Effects of OEF/OIF-Related Physical and Emotional Co-Morbidities on Associative Learning: Concurrent Delay and Trace Eyeblink Classical Conditioning
Abstract
:1. Introduction
| Demographic Variables | Overall | Deployed Control | PTSD 1 Only | Comorbid PTSD + mTBI 2 | mTBI Only | |
|---|---|---|---|---|---|---|
| Age * | N | 81 | 25 | 25 | 31 | 7 |
| Mean | 35.2 | 37.6 * | 37.3 * | 31.6 * | 34.4 | |
| S.E. | 1.1 | 2 | 2.1 | 1.4 | 3.2 | |
| Minimum | 20 | 23 | 21 | 20 | 22 | |
| Maximum | 62 | 62 | 58 | 46 | 46 | |
| Education 3 | N | 81 | 25 | 25 | 31 | 7 |
| Mean | 13.4 | 13.8 | 13.4 | 13 | 15.1 | |
| S.E. | 0.2 | 0.3 | 0.3 | 0.3 | 1 | |
| Minimum | 12 | 12 | 12 | 12 | 12 | |
| Maximum | 17 | 17 | 16 | 17 | 19 | |
| WTAR/ Estimated IQ 4,5 | N | 80 | 25 | 25 | 30 | 7 |
| Mean | 97.6 | 100.1 | 96.5 | 96.4 | 100.6 | |
| S.E. | 1.2 | 2.3 | 2.7 | 1.5 | 3.6 | |
| Minimum | 66 | 75 | 66 5 | 83 | 91 | |
| Maximum | 123 | 123 | 121 | 114 | 119 | |
| Gender * | Male | 77 | 22 | 18 | 30 | 7 |
| Female | 11 | 3 | 7 | 1 | 0 | |
| Ethnicity/ Race | Black or African American | 4 | 2 | 1 | 1 | 0 |
| Hispanic or Latino | 9 | 5 | 1 | 2 | 1 | |
| American Indian | 1 | 0 | 1 | 0 | 0 | |
| Asian | 1 | 0 | 1 | 0 | 0 | |
| White | 72 | 18 | 21 | 27 | 6 | |
| Smoking 6 | Smokers | 23 | 4 | 8 | 11 | 1 |
| Non-smokers | 62 | 20 | 17 | 19 | 6 | |
| Gender: | Frequency | Percent |
|---|---|---|
| Male | 70 | 86.4% |
| Female | 11 | 13.6% |
| Ethnicity/Race | ||
| Black or African American | 4 | 4.9% |
| Hispanic or Latino | 9 | 9.9% |
| American Indian | 1 | 1.2% |
| Asian | 1 | 1.2% |
| White | 66 | 81.5% |
| Deployment Variables | Overall | Deployed Control | PTSD 1 Only | Comorbid PTSD + mTBI 2 | mTBI Only | |
|---|---|---|---|---|---|---|
| Number of OEF/OIF Deployments | N | 81 | 25 | 25 | 31 | 7 |
| Mean | 1.28 | 1.36 | 1.32 | 1.19 | 1.43 | |
| Median | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |
| S.E. | 0.056 | 0.114 | 0.111 | 0.072 | 0.202 | |
| Minimum | 1 | 1 | 1 | 1 | 1 | |
| Maximum | 3 | 3 | 3 | 2 | 2 | |
| Total Duration of Deployments (months) | N | 81 | 25 | 25 | 31 | 7 |
| Mean | 13.94 | 15.28 | 14.20 | 12.65 | 16.29 | |
| Median | 12.00 | 12.00 | 12.00 | 12.00 | 14.00 | |
| S.E. | 0.852 | 1.618 | 1.579 | 1.285 | 3.708 | |
| Minimum | 3 | 3 | 5 | 4 | 3 | |
| Maximum | 38 | 29 | 34 | 38 | 28 | |
| Time Since Last Deployment (months) | N | 79 | 25 | 25 | 31 | 7 |
| Mean | 30.652 | 24.20 | 31.04 | 36.16 | 20.57 | |
| Median | 24.00 | 22.00 | 34.00 | 31.00 | 25.00 | |
| S.E. | 2.842 | 3.985 | 4.738 | 5.226 | 4.674 | |
| Minimum | 1 | 1 | 2 | 1 | 3 | |
| Maximum | 99 | 80 | 75 | 99 | 34 | |
| Physical/Emotional Variables | Overall | Deployed Control | PTSD 1 Only | Comorbid PTSD + mTBI 2 | mTBI Only | |
|---|---|---|---|---|---|---|
| Number of Blast Exposures | N | 81 | 25 | 25 | 31 | 7 |
| Median | 2.00 | 1.00 | 2.00 | 5.00 | 4.00 | |
| S.E. | 2.936 | 2.631 | 8.322 | 3.081 | 72.360 | |
| Minimum | 0 | 0 | 0 | 0 | 1 | |
| Maximum | 180 | 60 | 180 | 61 | 511 | |
| Number of mTBI (blast or blunt) | N | 81 | 25 | 25 | 31 | 7 |
| Median | 0.0 | 0.0 | 0.0 | 1.00 | 1.00 | |
| S.E. | 0.095 | 0.0 | 0.0 | 0.138 | 0.143 | |
| Minimum | 0 | 0.0 | 0.0 | 1 | 1 | |
| Maximum | 3 | 0.0 | 0.0 | 3 | 2 | |
| CAPS 3 Current *** | N | 81 | 25 | 25 | 31 | 7 |
| Mean | 51.75 | 17.52 *** | 64.92 *** | 68.74 *** | 28.14 | |
| S.E. | 3.185 | 2.106 | 3.956 | 3.457 | 7.551 | |
