Ketogenic Diet for Obesity: Friend or Foe?
Abstract
:1. Introduction
2. Ketogenic Diets in the Clinic
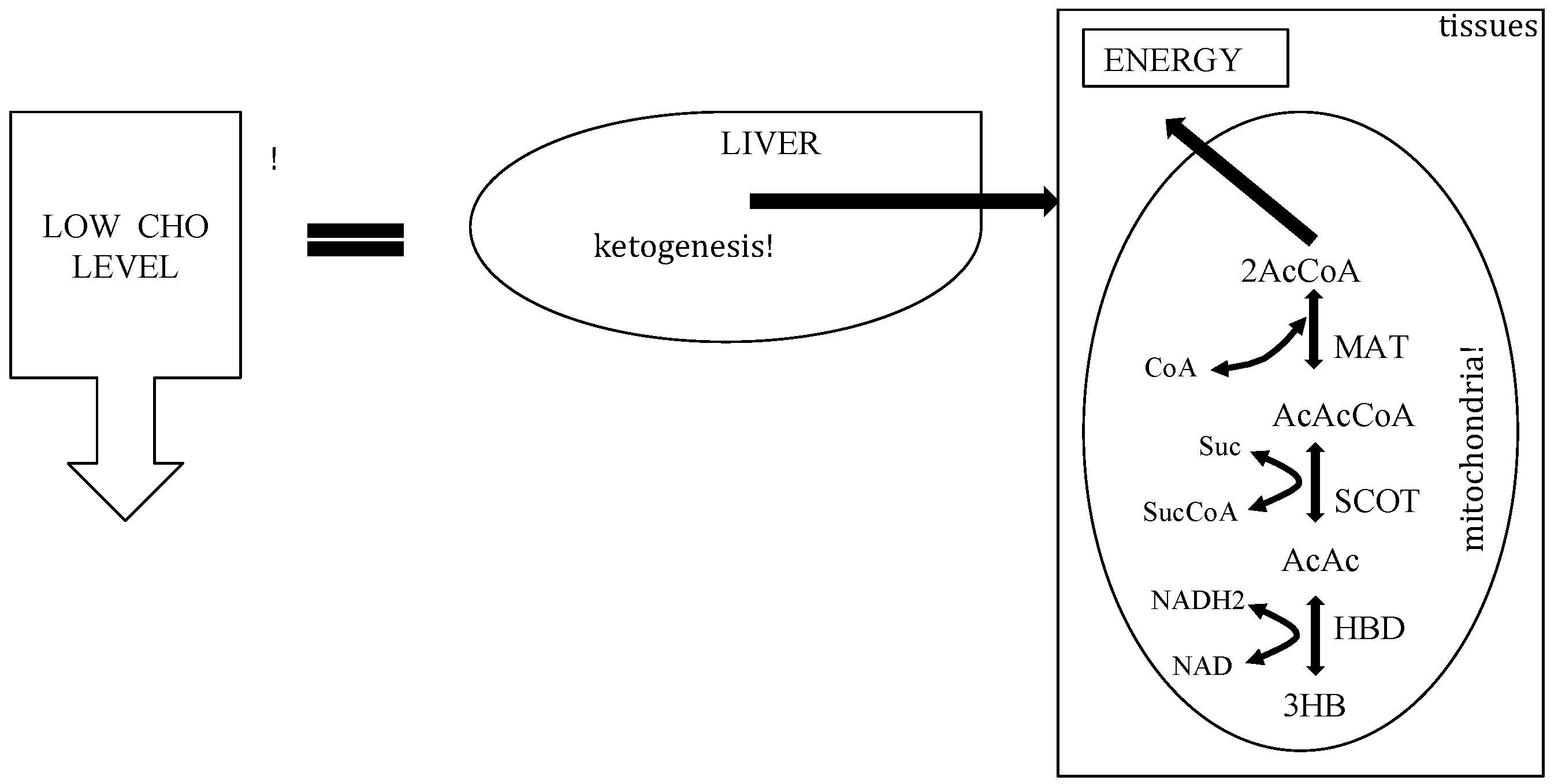
3. The Physiology of Ketosis
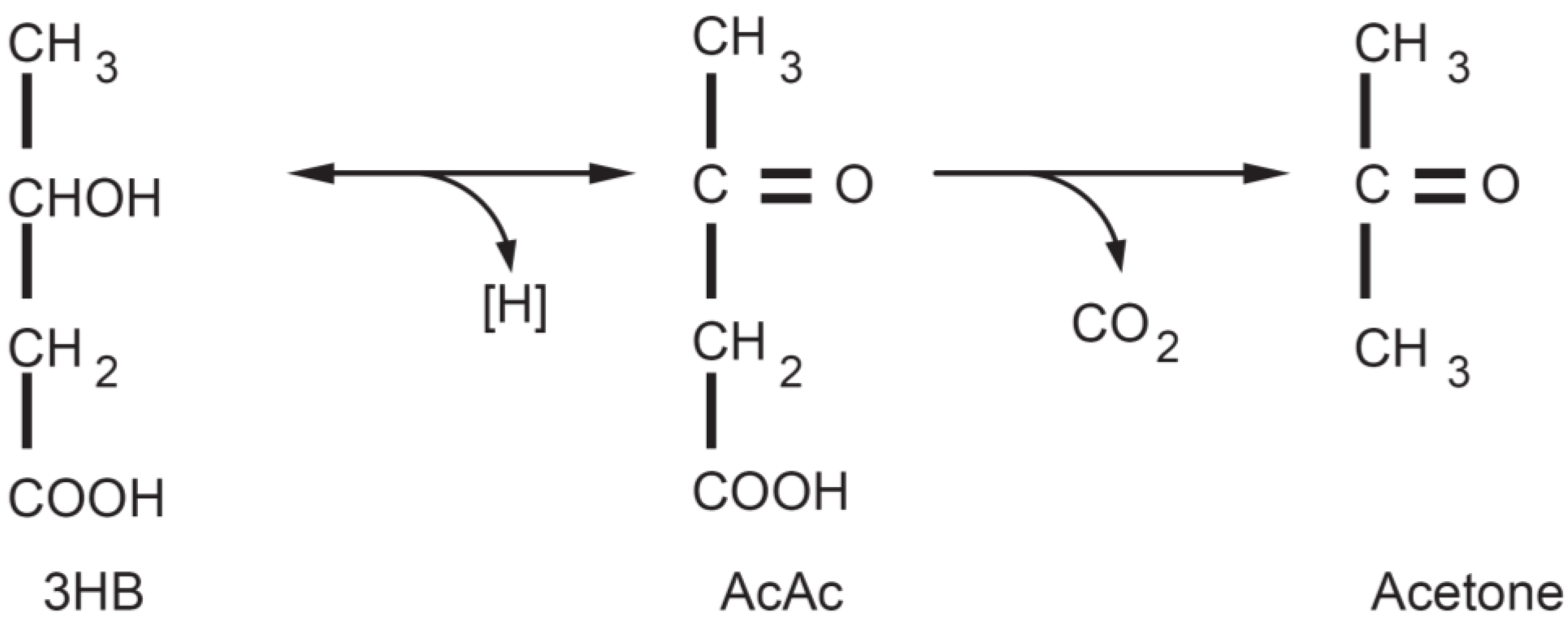

| Blood Levels | Normal Diet | Ketogenic Diet | Diabetic Ketoacidosis |
|---|---|---|---|
| Glucose (mg/dL) | 80–120 | 65–80 | >300 |
| Insulin (µU/L) | 6–23 | 6.6–9.4 | ≈0 |
| KB conc (mmol/L) | 0.1 | 7/8 | >25 |
| pH | 7.4 | 7.4 | <7.3 |
4. Do Ketogenic Diet Work?
- (1)
- Do ketogenic diets works?
- (2)
- Is there a yo-yo effect?
- (3)
- Is a KD safe for obese subjects?
- (1)
- (2)
- (3)
- (4)
5. Other Beneficial Effects in Obesity
6. Is There a Yo-Yo Effect?
7. It Is Safe for Obese Subjects?
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Olshansky, S.J.; Passaro, D.J.; Hershow, R.C.; Layden, J.; Carnes, B.A.; Brody, J.; Hayflick, L.; Butler, R.N.; Allison, D.B.; Ludwig, D.S. A potential decline in life expectancy in the United States in the 21st century. N. Engl. J. Med. 2005, 352, 1138–1145. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight, Factsheet No. 311, Updated March 2013. Available online: http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed on 24 January 2014).
