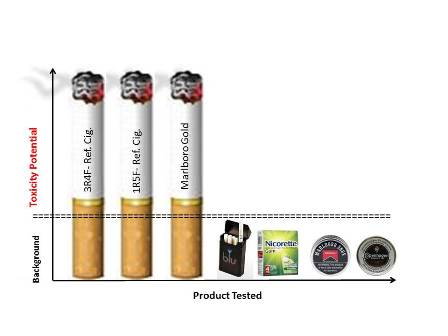
Comparative In Vitro Toxicity Profile of Electronic and Tobacco Cigarettes, Smokeless Tobacco and Nicotine Replacement Therapy Products: E-Liquids, Extracts and Collected Aerosols
Abstract
:1. Introduction
- Toxicity of e-cig liquids;
- Toxicity of SLT products;
- Toxicity of a NRT lozenge product;
- Toxicity of pad-collected particulate matter from freshly-generated smoke and aerosols from tobacco cigarettes and e-cigs, respectively.
2. Materials and Methods
2.1. Chemicals and Methods
2.2. Product Characterization
2.3. E-Liquid Extraction
| Product Class | Name/Description | Abbreviation | Lot # | Product Label Nicotine (mg) | Nicotine Measured in Samples (mg/mL) |
|---|---|---|---|---|---|
| Tobacco Cigarettes | Kentucky Reference Cigarettes | 3R4F | -- | 0.8 | 2.08 * |
| 1R5F | -- | 0.2 | 1.27 ± 0.10 | ||
| Marlboro Gold, 72 mm | Marlboro Gold | V128Z33B4 | 0.68 | 1.96 ± 0.08 | |
| Electronic Cigarettes (e-cig) | Control e-cig | N/A | -- | 0 | 0 ± 0 |
| blu™ e-cigs Classic Tobacco No Nicotine (Rechargeable) | blu CT-Ø | 0248 | 0 | 0 ± 0 | |
| blu™ e-cigs Classic Tobacco High Nicotine (Cigalike) | blu CT-High | 270/404 | 24.0 | 17.93 ± 0.34 | |
| blu™ e-cigs Magnificent Menthol No Nicotine (Rechargeable) | blu MM-Ø | 237 | 0 | 0 ± 0 | |
| blu™ e-cigs Magnificent Menthol High Nicotine (Cigalike) | blu MM-High | 404 | 24.0 | 20.43 ± 0.41 | |
| Smokeless Tobacco (SLT) | Marlboro® Snus | N/A | N335X0X50 | 15.7 | 0.42 ± 0.14 |
| Copenhagen® Snuff | N/A | NEI31755H | 10.6 | 0.46 ± 0.03 | |
| Nicotine Replacement Therapy (NRT) | Nicorette® Lozenge | N/A | 13780 | 4.0 | 0.10 ± 0.03 |
2.4. Pad-Collected Aerosols for Tobacco Cigarettes and E-Cigs
2.5. Aqueous Extract of Smokeless Tobacco (SLT) and Nicotine Replacement Therapy (NRT) Products
2.6. Nicotine Measurement
2.7. Cell Culture
2.8. Cell Treatment
2.9. Cytotoxicity and IL-8 Assay
2.10. Bacterial Mutagenesis Assay
2.11. Micronucleus Assay
3. Results and Discussion
3.1. E-Liquids, Smokeless Tobaccos (SLTs) and Nicotine Replacement Therapy (NRT): Cytotoxicity
 ) blu CT-Ø; (
) blu CT-Ø; (  ) blu CT-High; (
) blu CT-High; (  ) blu MM-Ø; (
) blu MM-Ø; (  ) blu MM-High; (
) blu MM-High; (  ) Marlboro Snus; (
) Marlboro Snus; (  ) Copenhagen Snuff; (
) Copenhagen Snuff; (  ) Nicorette Lozenge; (
) Nicorette Lozenge; (  ) Control e-cig.
) Control e-cig.
 ) blu CT-Ø; (
) blu CT-Ø; (  ) blu CT-High; (
) blu CT-High; (  ) blu MM-Ø; (
) blu MM-Ø; (  ) blu MM-High; (
) blu MM-High; (  ) Marlboro Snus; (
) Marlboro Snus; (  ) Copenhagen Snuff; (
) Copenhagen Snuff; (  ) Nicorette Lozenge; (
) Nicorette Lozenge; (  ) Control e-cig.