| Minimum | 2 | 2 | 33 | 38 | 6 | |
| Maximum | 114 | 44 | 102 | 114 | 63 | |
| DASS 4 *** | N | 81 | 25 | 25 | 31 | 7 |
| Mean | 30.14 | 9.28 *** | 40.16 *** | 41.35 *** | 19.14 | |
| S.E. | 2.873 | 2.091 | 5.694 | 4.4861 | 10.10 | |
| Minimum | 0 | 0 | 8 | 4 | 0 | |
| Maximum | 122 | 34 | 122 | 94 | 78 | |
| SMAST 5,† (Past 12 months) | N | 79 | 25 | 25 | 29 | 6 |
| Mean | 1.59 | 0.64 † | 2.12 † | 1.97 † | 0.50 | |
| S.E. | 0.297 | 0.305 | 0.667 | 0.482 | 0.342 | |
| Minimum | 0 | 0 | 0 | 0 | 0 | |
| Maximum | 12 | 7 | 12 | 11 | 2 | |
| SMAST (Lifetime) | N | 51 | 15 | 16 | 20 | 4 |
| Mean | 2.73 | 2.60 | 3.75 | 2.00 | 1.75 | |
| S.E. | 0.482 | 0.920 | 1.101 | 0.503 | 0.479 | |
| Minimum | 0 | 0 | 0 | 0 | 1 | |
| Maximum | 13 | 13 | 12 | 8 | 3 | |
| LDH 6 Total | N | 77 | 25 | 24 | 28 | 7 |
| Mean | 9,377.62 | 9,269.84 | 10,622.60 | 8,406.71 | 6,588.13 | |
| S.E. | 1,201.22 | 2,052.92 | 2,285.66 | 1,981.29 | 1,723.93 | |
| Minimum | 0 | 49.00 | 439.00 | 0.00 | 730.00 | |
| Maximum | 51,538.00 | 35,622.0 | 39,010.00 | 51,538.00 | 14,577.00 | |
2. Experimental Section
2.1. Participants
2.2. Procedures
2.2.1. TBI Diagnosis
2.2.2. PTSD Assessment
2.2.3. Alcohol Assessment
2.2.4. Substance Use Disorders
2.2.5. Concurrent Delay/Trace Eyeblink Classical Conditioning

3. Results and Discussion
3.1. Acquisition of Concurrent Delay/Trace Eyeblink Classical Conditioning

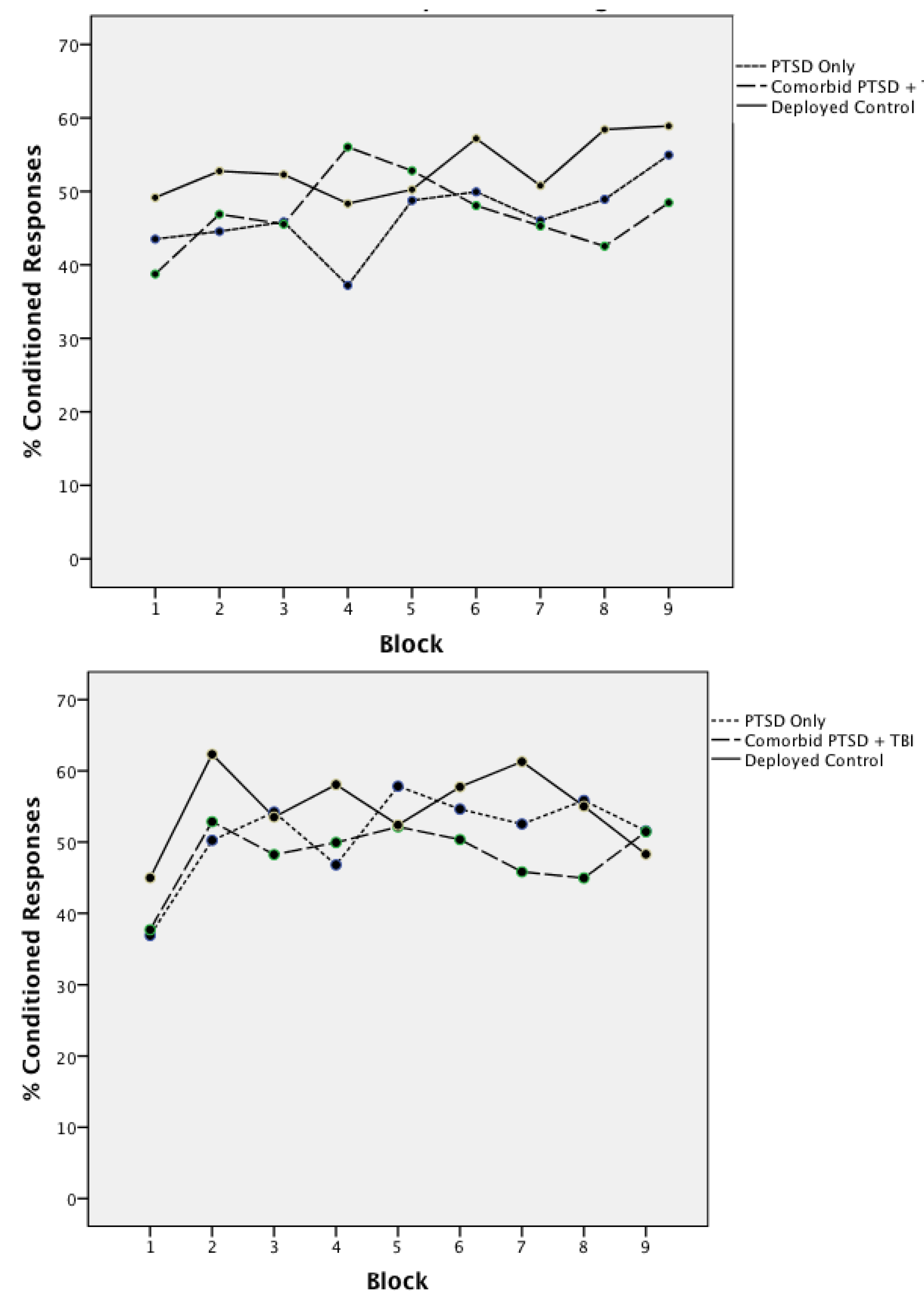
| Mean | Standard Error | F(2,80) | |
|---|---|---|---|
| Delay CR 1 Onset | 340.15 | 9.044 | 0.09 |
| Delay CR Peak Latency | 462.77 | 9.074 | 0.22 |
| Delay CR Amplitude | 26.05 | 1.72 | 0.73 |
| Delay UR 2 Amplitude | 43.36 | 1.89 | 0.08 |
| Delay Alpha Responses | 6.30 | 0.61 | 0.23 |
| Trace CR Onset | 344.03 | 9.80 | 0.35 |
| Trace CR Peak latency | 481.52 | 10.16 | 0.20 |
| Trace CR Amplitude | 29.24 | 1.81 | 0.82 |
| Trace UR Amplitude | 42.39 | 1.80 | 0.10 |
| Trace Alpha Responses | 6.21 | 0.55 | 0.58 |
3.2. Extinction of Concurrent Delay/Trace Eyeblink Classical Conditioning
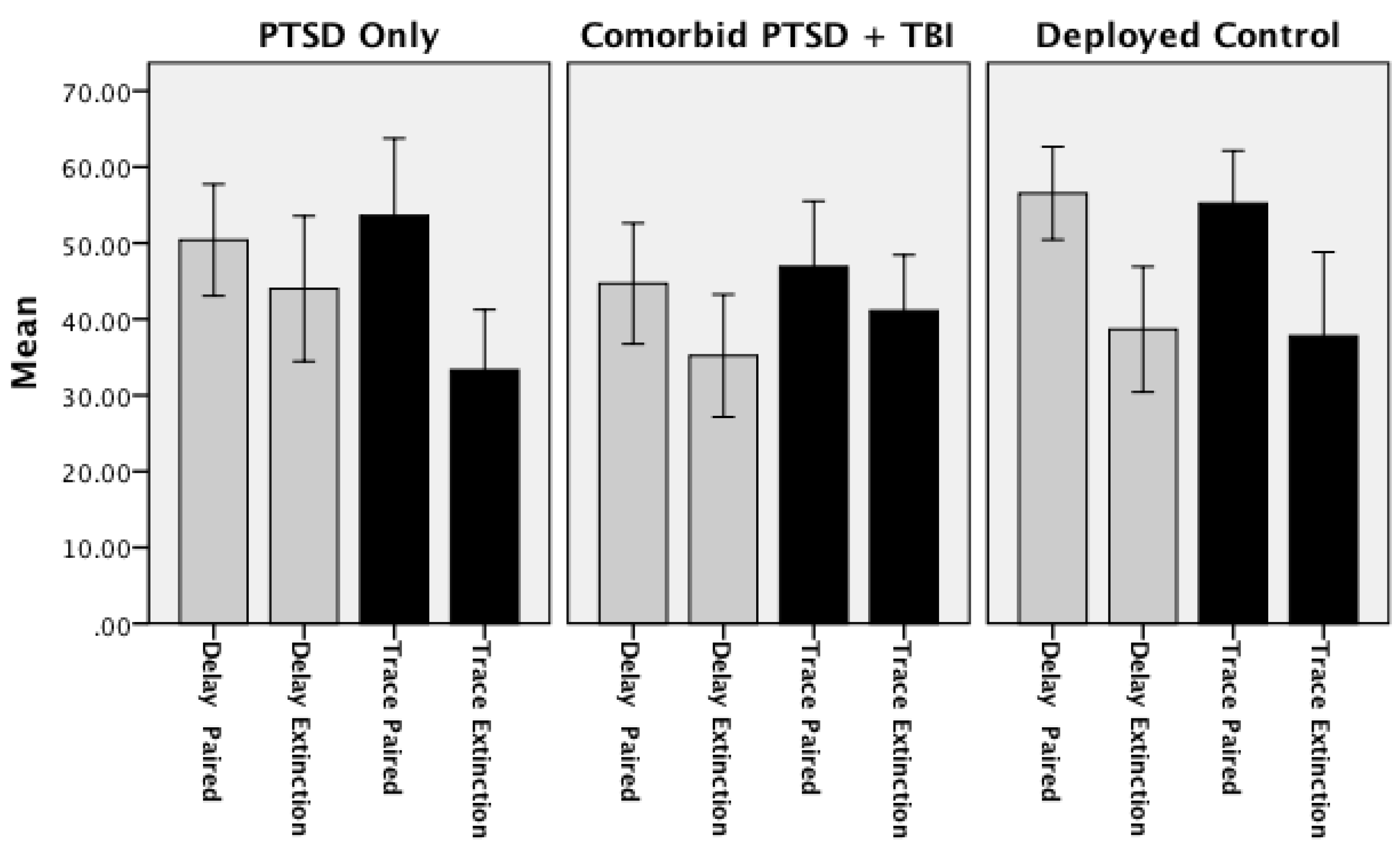
| Delay Conditioning | Trace Conditioning | |||
|---|---|---|---|---|
| Final 9 Paired Trials | Extinction Trials | Final 9 Paired Trials | Extinction Trials | |
| Deployed Controls *** | 58.22 | 38.67 *** | 46.22 | 37.78 |
| (3.86) | (4.11) | (4.04) | (5.52) | |
| PTSD Only †,* | 53.33 | 44.00 † | 49.33 | 33.33 * |
| (3.14) | (4.78) | (5.63) | (3.95) | |
| Comorbid PTSD/TBI * | 45.16 * | 35.48 | 41.21 | 40.86 |
| (4.15) | (3.90) | (4.87) | (3.55) | |
3.3. Associations between Conditioning Performance with Physical and Emotional Measures of Trauma and Deployment Statistics
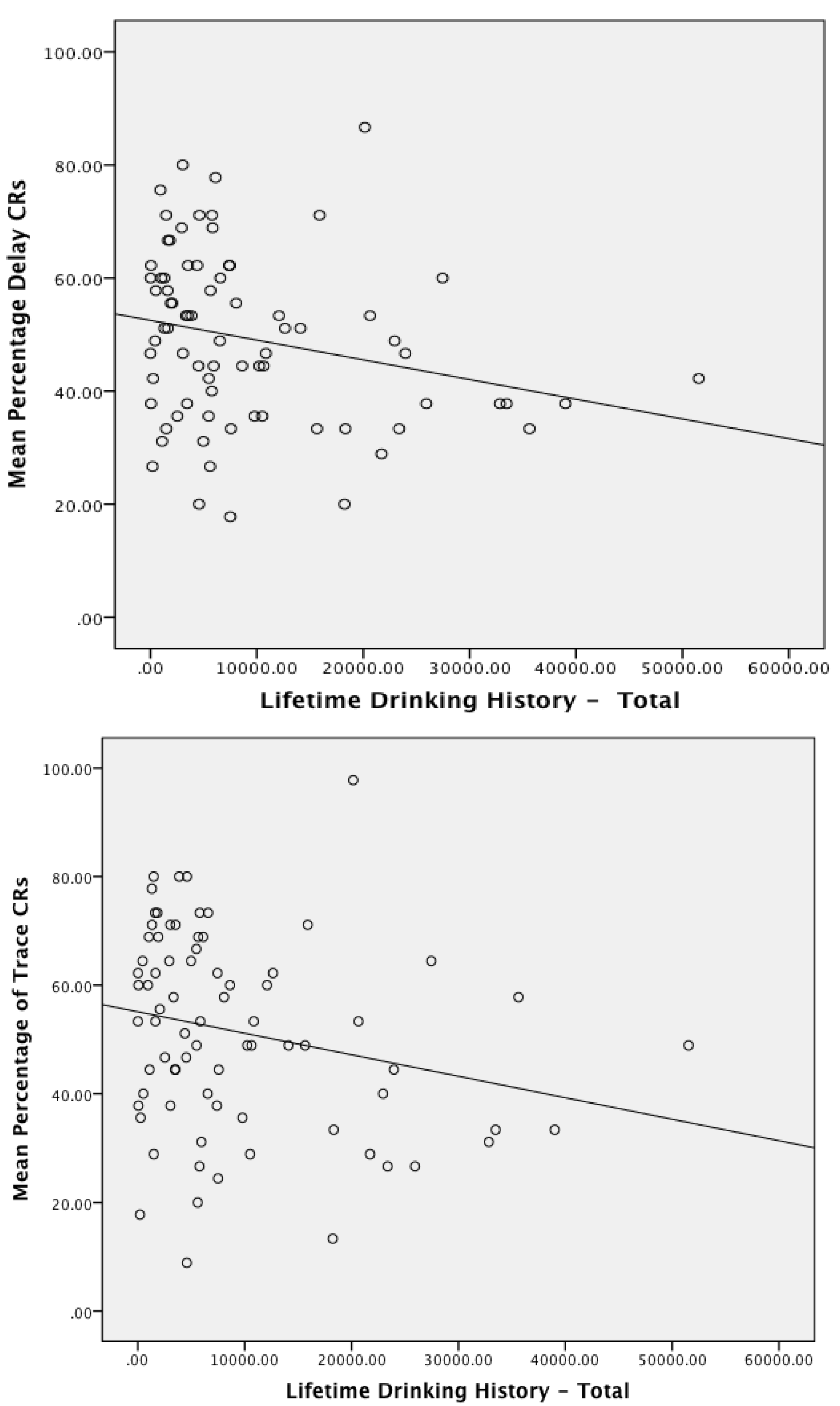
3.4. Acquisition of Delay and Trace Conditioned Responses
3.5. Extinction of Delay and Trace Conditioned Responses
3.6. Association of Alcohol Use and Performance on Eyeblink Classical Conditioning
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vythilingam, M.; Lawley, M.; Collin, C.; Bonne, O.; Agarwal, R.; Hadd, K.; Charney, D.S.; Grillon, C. Hydrocortisone impairs hippocampal-dependent trace eyeblink conditioning in post-traumatic stress disorder. Neuropsychopharmacology 2006, 31, 182–188. [Google Scholar]
- Woodruff-Pak, D.S.; Steinmetz, J.E. Eyeblink Classical Conditioning: Volume I—Applications in Humans; Kluwer Academic Publishers: Boston, MA, USA, 2000. [Google Scholar]