- Koh-Banerjee, P.; Wang, Y.; Hu, F.B.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Changes in bodyweight and body fat distribution as risk factors for clinical diabetes in US men. Amer. J. Epidemiol. 2004, 159, 1150–1159. [Google Scholar] [CrossRef]
- Thompson, W.G.; Cook, D.A.; Clark, M.M.; Bardia, A.; Levine, J.A. Treatment of obesity. Mayo Clin. Proc. 2007, 82, 93–101. [Google Scholar]
- Paoli, A.; Moro, T.; Marcolin, G.; Neri, M.; Bianco, A.; Palma, A.; Grimaldi, K. High-intensity interval resistance training (HIRT) influences resting energy expenditure and respiratory ratio in non-dieting individuals. J. Transl. Med. 2012, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S., Jr.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs. low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef]
- Chahoud, G.; Aude, Y.W.; Mehta, J.L. Dietary recommendations in the prevention and treatment of coronary heart disease: Do we have the ideal diet yet? Amer. J. Cardiol. 2004, 94, 1260–1267. [Google Scholar] [CrossRef]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef]
- Drewnowski, A.; Krahn, D.D.; Demitrack, M.A.; Nairn, K.; Gosnell, B.A. Taste responses and preferences for sweet high-fat foods: Evidence for opioid involvement. Physiol. Behav. 1992, 51, 371–379. [Google Scholar] [CrossRef]
- Yeomans, M.R. Psychological approaches to under standing satiation and satiety. Agro Food Ind. Hi-Tech 2010, 21, 16–19. [Google Scholar]
- Paoli, A.; Rubini, A.; Volek, J.S.; Grimaldi, K.A. Beyond weight loss: A review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur. J. Clin. Nutr. 2013, 67, 789–796. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Rho, J.M. Ketogenic diets: An update for child neurologists. J. Child Neurol. 2009, 24, 979–988. [Google Scholar] [CrossRef]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. Low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef]
- Al-Khalifa, A.; Mathew, T.C.; Al-Zaid, N.S.; Mathew, E.; Dashti, H.M. Therapeutic role of low-carbohydrate ketogenic diet in diabetes. Nutrition 2009, 25, 1177–1185. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Khadada, M.; Al-Mousawi, M.; Talib, H.; Asfar, S.K.; Behbahani, A.I.; Al-Zaid, N.S. Beneficial effects of ketogenic diet in obese diabetic subjects. Mol. Cell. Biochem. 2007, 302, 249–256. [Google Scholar] [CrossRef]
- Sharman, M.J.; Kraemer, W.J.; Love, D.M.; Avery, N.G.; Gomez, A.L.; Scheett, T.P.; Volek, J.S. A ketogenic diet favorably affects serum biomarkers for cardiovascular disease in normal-weight men. J. Nutr. 2002, 132, 1879–1885. [Google Scholar]
- Freedman, M.R.; King, J.; Kennedy, E. Popular diets: A scientific review. Obes. Res. 2001, 9, S1–S40. [Google Scholar] [CrossRef]
- Krebs, H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzyme Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef]
- Felig, P.; Owen, O.E.; Wahren, J.; Cahill, G.F., Jr. Amino acid metabolism during prolonged starvation. J. Clin. Invest. 1969, 48, 584–594. [Google Scholar] [CrossRef]
- Owen, O.E. Ketone bodies as a fuel for the brain during starvation. Biochem. Mol. Biol. Educ. 2005, 33, 246–251. [Google Scholar] [CrossRef]
- Owen, O.E.; Felig, P.; Morgan, A.P.; Wahren, J.; Cahill, G.F., Jr. Liver and kidney metabolism during prolonged starvation. J. Clin. Invest. 1969, 48, 574–583. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F., Jr. Brain metabolism during fasting. J. Clin. Invest. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- Jitrapakdee, S.; Vidal-Puig, A.; Wallace, J.C. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell. Mol. Life Sci. 2006, 63, 843–854. [Google Scholar] [CrossRef]