) Control e-cig.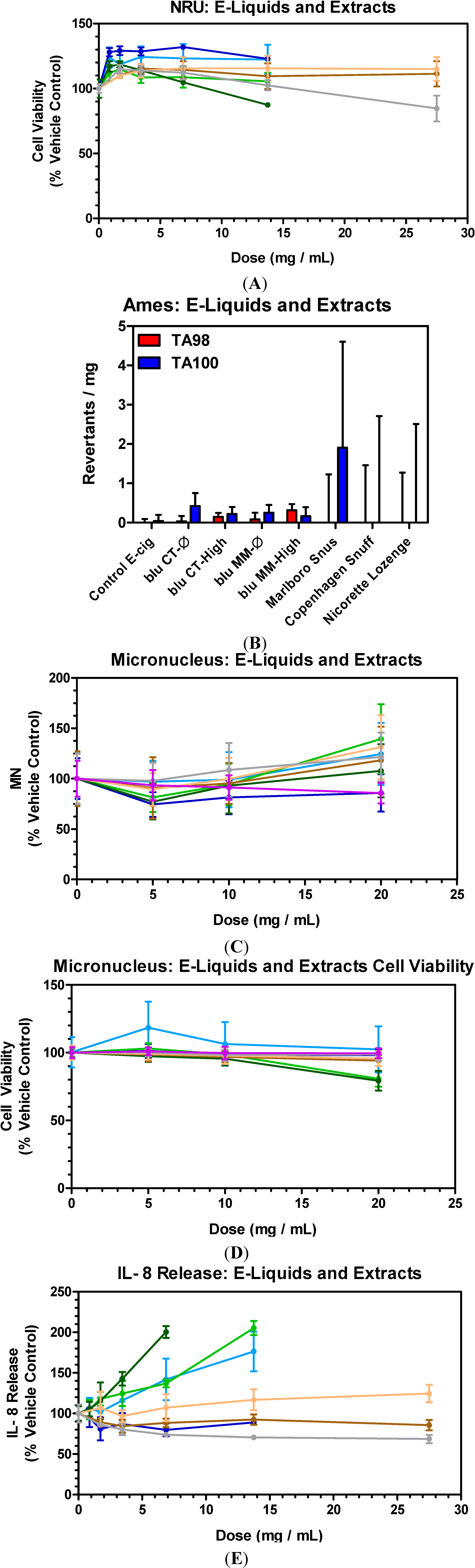
3.2. E-Liquids, Smokeless Tobaccos (SLTs) and Nicotine Replacement Therapy (NRT): Mutagenicity
3.3. E-Liquids, Smokeless Tobaccos (SLTs) and Nicotine Replacement Therapy (NRT): Genotoxicity
3.4. E-Liquids, Smokeless Tobaccos (SLTs) and Nicotine Replacement Therapy (NRT): Inflammation
 ) 3R4F; (
) 3R4F; (  ) 1R5F; (
) 1R5F; (  ) Marlboro Gold; (
) Marlboro Gold; (  ) blu CT-Ø; (
) blu CT-Ø; (  ) blu CT-High; (
) blu CT-High; (  ) blu MM-Ø; (
) blu MM-Ø; (  ) blu MM-High; (
) blu MM-High; (  ) Control e-cig.
) Control e-cig.
 ) 3R4F; (
) 3R4F; (  ) 1R5F; (
) 1R5F; (  ) Marlboro Gold; (
) Marlboro Gold; (  ) blu CT-Ø; (
) blu CT-Ø; (  ) blu CT-High; (
) blu CT-High; (  ) blu MM-Ø; (
) blu MM-Ø; (  ) blu MM-High; (
) blu MM-High; (  ) Control e-cig.
) Control e-cig.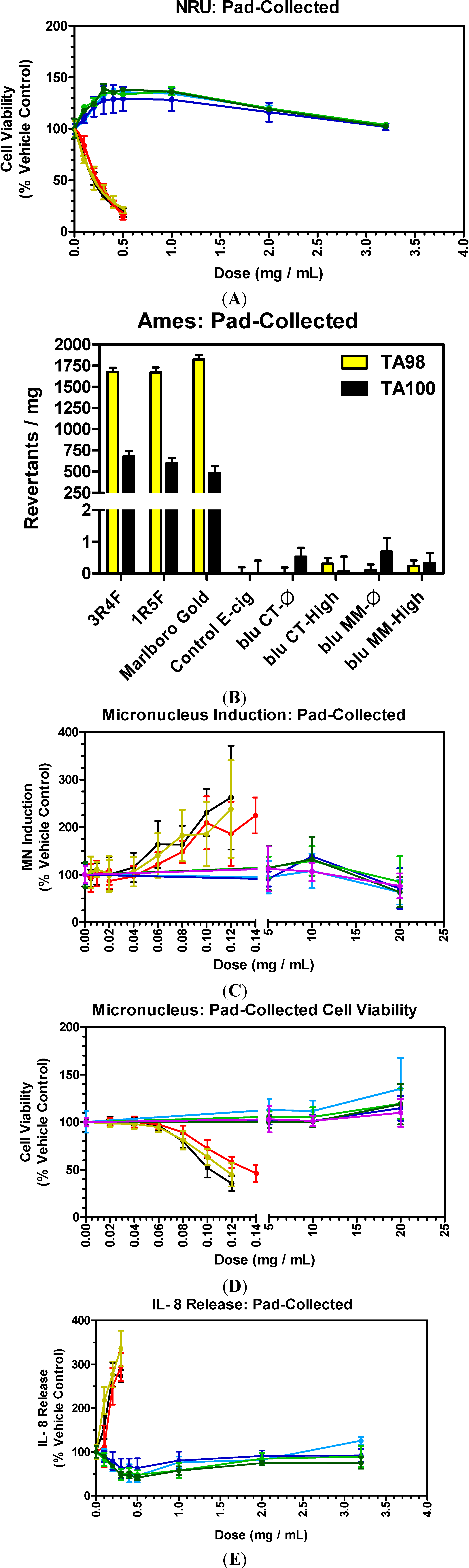
3.5. E-Cigarettes and Conventional Cigarettes
3.6. E-Cigarettes and Conventional Cigarettes: Cytotoxicity
| Pad-Collected Matter: Smoke and Aerosols | ||
|---|---|---|
| Sample | NRU EC50 (mg/mL) | S.E. |
| 3R4F | 0.196 | 0.010 |
| 1R5F | 0.237 | 0.014 |
| Marlboro Gold | 0.204 | 0.009 |
| Control e-cig | ND † | -- |
| blu CT-Ø | ND † | -- |
| blu CT-High | ND † | -- |
| blu MM- Ø | ND † | -- |
| blu MM-High | ND † | -- |
3.7. E-Cigarettes and Conventional Cigarettes: Mutagenicity
3.8. E-Cigarettes and Conventional Cigarettes: Genotoxicity
3.9. E-Cigarettes and Conventional Cigarettes: Inflammation
3.10. Nicotine Equivalence
3.11. Comparable Human Exposure: Conventional and E-Cig
3.12. Contribution of Findings to Tobacco Harm Reduction and E-Cigs
4. Conclusions
- (1)