- Tanielian, T.; Jaycox, L.H. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery; RAND Corp: Santa Monica, CA, USA, 2008. [Google Scholar]
- Stein, M.B.; McAllister, T.W. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Amer. J. Psychiat. 2009, 166, 768–776. [Google Scholar] [CrossRef]
- Vasterling, J.J.; Verfaellie, M.; Sullivan, K.D. Mild traumatic brain injury and posttraumatic stress disorder in returning veterans: Perspectives from cognitive neuroscience. Clin. Psychol. Rev. 2009, 29, 674–684. [Google Scholar] [CrossRef]
- Vanderploeg, R.D.; Belanger, H.G.; Curtiss, G. Mild traumatic brain injury and posttraumatic stress disorder and their associations with health symptoms. Arch. Phys. Med. Rehabil. 2009, 90, 1084–1093. [Google Scholar] [CrossRef]
- Kim, E.; Lauterbach, E.C.; Reeve, A.; Arciniegas, D.B.; Coburn, K.L.; Mendez, M.F.; Rummans, T.A.; Coffey, E.C. Neuropsychiatric complications of traumatic brain injury: A critical review of the literature (a report by the ANPA committee on research). J. NNeuropsychiatr. Clin. Neurosc. 2007, 19, 106–127. [Google Scholar] [CrossRef]
- National, R.C. Long-term Consequences of Traumatic Brain Injury. In Gulf War and Health; The National Academies Press: Washington, D.C., USA, 2008; Volume 7. [Google Scholar]
- Graham, D.P.; Cardon, A.L. An update on substance use and treatment following traumatic brain injury. Ann. N. Y. Acad. Sci. 2008, 1141, 148–162. [Google Scholar] [CrossRef]
- Mills, K.L.; Teesson, M.; Ross, J.; Peters, L. Trauma, ptsd, and substance use disorders: Findings from the australian national survey of mental health and well-being. Amer. J. Psychiat. 2006, 163, 652–658. [Google Scholar] [CrossRef]
- Desai, R.A.; Dausey, D.; Rosenheck, R.A. Suicide among discharged psychiatric inpatients in the department of veterans affairs. Mil. Med. 2008, 173, 721–728. [Google Scholar]
- Gutierrez, P.M.; Brenner, L.A.; Huggins, J.A. A preliminary investigation of suicidality in psychiatrically hospitalized veterans with traumatic brain injury. Arch. Suicide Res. 2008, 12, 336–343. [Google Scholar] [CrossRef]
- Warden, D. Military tbi during the iraq and afghanistan wars. J. Head Trauma Rehabil. 2006, 21, 398–402. [Google Scholar] [CrossRef]
- Murray, C.K.; Reynolds, J.C.; Schroeder, J.M.; Harrison, M.B.; Evans, O.M.; Hospenthal, D.R. Spectrum of care provided at an echelon ii medical unit during operation iraqi freedom. Mil. Med. 2005, 170, 516–520. [Google Scholar]
- Morrow, C.E.; Bryan, C.J.; Isler, W.C. Concussive and psychological symptom predictors of aeromedical evacuation following possible brain injury among deployed military personnel. Psychol. Serv. 2011, 8, 224–235. [Google Scholar] [CrossRef]
- Schneiderman, A.I.; Braver, E.R.; Kang, H.K. Understanding sequelae of injury mechanisms and mild traumatic brain injury incurred during the conflicts in iraq and afghanistan: Persistent postconcussive symptoms and posttraumatic stress disorder. Amer. J. Epidemiol. 2008, 167, 1446–1452. [Google Scholar] [CrossRef]
- Terrio, H.; Brenner, L.A.; Ivins, B.J.; Cho, J.M.; Helmick, K.; Schwab, K.; Scally, K.; Bretthauer, R.; Warden, D. Traumatic brain injury screening: Preliminary findings in a U.S. army brigade combat team. J. Head Trauma Rehabil. 2009, 24, 14–23. [Google Scholar] [CrossRef]
