ETS in the general population. Environ. Int. 2010, 36, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Eberwein, G.; Hölzer, J.; Gladtke, D.; Angerer, J.; Marczynski, B.; Behrendt, H.; Ring, J.; Sugiri, D.; Ranft, U. Influence of industrial sources on children’s health—Hot spot studies in North Rhine Westphalia, Germany. Int. J. Hyg. Environ. Health 2007, 210, 591–599. [Google Scholar] [CrossRef] [PubMed]
- ATSDR. Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs); U.S. Department of Health and Human Services, U.S. Public Health Service, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1995; p. 271. [Google Scholar]
- Takahashi, G.; Kinoshita, K.; Hashimoto, K.; Yasuhira, K. Identification of benzo(a)pyrene metabolites by gas chromatograph-mass spectrometer. Cancer Res. 1979, 39, 1814–1818. [Google Scholar] [PubMed]
- Lee, W.; Shin, H.S.; Hong, J.E.; Pyo, H.; Kim, Y. Studies on the analysis of benzo(a)pyrene and its metabolites in biological samples by using high performance liquid chromatography-fluorescence detection and gas chromatography/mass spectrometry. Bull. Korean Chem. Soc. 2003, 24, 559–565. [Google Scholar] [CrossRef]
- Duarte-Salles, T.; Mendez, M.A.; Pessoa, V.; Guxens, M.; Aguilera, I.; Kogevinas, M.; Sunyer, J. Smoking during pregnancy is associated with higher dietary intake of polycyclic aromatic hydrocarbons and poor diet quality. Public Health Nutr. 2010, 13, 2034–2043. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Vinyals, G.; D’Errico, A.; Malats, N.; Kogevinas, M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup. Environ. Med. 2004, 61. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, J.; Carmella, S.G.; Hochalter, J.B.; Rauch, D.; Oliver, A.; Jensen, J.; Hatsukami, D.K.; Upadhyaya, P.; Zimmerman, C.; et al. Metabolism of [D10] phenanthrene to tetraols in smokers for potential lung cancer susceptibility assessment: Comparison of oral and inhalation routes of administration. J. Pharmacol. Exp. Ther. 2011, 338, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, A.; Moorthy, B.; Ghose, R. Drug disposition in pathophysiological conditions. Curr. Drug Metab. 2012, 9, 1327–1344. [Google Scholar] [CrossRef]
- Cook, D.S.; Braithwaite, R.A.; Hale, K.A. Estimating antemortem drug concentrations from postmortem blood samples: The influence of postmortem redistribution. J. Clin. Pathol. 2000, 53, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Richardson, T. Pitfalls in forensic toxicology. Ann. Clin. Biochem. 2000, 37, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Linnet, K. Two stage transformation systems for normalization of reference distributions evaluated. Clin. Chem. 1987, 33, 381–386. [Google Scholar] [PubMed]
- Grainger, J.; Huang, W.; Patterson, D.G., Jr.; Turner, W.E.; Pirkle, J.; Caudill, S.P.; Wang, R.Y.; Needham, L.L.; Sampson, E.J. Reference range levels of polycyclic aromatic hydrocarbons in the U.S. population by measurement of urinary monohydroxy metabolites. Environ. Res. 2006, 100, 394–423. [Google Scholar] [CrossRef] [PubMed]
- Seto, H.; Ohkubo, T.; Kanoh, T.; Koike, M.; Nakamura, K.; Kawahara, Y. Determination of polycyclic aromatic hydrocarbons in the lung. Arch. Environ. Contam. Toxicol. 1993, 24, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Beach, J.B.; Pellizzari, E.; Keever, J.T.; Ellis, L. Determination of benzo[a]pyrene and other polycyclic aromatic hydrocarbons (PAHs) at trace levels in human tissues. J. Anal. Toxicol. 2000, 24, 670–677. [Google Scholar] [CrossRef]