- E-cigs vs. Tobacco WTPM: At doses up to approximately 100-fold higher than typical cigarette smoke exposures, blu e-cig liquids and pad-collected aerosols had no-to-extremely low in vitro activity (NRU, Ames, MN and IL-8) when compared to WTPM from tobacco burning cigarettes. WTPM activity was up to approximately 6,000 times higher than e-cigs.
- (2)
- E-cigs vs. SLT and NRT: blu e-cig liquids demonstrated similar no-to-extremely low in vitro activity as aqueous extracts from a commercial nicotine lozenge (NRT) and commercial SLT products (snus and snuff).
- (3)
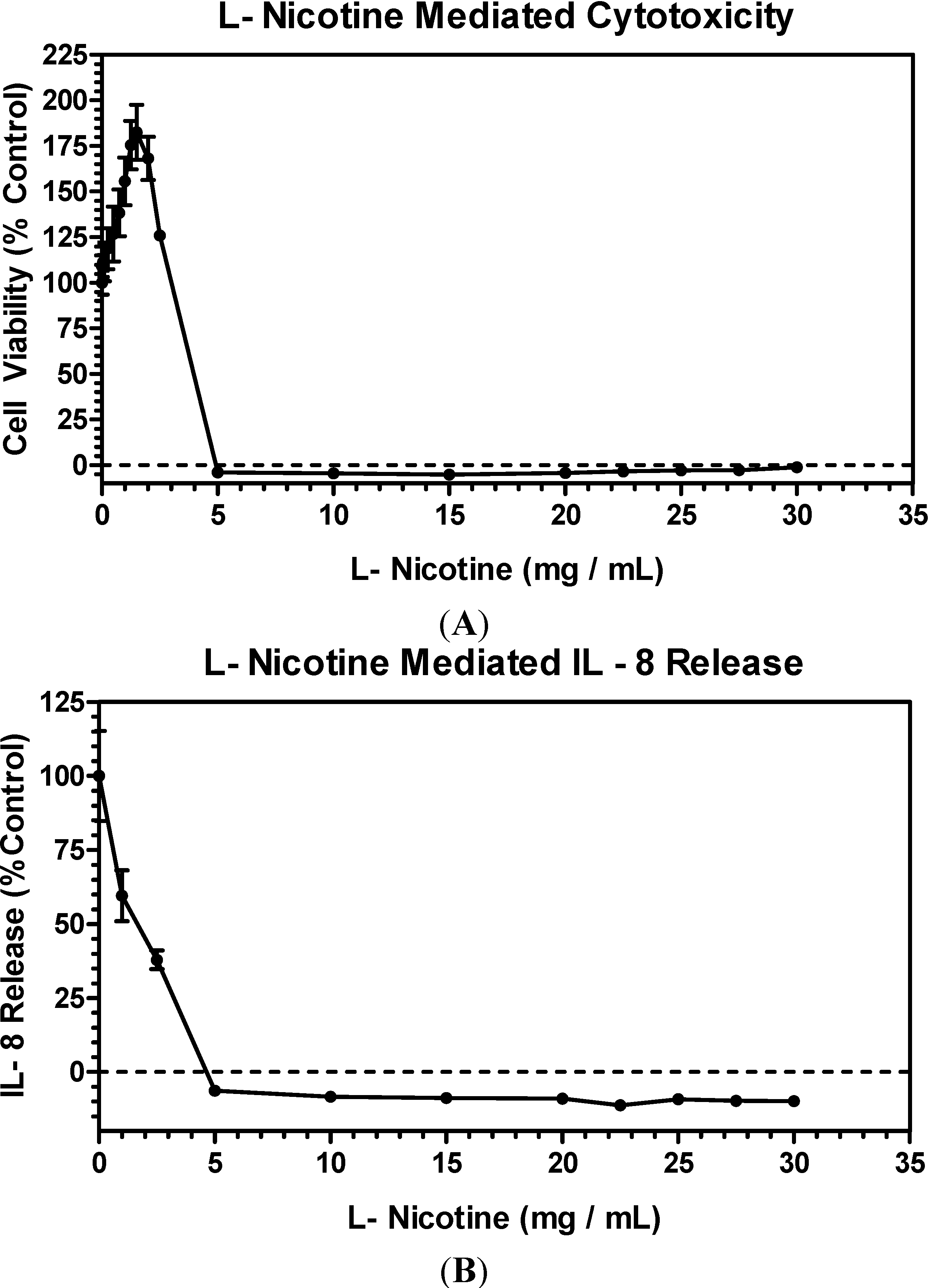
- Effect of Nicotine: In vitro activities (NRU, Ames, MN and IL-8) measured for blu e-cig exposures, with and without nicotine, were similar for all sample types, indicating that the presence of nicotine, at the levels tested, did not contribute to any toxicological effects, confirmed by the lack of cytotoxicity and inflammation response of L-nicotine at comparative levels.
- (4)
- Effect of Flavors: In vitro activities (NRU, Ames and MN) for the commercial blu e-cigs were indistinguishable from control (glycerol/water); indicating these flavors (CT and MM), at the levels tested, had no detectable impact on the cytotoxicity and genotoxicity endpoints utilized in this study. There was some observed IL-8 induction for some e-liquids, albeit at the highest doses tested.
- (5)
- Liquid vs. Pad-Collected Aerosol: In vitro results for blu e-cigs, in this study, were similar for the different exposure methods (e-liquids and pad-collected aerosol); demonstrating no detectable impact on the in vitro toxicological responses when the e-liquids were aerosolized.
- (6)