- Hoge, C.W.; Castro, C.A.; Messer, S.C.; McGurk, D.; Cotting, D.I.; Koffman, R.L. Combat duty in iraq and afghanistan, mental health problems, and barriers to care. N. Engl. J. Med. 2004, 351, 13–22. [Google Scholar] [CrossRef]
- Vasterling, J.J.; Proctor, S.P.; Amoroso, P.; Kane, R.; Heeren, T.; White, R.F. Neuropsychological outcomes of army personnel following deployment to the iraq war. JAMA 2006, 296, 519–529. [Google Scholar] [CrossRef]
- Atkinson, M.P.; Guetz, A.; Wein, L.M. A dynamic model for post traumatic stress disorder among us troops in operation iraqi freedom. Manag. Sci. 2009, 55, 1454–1468. [Google Scholar] [CrossRef]
- Hoge, C.W.; McGurk, D.; Thomas, J.L.; Cox, A.L.; Engel, C.C.; Castro, C.A. Mild traumatic brain injury in U.S. Soldiers returning from iraq. N. Engl. J. Med. 2008, 358, 453–463. [Google Scholar] [CrossRef]
- Harvey, A.G.; Bryant, R.A. Two-year prospective evaluation of the relationship between acute stress disorder and posttraumatic stress disorder following mild traumatic brain injury. Amer. J. Psychiat. 2000, 157, 626–628. [Google Scholar] [CrossRef]
- Chemtob, C.M.; Muraoka, M.Y.; Wu-Holt, P.; Fairbank, J.A.; Hamada, R.S.; Keane, T.M. Head injury and combat-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 1998, 186, 701–708. [Google Scholar] [CrossRef]
- Lippa, S.M.; Pastorek, N.J.; Benge, J.F.; Thornton, G.M. Postconcussive symptoms after blast and nonblast-related mild traumatic brain injuries in afghanistan and iraq war veterans. J. Int. Neuropsychol. Soc. 2010, 16, 856–866. [Google Scholar] [CrossRef]
- Vasterling, J.J.; Dikmen, S. Mild traumatic brain injury and posttraumatic stress disorder: Clinical and conceptual complexities. J. Int. Neuropsychol. Soc. 2012, 18, 390–393. [Google Scholar] [CrossRef]
- Marx, B.P.; Brailey, K.; Proctor, S.P.; Macdonald, H.Z.; Graefe, A.C.; Amoroso, P.; Heeren, T.; Vasterling, J.J. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following iraq war deployment. Arch. Gen. Psychiat. 2009, 66, 996–1004. [Google Scholar] [CrossRef]
- Vasterling, J.J.; Brailey, K.; Proctor, S.P.; Kane, R.; Heeren, T.; Franz, M. Neuropsychological outcomes of mild traumatic brain injury, post-traumatic stress disorder and depression in iraq-deployed us army soldiers. Br. J. Psychiat. 2012, 201, 186–192. [Google Scholar] [CrossRef]
- Brenner, L.A.; Terrio, H.; Homaifar, B.Y.; Gutierrez, P.M.; Staves, P.J.; Harwood, J.E.; Reeves, D.; Adler, L.E.; Ivins, B.J.; Helmick, K.; et al. Neuropsychological test performance in soldiers with blast-related mild TBI. Neuropsychology 2010, 24, 160–167. [Google Scholar] [CrossRef]
- Cooper, D.B.; Mercado-Couch, J.M.; Critchfield, E.; Kennedy, J.; Vanderploeg, R.D.; DeVillibis, C.; Gaylord, K.M. Factors influencing cognitive functioning following mild traumatic brain injury in OIF/OEF burn patients. Neurorehabilitation 2010, 26, 233–238. [Google Scholar]
- Levin, H.S.; Wilde, E.; Troyanskaya, M.; Petersen, N.J.; Scheibel, R.; Newsome, M.; Radaideh, M.; Wu, T.; Yallampalli, R.; Chu, Z.; et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J. Neurotrauma 2010, 27, 683–694. [Google Scholar] [CrossRef]
- Nelson, L.A.; Yoash-Gantz, R.E.; Pickett, T.; Campbell, T.A. Relationship between processing speed and executive functioning performance among OIF/OEF veterans: Implications for postdeployment rehabilitation. J. Head Trauma Rehabil. 2009, 24, 32–40. [Google Scholar] [CrossRef]