- Hartman, A.L.; Gasior, M.; Vining, E.P.; Rogawski, M.A. The neuropharmacology of the ketogenic diet. Pediatr. Neurol. 2007, 36, 281–292. [Google Scholar] [CrossRef]
- Fukao, T.; Lopaschuk, G.D.; Mitchell, G.A. Pathways and control of ketone body metabolism: On the fringe of lipid biochemistry. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 243–251. [Google Scholar] [CrossRef]
- Laeger, T.; Metges, C.C.; Kuhla, B. Role of beta-hydroxybutyric acid in the central regulation of energy balance. Appetite 2010, 54, 450–455. [Google Scholar] [CrossRef]
- Veech, R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fatty Acids 2004, 70, 309–319. [Google Scholar] [CrossRef]
- Leino, R.L.; Gerhart, D.Z.; Duelli, R.; Enerson, B.E.; Drewes, L.R. Diet-induced ketosis increases monocarboxylate transporter (MCT1) levels in rat brain. Neurochem. Int. 2001, 38, 519–527. [Google Scholar] [CrossRef]
- Manninen, A.H. Metabolic effects of the very-low-carbohydrate diets: Misunderstood “villains” of human metabolism. J. Int. Soc. Sport. Nutr. 2004, 1, 7–11. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Pt. A 2010, 156, 1–18. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P. Targeting energy metabolism in brain cancer: Review and hypothesis. Nutr. Metab. 2005, 2. [Google Scholar] [CrossRef]
- Vazquez, J.A.; Kazi, U. Lipolysis and gluconeogenesis from glycerol during weight reduction with very-low-calorie diets. Metabolism 1994, 43, 1293–1299. [Google Scholar] [CrossRef]
- Veldhorst, M.A.; Westerterp-Plantenga, M.S.; Westerterp, K.R. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Amer. J. Clin. Nutr. 2009, 90, 519–526. [Google Scholar] [CrossRef]
- Bortz, W.M.; Paul, P.; Haff, A.C.; Holmes, W.L. Glycerol turnover and oxidation in man. J. Clin. Invest. 1972, 51, 1537–1546. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; Toniolo, L.; Canato, M.; Bianco, A.; Fratter, A. Nutrition and acne: Therapeutic potential of ketogenic diets. Skin Pharmacol. Physiol. 2012, 25, 111–117. [Google Scholar] [CrossRef]
- Atkins, R.C. Dr. Atkins’ Diet Revolution. In The High Calorie Way to Stay Thin Forever; D. McKay Co.: New York, NY, USA, 1972. [Google Scholar]
- Feinman, R.D.; Fine, E.J. Nonequilibrium thermodynamics and energy efficiency in weight loss diets. Theor. Biol. Med. Model. 2007, 4. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S.; Nieuwenhuizen, A.; Tome, D.; Soenen, S.; Westerterp, K.R. Dietary protein, weight loss, and weight maintenance. Annu. Rev. Nutr. 2009, 29, 21–41. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; Bianco, A.; Lodi, A.; Cenci, L.; Parmagnani, A. Medium term effects of a ketogenic diet and a mediterranean diet on resting energy expenditure and respiratory ratio. BMC Proc. 2012, 6. [Google Scholar] [CrossRef]
- Veldhorst, M.; Smeets, A.; Soenen, S.; Hochstenbach-Waelen, A.; Hursel, R.; Diepvens, K.; Lejeune, M.; Luscombe-Marsh, N.; Westerterp-Plantenga, M. Protein-induced satiety: Effects and mechanisms of different proteins. Physiol. Behav. 2008, 94, 300–307. [Google Scholar] [CrossRef]
- Sumithran, P.; Prendergast, L.A.; Delbridge, E.; Purcell, K.; Shulkes, A.; Kriketos, A.; Proietto, J. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 2013, 67, 759–764. [Google Scholar] [CrossRef]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Amer. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar]
- Cahill, G.F., Jr. Fuel metabolism in starvation. Annu. Rev. Nutr. 2006, 26, 1–22. [Google Scholar] [CrossRef]
- Paoli, A.; Cenci, L.; Fancelli, M.; Parmagnani, A.; Fratter, A.; Cucchi, A.; Bianco, A. Ketogenic diet and phytoextracts comparison of the efficacy of mediterranean, zone and tisanoreica diet on some health risk factors. Agro Food Ind. Hi-Tech 2010, 21, 24–29. [Google Scholar]