- Gitipour, S.; Firouzbakht, S.; Mirzaee, E.; Alimohammadi, M. Assessment of soil screening levels due to ingestion and dermal absorption of chrysene and benzo[k]fluoranthene and appropriate remediation method for Dorson Abad. Environ. Monit. Assess. 2014, 186, 3541–3552. [Google Scholar] [CrossRef] [PubMed]
- Spink, D.C.; Wu, S.J.; Spink, B.C.; Hussain, M.M.; Vakharia, D.D.; Pentecost, B.T.; Kaminsky, L.S. Induction of CYP1A1 and CYP1B1 by benzo(k)fluoranthene and benzo(a)pyrene in T-47D human breast cancer cells: Roles of PAH interactions and PAH metabolites. Toxicol. Appl. Pharmacol. 2008, 226, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Liu, B.; Lovinsky-Desir, S.; Yan, B.; Camann, D.; Sjodin, A.; Li, Z.; Perera, F.; Kinney, P.; Chillrud, S.; et al. Time trends of polycyclic aromatic hydrocarbon exposure in New York City from 2001 to 2012: Assessed by repeat air and urine samples. Environ. Res. 2014, 131, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Merlo, F.; Bolognesi, C.; Peluso, M.; Valerio, F.; Abbondandolo, A.; Puntoni, R. Airborne levels of polycyclic aromatic hydrocarbons: 32P-postlabeling DNA adducts and micronuclei in white blood cells from traffic police workers and urban residents. J. Environ. Pathol. Toxicol. Oncol. 1997, 16, 157–162. [Google Scholar] [PubMed]
- Qin, Y.Y.; Leung, C.K.; Lin, C.K.; Leung, A.O.; Wang, H.S.; Giesy, J.P.; Wong, M.H. Halogenated POPs and PAHs in blood plasma of Hong Kong residents. Environ. Sci. Technol. 2011, 45, 1630–1637. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.G.; Li, Y.; O’Sullivan, M.M.; Demopoulos, R.; Garte, S.; Taioli, E.; Brandt-Rauf, P.W. Glutathione S-transferase M1 polymorphism and lung cancer risk in African-Americans. Carcinogenesis 2000, 21, 1971–1975. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J.; Chung, D.; Mahmoud, N.; Sepulveda, A.R.; Manafe, M.; Arch, J.; Adada, H.; van der Merwe, T. Why do African Americans get more colon cancer than Native Africans? J. Nutr. 2007, 137, S175–S182. [Google Scholar]
- Tang, D.; Kryvenko, O.N.; Wang, Y.; Jankowski, M.; Trudeau, S.; Rundle, A.; Rybicki, B.A. Elevated polycyclic aromatic hydrocarbon-DNA adducts in benign prostate and risk of prostate cancer in African Americans. Carcinogenesis 2013, 34, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Steck, S.E.; Butler, L.M.; Keku, T.; Antwi, S.; Galanko, J.; Sandler, R.S.; Hu, J.J. Nucleotide excision repair gene polymorphisms, meat intake and colon cancer risk. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 762, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Tsang, H.L.; Wu, S.; Leung, C.K.; Tao, S.; Wong, M.H. Body burden of POPs of Hong Kong residents, based on human milk, maternal and cord serum. Environ. Int. 2011, 37, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chanock, S.; Tang, D.; Li, Z.; Edwards, S.; Jedrychowski, W.; Perera, F.P. Effect of gene-environment Interactions on mental development in African American, Dominican, and Caucasian mothers and newborns. Ann. Hum. Genet. 2010, 74, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Neal, M.S.; Zhu, J.; Foster, W.G. Quantification of benzo[a]pyrene and other PAHs in the serum and follicular fluid of smokers versus non-smokers. Reprod. Toxicol. 2008, 25, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Pleil, J.D.; Stiegel, M.A.; Sobus, J.R.; Tabucchi, S.; Ghio, A.J.; Madden, M.C. Cumulative exposure assessment for trace-level polycyclic aromatic hydrocarbons (PAHs) using human blood and plasma analysis. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2010, 878, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Alokail, M.S.; Abd-Alrahman, S.H.; Draz, H.M. Polycyclic aromatic hydrocarbon distribution in serum of Saudi children using HPLC-FLD: Marker elevations in children with asthma. Environ. Sci. Pollut. Res. Int. 2014, 20, 12085–12090. [Google Scholar] [CrossRef]