- Thompson, R.F. The neurobiology of learning and memory. Science 1986, 233, 941–947. [Google Scholar]
- Thompson, R.F. The neural basis of basic associative learning of discrete behavioral responses. Trends Neurosci. 1988, 11, 152–155. [Google Scholar] [CrossRef]
- Kim, J.J.; Thompson, R.F. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997, 20, 177–181. [Google Scholar] [CrossRef]
- Raymond, J.L.; Lisberger, S.G.; Mauk, M.D. The cerebellum: A neuronal learning machine? Science 1996, 272, 1126–1131. [Google Scholar]
- Cheng, D.T.; Disterhoft, J.F.; Power, J.M.; Ellis, D.A.; Desmond, J.E. Neural substrates underlying human delay and trace eyeblink conditioning. Proc. Natl. Acad. Sci. USA 2008, 105, 8108–8113. [Google Scholar] [CrossRef]
- Weible, A.P.; McEchron, M.D.; Disterhoft, J.F. Cortical involvement in acquisition and extinction of trace eyeblink conditioning. Behav. Neurosci. 2000, 114, 1058–1067. [Google Scholar] [CrossRef]
- McLaughlin, J.; Skaggs, H.; Churchwell, J.; Powell, D.A. Medial prefrontal cortex and pavlovian conditioning: Trace versus delay conditioning. Behav. Neurosci. 2002, 11, 37–47. [Google Scholar]
- Arndt, T.L.; Stodgell, C.J.; Rodier, P.M. The teratology of autism. Int. J. Dev. Neurosci. 2005, 23, 189–199. [Google Scholar] [CrossRef]
- Bolbecker, A.R.; Mehta, C.S.; Edwards, C.R.; Steinmetz, J.E.; O’Donnell, B.F.; Hetrick, W.P. Eye-blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophr. Res. 2009, 111, 182–191. [Google Scholar] [CrossRef]
- Bolbecker, A.R.; Steinmetz, A.B.; Mehta, C.S.; Forsyth, J.K.; Klaunig, M.J.; Lazar, E.K.; Steinmetz, J.E.; O’Donnell, B.F.; Hetrick, W.P. Exploration of cerebellar-dependent associative learning in schizophrenia: Effects of varying and shifting interstimulus interval on eyeblink conditioning. Behav. Neurosci. 2011, 125, 687–698. [Google Scholar] [CrossRef]
- Brown, S.M.; Kieffaber, P.D.; Carroll, C.A.; Vohs, J.L.; Tracy, J.A.; Shekhar, A.; O’ÄôDonnell, B.F.; Steinmetz, J.E.; Hetrick, W.P. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cognition 2005, 58, 94–108. [Google Scholar] [CrossRef]
- Coffin, J.M.; Baroody, S.; Schneider, K.; O’Neill, J. Impaired cerebellar learning in children with prenatal alcohol exposure: A comparative study of eyeblink conditioning in children with adhd and dyslexia. Cortex 2005, 41, 389–398. [Google Scholar] [CrossRef]
- Edwards, C.R.; Newman, S.; Bismark, A.; Skosnik, P.D.; O’Donnell, B.F.; Shekhar, A.; Steinmetz, J.E.; Hetrick, W.P. Cerebellum volume and eyeblink conditioning in schizophrenia. Psychiatry Res. Neuroimaging 2008, 162, 185–194. [Google Scholar] [CrossRef]
- Fortier, C.; Maksimovskiy, A.L.; Venne, J.R.; LaFleche, G.; McGlinchey, R.E. Silent trace eliminates differential eyeblink learning in abstinent alcoholics. Int. J. Environ. Res. Public Health 2009, 6, 2007–2027. [Google Scholar] [CrossRef]
- Fortier, C.B.; Disterhoft, J.D.; McGlinchey-Berroth, R. Cerebellar cortical degeneration disrupts discrimination learning but not delay or trace eyeblink conditioning. Neuropsychology 2000, 14, 537–550. [Google Scholar] [CrossRef]
- Gabrieli, J.D.E.; McGlinchey-Berroth, R.; Carrillo, M.C.; Gluck, M.A.; Cermak, L.S.; Disterhoft, J.F. Intact delay-eyeblink classical conditioning in amnesia. Behav. Neurosci. 1995, 109, 819–827. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Stanton, M.E.; Dodge, N.C.; Pienaar, M.; Fuller, D.S.; Molteno, C.D.; Meintjes, E.M.; Hoyme, H.E.; Robinson, L.K.; Khaole, N.; et al. Impaired delay and trace eyeblink conditioning in school-age children with fetal alcohol syndrome. Alcohol. Clin. Exp. Res. 2011, 35, 250–264. [Google Scholar] [CrossRef]