- Tagliabue, A.; Bertoli, S.; Trentani, C.; Borrelli, P.; Veggiotti, P. Effects of the ketogenic diet on nutritional status, resting energy expenditure, and substrate oxidation in patients with medically refractory epilepsy: A 6-month prospective observational study. Clin. Nutr. 2012, 31, 246–249. [Google Scholar] [CrossRef]
- Fine, E.J.; Feinman, R.D. Thermodynamics of weight loss diets. Nutr. Metab. 2004, 1. [Google Scholar] [CrossRef]
- Davidson, T.L.; Hargrave, S.L.; Swithers, S.E.; Sample, C.H.; Fu, X.; Kinzig, K.P.; Zheng, W. Inter-relationships among diet, obesity and hippocampal-dependent cognitive function. Neuroscience 2013, 253, 110–122. [Google Scholar] [CrossRef]
- Brinkworth, G.D.; Noakes, M.; Clifton, P.M.; Buckley, J.D. Effects of a low carbohydrate weight loss diet on exercise capacity and tolerance in obese subjects. Obesity 2009, 17, 1916–1923. [Google Scholar] [CrossRef]
- Yancy, W.S., Jr.; Almirall, D.; Maciejewski, M.L.; Kolotkin, R.L.; McDuffie, J.R.; Westman, E.C. Effects of two weight-loss diets on health-related quality of life. Qual. Life Res. 2009, 18, 281–289. [Google Scholar] [CrossRef]
- Vining, E.P.; Freeman, J.M.; Ballaban-Gil, K.; Camfield, C.S.; Camfield, P.R.; Holmes, G.L.; Shinnar, S.; Shuman, R.; Trevathan, E.; Wheless, J.W. A multicenter study of the efficacy of the ketogenic diet. Arch. Neurol. 1998, 55, 1433–1437. [Google Scholar] [CrossRef]
- Lefevre, F.; Aronson, N. Ketogenic diet for the treatment of refractory epilepsy in children: A systematic review of efficacy. Pediatrics. 2000, 105, p. e46. Available online: http://pediatrics.aappublications.org/content/105/4/e46.full.pdf (accessed on 14 February 2014).
- McLaughlin, T.; Allison, G.; Abbasi, F.; Lamendola, C.; Reaven, G. Prevalence of insulin resistance and associated cardiovascular disease risk factors among normal weight, overweight, and obese individuals. Metabolism 2004, 53, 495–499. [Google Scholar] [CrossRef]
- Rabinowitz, D.; Zierler, K.L. Forearm metabolism in obesity and its response to intra-arterial insulin. Characterization of insulin resistance and evidence for adaptive hyperinsulinism. J. Clin. Invest. 1962, 41, 2173–2181. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D.; Forsythe, C.E.; Quann, E.E.; Wood, R.J.; Puglisi, M.J.; Kraemer, W.J.; Bibus, D.M.; Fernandez, M.L.; Feinman, R.D. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids 2009, 44, 297–309. [Google Scholar] [CrossRef]
- McDaniel, S.S.; Rensing, N.R.; Thio, L.L.; Yamada, K.A.; Wong, M. The ketogenic diet inhibits the mammalian target of rapamycin (mTOR) pathway. Epilepsia 2011, 52. [Google Scholar] [CrossRef]
- Freedland, S.J.; Mavropoulos, J.; Wang, A.; Darshan, M.; Demark-Wahnefried, W.; Aronson, W.J.; Cohen, P.; Hwang, D.; Peterson, B.; Fields, T.; et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate 2008, 68, 11–19. [Google Scholar] [CrossRef]
- Srivastava, S.; Kashiwaya, Y.; King, M.T.; Baxa, U.; Tam, J.; Niu, G.; Chen, X.; Clarke, K.; Veech, R.L. Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet. FASEB J. 2012, 26, 2351–2362. [Google Scholar] [CrossRef]
- Chang, K.T.; Min, K.T. Regulation of lifespan by histone deacetylase. Ageing Res. Rev. 2002, 1, 313–326. [Google Scholar] [CrossRef]
- Yoo, Y.E.; Ko, C.P. Treatment with trichostatin a initiated after disease onset delays disease progression and increases survival in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2011, 231, 147–159. [Google Scholar] [CrossRef]
- Kang, H.L.; Benzer, S.; Min, K.T. Life extension in drosophila by feeding a drug. Proc. Natl. Acad. Sci. USA 2002, 99, 838–843. [Google Scholar] [CrossRef]