- Hussar, E.; Richards, S.; Lin, Z.Q.; Dixon, R.P.; Johnson, K.A. Human health risk assessment of 16 priority polycyclic aromatic hydrocarbons in soils of Chattanooga, Tennessee, USA. Water Air Soil Pollut. 2012, 223, 5535–5548. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yamaguchi, K.; Lee, S.H.; Tithof, P.K.; Sayler, G.S.; Yoon, J.H.; Baek, S.J. Evaluation of polycyclic aromatic hydrocarbons in the activation of early growth response-1 and peroxisome proliferator activated receptors. Toxicol. Sci. 2005, 85, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.D.; Ladd, D.E.; Farmer, J.J. Fate and Transport of Petroleum Hydrocarbons in Soil and Ground Water at Big South Fork National River and Recreation Area, Tennessee and Kentucky, 2002–2003; U.S. Geological Survey Scientific Investigations Report 2005-5104; U.S. Geological Survey: Reston, VA, USA, 2006; p. 29. [Google Scholar]
- Wang, N.; Ingersoll, C.G.; Kunz, J.L.; Brumbaugh, W.G.; Kane, C.M.; Evans, R.B.; Alexander, S.; Walker, C.; Bakaletz, S. Toxicity of sediments potentially contaminated by coal mining and natural gas extraction to unionid mussels and commonly tested benthic invertebrates. Environ. Toxicol. Chem. 2013, 32, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Ruhl, L.; Vengosh, A.; Dwyer, G.S.; Hsu-Kim, H.; Deonarine, A.; Bergin, M.; Kravchenko, J. Survey of the potential environmental and health impacts in the immediate aftermath of the coal ash spill in Kingston, Tennessee. Environ. Sci. Technol. 2009, 43, 6326–6333. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Shrubsole, M.J.; Li, G.; Smalley, W.E.; Hein, D.W.; Cai, Q.; Ness, R.M.; Zheng, W. Interaction of cigarette smoking and carcinogen-metabolizing polymorphisms in the risk of colorectal polyps. Carcinogenesis 2013, 34, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Shrubsole, M.J.; Smalley, W.E.; Wu, H.; Chen, Z.; Shyr, Y.; Ness, R.M.; Zheng, W. Association of meat intake and meat-derived mutagen exposure with the risk of colorectal polyps by histologic type. Cancer Prev. Res. 2011, 4, 1686–1697. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, A.; Kumar, A.; Aramandla, M.P.; Nyanda, A.M. Polycyclic Aromatic Hydrocarbon Residues in Serum Samples of Autopsied Individuals from Tennessee. Int. J. Environ. Res. Public Health 2015, 12, 322-334. https://doi.org/10.3390/ijerph120100322
Ramesh A, Kumar A, Aramandla MP, Nyanda AM. Polycyclic Aromatic Hydrocarbon Residues in Serum Samples of Autopsied Individuals from Tennessee. International Journal of Environmental Research and Public Health. 2015; 12(1):322-334. https://doi.org/10.3390/ijerph120100322
Chicago/Turabian StyleRamesh, Aramandla, Anil Kumar, Mounika P. Aramandla, and Alfred M. Nyanda. 2015. "Polycyclic Aromatic Hydrocarbon Residues in Serum Samples of Autopsied Individuals from Tennessee" International Journal of Environmental Research and Public Health 12, no. 1: 322-334. https://doi.org/10.3390/ijerph120100322