- Jacobson, S.W.; Stanton, M.E.; Molteno, C.D.; Burden, M.J.; Fuller, D.S.; Hoyme, H.E.; Robinson, L.K.; Khaole, N.; Jacobson, J.L. Impaired eyeblink conditioning in children with fetal alcohol syndrome. Alcohol.: Clin. Exp. Res. 2008, 32, 365–372. [Google Scholar] [CrossRef]
- McGlinchey-Berroth, R.; Carrillo, M.C.; Gabrieli, J.D.E.; Brawn, C.M.; Disterhoft, J.F. Impaired trace eyeblink conditioning in bilateral medial temporal lobe amnesia. Behav. Neurosci. 1997, 111, 873–882. [Google Scholar] [CrossRef]
- McGlinchey-Berroth, R.; Cermak, L.S.; Carrillo, M.; Armfield, S.; Gabrieli, J.D.E.; Disterhoft, J.F. Impaired delay eyeblink conditioning in amnesic korsakoff’s patients and recovered alcoholics. Alcohol.: Clin. Exp. Res. 1995, 19, 1127–1132. [Google Scholar] [CrossRef]
- McGlinchey-Berroth, R.; Fortier, C.; Tangle, L.; Disterhoft, J.F. Trace eyeblink conditioning in naïve and trained recovered alcoholics. Soc. Neurosci. Abstr. 2000, 26, 41–47. [Google Scholar]
- Oristaglio, J.; Hyman West, S.; Ghaffari, M.; Lech, M.S.; Verma, B.R.; Harvey, J.A.; Welsh, J.P.; Malone, R.P. Children with autism spectrum disorders show abnormal conditioned response timing on delay, but not trace, eyeblink conditioning. Neuroscience 2013, 248, 708–718. [Google Scholar] [CrossRef]
- Neylan, T.C.; Lenoci, M.; Rothlind, J.; Metzler, T.J.; Schuff, N.; Du, A.T.; Franklin, K.W.; Weiss, D.S.; Weiner, M.W.; Marmar, C.R. Attention, learning, and memory in posttraumatic stress disorder. J. Trauma. Stress 2004, 17, 41–46. [Google Scholar] [CrossRef]
- Pederson, C.L.; Maurer, S.H.; Kaminski, P.L.; Zander, K.A.; Peters, C.M.; Stokes-Crowe, L.A.; Osborn, R.E. Hippocampal volume and memory performance in a community-based sample of women with posttraumatic stress disorder secondary to child abuse. J. Trauma. Stress 2004, 17, 37–40. [Google Scholar]
- Vasterling, J.J.; Brailey, K.; Constans, J.I.; Sutker, P.B. Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 1998, 12, 125–133. [Google Scholar] [CrossRef]
- Ayers, E.D.; White, J.; Powell, D.A. Pavlovian eyeblink conditioning in combat veterans with and without post-traumatic stress disorder. Integr. Physiol. Behav. Sci. 2003, 38, 230–247. [Google Scholar] [CrossRef]
- Burriss, L.; Ayers, E.; Powell, D.A. Combat veterans show normal discrimination during differential trace eyeblink conditioning, but increased responsivity to the conditioned and unconditioned stimulus. J. Psychiatr. Res. 2007, 41, 785–794. [Google Scholar] [CrossRef]
- Ginsberg, J.P.; Ayers, E.; Burriss, L.; Powell, D.A. Discriminative delay pavlovian eyeblink conditioning in veterans with and without posttraumatic stress disorder. J. Anxiety Disord. 2008, 22, 809–823. [Google Scholar]
- Bremner, J.D.; Elzinga, B.; Schmahl, C.; Vermetten, E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog. Brain Res. 2008, 167, 171–186. [Google Scholar]
- Gilbertson, M.W.; Shenton, M.E.; Ciszewski, A.; Kasai, K.; Lasko, N.B.; Orr, S.P.; Pitman, R.K. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat. Neurosci. 2002, 5, 1242–1247. [Google Scholar] [CrossRef]