- Mammucari, C.; Schiaffino, S.; Sandri, M. Downstream of Akt: Foxo3 and mTOR in the regulation of autophagy in skeletal muscle. Autophagy 2008, 4, 524–526. [Google Scholar]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef]
- Sandri, M.; Barberi, L.; Bijlsma, A.Y.; Blaauw, B.; Dyar, K.A.; Milan, G.; Mammucari, C.; Meskers, C.G.; Pallafacchina, G.; Paoli, A.; et al. Signalling pathways regulating muscle mass in ageing skeletal muscle. The role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology 2013, 14, 303–323. [Google Scholar] [CrossRef]
- Janda, M.; Zeidler, D.; Bohm, G.; Schoberberger, R. An instrument to measure adherence to weight loss programs: The compliance praxis survey-diet (COMPASS-diet). Nutrients 2013, 5, 3828–3838. [Google Scholar] [CrossRef]
- Jeffery, R.W. Does weight cycling present a health risk? Amer. J. Clin. Nutr. 1996, 63, S452–S455. [Google Scholar]
- Sumithran, P.; Proietto, J. The defence of body weight: A physiological basis for weight regain after weight loss. Clin. Sci. 2013, 124, 231–241. [Google Scholar] [CrossRef]
- Wing, R.R.; Hill, J.O. Successful weight loss maintenance. Annu. Rev. Nutr. 2001, 21, 323–341. [Google Scholar] [CrossRef]
- Thomas, P.R. Weighing the Options: Criteria for Evaluating Weight-Management Programs; National Academies Press: Washington, DC, USA, 1995. [Google Scholar]
- Paoli, A.; Bianco, A.; Grimaldi, K.A.; Lodi, A.; Bosco, G. Long term successful weight loss with a combination biphasic ketogenic mediterranean diet and mediterranean diet maintenance protocol. Nutrients 2013, 5, 5205–5217. [Google Scholar] [CrossRef]
- Volek, J.S.; Sharman, M.J.; Forsythe, C.E. Modification of lipoproteins by very low-carbohydrate diets. J. Nutr. 2005, 135, 1339–1342. [Google Scholar]
- Welle, S.; Nair, K.S. Relationship of resting metabolic rate to body composition and protein turnover. Amer. J. Physiol. 1990, 258, 990–998. [Google Scholar]
- Praga, M. Synergy of low nephron number and obesity: A new focus on hyperfiltration nephropathy. Nephrol. Dial. Transplant. 2005, 20, 2594–2597. [Google Scholar] [CrossRef]
- Westerterp-Plantenga, M.S. How are normal, high- or low-protein diets defined? Br. J. Nutr. 2007, 97, 217–218. [Google Scholar] [CrossRef]
- Eisenstein, J.; Roberts, S.B.; Dallal, G.; Saltzman, E. High-protein weight-loss diets: Are they safe and do they work? A review of the experimental and epidemiologic data. Nutr. Rev. 2002, 60, 189–200. [Google Scholar] [CrossRef]
- Pijls, L.T.; de Vries, H.; Donker, A.J.; van Eijk, J.T. The effect of protein restriction on albuminuria in patients with type 2 diabetes mellitus: A randomized trial. Nephrol. Dial. Transplant. 1999, 14, 1445–1453. [Google Scholar] [CrossRef]
- Pijls, L.T.; de Vries, H.; van Eijk, J.T.; Donker, A.J. Protein restriction, glomerular filtration rate and albuminuria in patients with type 2 diabetes mellitus: A randomized trial. Eur. J. Clin. Nutr. 2002, 56, 1200–1207. [Google Scholar] [CrossRef]
- Poplawski, M.M.; Mastaitis, J.W.; Isoda, F.; Grosjean, F.; Zheng, F.; Mobbs, C.V. Reversal of diabetic nephropathy by a ketogenic diet. PLoS One 2011, 6. [Google Scholar] [CrossRef]
- Paoli, A.; Cenci, L.; Grimaldi, K.A. Effect of ketogenic mediterranean diet with phytoextracts and low carbohydrates/high-protein meals on weight, cardiovascular risk factors, body composition and diet compliance in Italian council employees. Nutr. J. 2011, 10. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9. [Google Scholar] [CrossRef]
- Paoli, A.; Canato, M.; Toniolo, L.; Bargossi, A.M.; Neri, M.; Mediati, M.; Alesso, D.; Sanna, G.; Grimaldi, K.A.; Fazzari, A.L.; et al. The ketogenic diet: An underappreciated therapeutic option? Clin. Ter. 2011, 162, 145–153. [Google Scholar]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS One 2013, 8. [Google Scholar] [CrossRef]