- Donald, C.L.M.; Johnson, A.M.; Cooper, D.; Nelson, E.C.; Werner, N.J.; Shimony, J.S.; Snyder, A.Z.; Raichle, M.E.; Witherow, J.R.; Fang, R.; et al. Detection of blast-related traumatic brain injury in U.S. Military personnel. N. Engl. J. Med. 2011, 364, 2091–2100. [Google Scholar] [CrossRef]
- Cheng, D.T.; Faulkner, M.L.; Disterhoft, J.F.; Desmond, J.E. The effects of aging in delay and trace human eyeblink conditioning. Psychol. Aging 2010, 25, 684–690. [Google Scholar] [CrossRef]
- Committee, M.T.B.I. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 1993, 8, 86–87. [Google Scholar] [CrossRef]
- Department of Veterans Affairs Office of Quality and Performance and Department of Defense Quality Management Directorate, USAMC. VA/DOD Clinical Practice Guidelines for Management Of Concussion/Mild Traumtic Brain Injury. Department of Veterans Affairs and Department of Defense: Washington, D.C., USA, 2009. [Google Scholar]
- Bailes, J.E.; Cantu, R.C. Head injury in athletes. Neurosurgery 2001, 48, 26–45. [Google Scholar]
- Fortier, C.B.; Amick, M.M.; Grande, L.; McGlynn, S.; Kenna, A.; Morra, L.; Clark, A.; Milberg, W.P.; McGlinchey, R.E. The boston assessment of traumatic brain injury-lifetime (BAT-L) semistructured interview: Evidence of research utility and validity. J. Head Trauma Rehabil. 2013. [Google Scholar] [CrossRef]
- Blake, D.D.; Weathers, F.W.; Nagy, L.M.; Kaloupek, D.G.; Gusman, F.D.; Charney, D.S.; Keane, T.M. The development of a clinician-administered ptsd scale. J. Trauma. Stress 1995, 8, 75–90. [Google Scholar] [CrossRef]
- Skinner, H.A.; Sheu, W.J. Reliability of alcohol use indices: The lifetime drinking history and the mast. J. Stud. Alcohol 1982, 43, 1157–1170. [Google Scholar]
- Fortier, C.B.; Steffen, E.M.; LaFleche, G.; Venne, J.R.; Disterhoft, J.F.; McGlinchey, R.E. Delay discrimination and reversal eyebink classical conditioning in abstinent chronic alcoholics. Neuropsychology 2008, 22, 196–208. [Google Scholar] [CrossRef]
- McGlinchey, R.E.; Capozzi, S.; Fortier, C.B.; Disterhoft, J.F. Procedural memory system supports single cue trace eyeblink conditioning in medial temporal lobe amnesia. Neuropsychology 2008, 22, 278–282. [Google Scholar] [CrossRef]
- Knuttinen, M.G.; Power, J.M.; Preston, A.R.; Disterhoft, J.F. Awareness in classical differential eyeblink conditioning in young and aging humans. Behav. Neurosci. 2001, 115, 747–757. [Google Scholar] [CrossRef]
- Solomon, P.R.; Pomerleau, D.; Morse, D.L. Acquisition of the classically conditioned eyeblink response in humans over the life span. Psychol. Aging 1989, 4, 34–41. [Google Scholar] [CrossRef]
- Gormezano, I. Classical Conditioning. In Experimental Methods and Instrumentation in Psychology; Sidowski, J.B., Ed.; McGraw-Hill: New York, NY, USA, 1966; pp. 385–420. [Google Scholar]
- Corp, I. Ibm Spss Statistics for Macintosh; IBM Corporation: Armonk, NY, USA, 2010. [Google Scholar]
- McGlinchey, R.; Fortier, C.B.; Capozzi, S.; Disterhoft, J.F. Trace eyeblink conditioning in abstinent alcoholics: Effects of complex task demands and prior conditioning. Neuropsychology 2005, 19, 159–170. [Google Scholar] [CrossRef]
- Kalmbach, B.E.; Mauk, M.D. Multiple sites of extinction for a single learned response. J. Neurophysiol. 2012, 107, 226–238. [Google Scholar] [CrossRef]
- Robleto, K.; Poulos, A.M.; Thompson, R.F. Brain mechanisms of extinction of the classically conditioned eyeblink response. Learn. Memory 2004, 11, 517–524. [Google Scholar] [CrossRef]