- Larsen, T.M.; Dalskov, S.M.; van Baak, M.; Jebb, S.A.; Papadaki, A.; Pfeiffer, A.F.; Martinez, J.A.; Handjieva-Darlenska, T.; Kunesova, M.; Pihlsgard, M.; et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N. Engl. J. Med. 2010, 363, 2102–2113. [Google Scholar] [CrossRef]
- Bielohuby, M.; Sisley, S.; Sandoval, D.; Herbach, N.; Zengin, A.; Fischereder, M.; Menhofer, D.; Stoehr, B.J.; Stemmer, K.; Wanke, R.; et al. Impaired glucose tolerance in rats fed low-carbohydrate, high-fat diets. Am. J. Physiol. Endocrinol. Metab. 2013, 305, 1059–1070. [Google Scholar] [CrossRef]
- Ellenbroek, J.H.; van Dijck, L.; Tons, H.A.; Rabelink, T.J.; Carlotti, F.; Ballieux, B.E.; de Koning, E.J. Long-term ketogenic diet causes glucose intolerance and reduced beta and alpha cell mass but no weight loss in mice. Am. J. Physiol. Endocrinol. Metab. 2014. [Google Scholar] [CrossRef]
- Bielohuby, M.; Menhofer, D.; Kirchner, H.; Stoehr, B.J.; Muller, T.D.; Stock, P.; Hempel, M.; Stemmer, K.; Pfluger, P.T.; Kienzle, E.; et al. Induction of ketosis in rats fed low-carbohydrate, high-fat diets depends on the relative abundance of dietary fat and protein. Am. J. Physiol. Endocrinol. Metab. 2011, 300, 65–76. [Google Scholar] [CrossRef]
- Demetrius, L. Aging in mouse and human systems: A comparative study. Ann. N. Y. Acad. Sci. 2006, 1067, 66–82. [Google Scholar]
- Demetrius, L. Of mice and men. When it comes to studying ageing and the means to slow it down, mice are not just small humans. EMBO Rep. 2005, 6, S39–S44. [Google Scholar] [CrossRef]
- Bielohuby, M.; Sawitzky, M.; Stoehr, B.J.; Stock, P.; Menhofer, D.; Ebensing, S.; Bjerre, M.; Frystyk, J.; Binder, G.; Strasburger, C.; et al. Lack of dietary carbohydrates induces hepatic growth hormone (GH) resistance in rats. Endocrinology 2011, 152, 1948–1960. [Google Scholar] [CrossRef]
- Bielohuby, M.; Matsuura, M.; Herbach, N.; Kienzle, E.; Slawik, M.; Hoeflich, A.; Bidlingmaier, M. Short-term exposure to low-carbohydrate, high-fat diets induces low bone mineral density and reduces bone formation in rats. J. Bone Miner. Res. 2010, 25, 275–284. [Google Scholar] [CrossRef]
- Bergqvist, A.G.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Amer. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef]
- Gower, B.A.; Casazza, K. Divergent effects of obesity on bone health. J. Clin. Densitom. 2013, 16, 450–454. [Google Scholar] [CrossRef]
- Clifton, P. Effects of a high protein diet on body weight and comorbidities associated with obesity. Br. J. Nutr. 2012, 108, S122–S129. [Google Scholar] [CrossRef]
- Tang, M.; O’Connor, L.E.; Campbell, W.W. Diet-induced weight loss: The effect of dietary protein on bone. J. Acad. Nutr. Diet. 2014, 114, 72–85. [Google Scholar] [CrossRef]
- Carter, J.D.; Vasey, F.B.; Valeriano, J. The effect of a low-carbohydrate diet on bone turnover. Osteoporos. Int. 2006, 17, 1398–1403. [Google Scholar] [CrossRef]
- Skov, A.R.; Haulrik, N.; Toubro, S.; Molgaard, C.; Astrup, A. Effect of protein intake on bone mineralization during weight loss: A 6-month trial. Obes. Res. 2002, 10, 432–438. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Paoli, A. Ketogenic Diet for Obesity: Friend or Foe? Int. J. Environ. Res. Public Health 2014, 11, 2092-2107. https://doi.org/10.3390/ijerph110202092
Paoli A. Ketogenic Diet for Obesity: Friend or Foe? International Journal of Environmental Research and Public Health. 2014; 11(2):2092-2107. https://doi.org/10.3390/ijerph110202092
Chicago/Turabian StylePaoli, Antonio. 2014. "Ketogenic Diet for Obesity: Friend or Foe?" International Journal of Environmental Research and Public Health 11, no. 2: 2092-2107. https://doi.org/10.3390/ijerph110202092