- McCormick, D.A.; Thompson, R.F. Locus coeruleus lesions and resistance to extinction of a classically conditioned response: Involvement of the neocortex and hippocampus. Brain Res. 1982, 245, 239–249. [Google Scholar] [CrossRef]
- Morgan, M.A.; Romanski, L.M.; LeDoux, J.E. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci. Lett. 1993, 163, 109–113. [Google Scholar]
- Myers, K.M.; Davis, M. Mechanisms of fear extinction. Mol. Psychiatr. 2007, 12, 120–150. [Google Scholar] [CrossRef]
- Fein, G.; di Sclafani, V.; Cardenas, V.A.; Goldmann, H.; Tolou-Shams, M.; Meyerhoff, D.J. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol. Clin. Exp. Res. 2002, 26, 558–564. [Google Scholar]
- Harper, C. Neuropathology of brain damage caused by alcohol. Med. J. Australia 1982, 2, 277–282. [Google Scholar]
- Harper, C.; Kril, J.; Daly, J. Are we drinking our neurons away? Br. Med. J. 1987, 294, 534–536. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Schafer, K.; Butters, N.; Cermak, L.S. Magnetic resonance imaging of alcoholic korsakoff patients. Int. J. Neuropsychopharmacol. 1991, 4, 175–186. [Google Scholar]
- Pfefferbaum, A.; Sullivan, E.V. Microstructural but not macrostructural disruption of white matter in women with chronic alcoholism. Neuroimage 2002, 15, 708–718. [Google Scholar]
- Pfefferbaum, A.; Sullivan, E.V.; Hedehus, M.; Adalsteinsson, E.; Lim, K.O.; Moselesy, M. In vivo detection and functional correlates of white matter microstructural disruption in chronic alcoholism. Alcohol. Clin. Exp. Res. 2000, 24, 1214–1221. [Google Scholar] [CrossRef]
- Fortier, C.B.; Leritz, E.C.; Salat, D.H.; Venne, J.R.; Maksimovskiy, A.L.; Williams, V.; Milberg, W.P.; McGlinchey, R.E. Reduced cortical thickness in abstinent alcoholics and association with alcoholic behavior. Alcohol. Clin. Exp. Res. 2011, 35, 2193–2201. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
McGlinchey, R.E.; Fortier, C.B.; Venne, J.R.; Maksimovskiy, A.L.; Milberg, W.P. Effects of OEF/OIF-Related Physical and Emotional Co-Morbidities on Associative Learning: Concurrent Delay and Trace Eyeblink Classical Conditioning. Int. J. Environ. Res. Public Health 2014, 11, 3046-3073. https://doi.org/10.3390/ijerph110303046
McGlinchey RE, Fortier CB, Venne JR, Maksimovskiy AL, Milberg WP. Effects of OEF/OIF-Related Physical and Emotional Co-Morbidities on Associative Learning: Concurrent Delay and Trace Eyeblink Classical Conditioning. International Journal of Environmental Research and Public Health. 2014; 11(3):3046-3073. https://doi.org/10.3390/ijerph110303046
Chicago/Turabian StyleMcGlinchey, Regina E., Catherine B. Fortier, Jonathan R. Venne, Arkadiy L. Maksimovskiy, and William P. Milberg. 2014. "Effects of OEF/OIF-Related Physical and Emotional Co-Morbidities on Associative Learning: Concurrent Delay and Trace Eyeblink Classical Conditioning" International Journal of Environmental Research and Public Health 11, no. 3: 3046-3073. https://doi.org/10.3390/ijerph110303046
APA StyleMcGlinchey, R. E., Fortier, C. B., Venne, J. R., Maksimovskiy, A. L., & Milberg, W. P. (2014). Effects of OEF/OIF-Related Physical and Emotional Co-Morbidities on Associative Learning: Concurrent Delay and Trace Eyeblink Classical Conditioning. International Journal of Environmental Research and Public Health, 11(3), 3046-3073. https://doi.org/10.3390/ijerph110303046