- SLT vs. Tobacco WTPM: SLT extracts added to the test systems at levels up to 54-fold higher than those used for Tobacco-WTPM generated by burning cigarettes was markedly less cytotoxic and mutagenic, and evoked a significantly lower IL-8 response at all dose levels evaluated. The effects of the SLT extracts in the assays were statistically indistinguishable from those of the e-cig and NRT preparations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ayers, J.W.; Ribisl, K.M.; Brownstein, J.S. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. Am. J. Prev. Med. 2011, 40, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.-F.; Bullen, C.; Flouris, A.D.; Laugesen, M.; Eissenberg, T. Electronic nicotine delivery systems: A research agenda. Tob. Control. 2011, 20, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Levitz, J.S.; Bradley, T.P.; Golden, A.L. Overview of smoking and all cancers. Med. Clin. North Am. 2004, 88, 1655–1675. [Google Scholar] [CrossRef] [PubMed]
- Polosa, R.; Rodu, B.; Caponnetto, P.; Maglia, M.; Raciti, C. A fresh look at tobacco harm reduction: The case for the electronic cigarette. Harm Reduct. J. 2013, 10. [Google Scholar] [CrossRef]
- Brown, B.; Beard, E.; Kotz, D.; Michie, S.; West, R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: A cross-sectional population study. 2014, 109, 1531–1540. [Google Scholar]
- Statement from Specialists in Nicotine Science and Public Health Policy. Available online: http://www.nicotinepolicy.net/documents/letters/MargaretChan.pdf (accessed on 27 October 2014).
- Goniewicz, M.L.; Knysak, J.; Gawron, M.; Kosmider, L.; Sobczak, A.; Kurek, J.; Prokopowicz, A.; Jablonska-Czapla, M.; Rosik-Dulewska, C.; Havel, C.; et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control 2013, 23, 1–7. [Google Scholar]
- Farsalinos, K.E.; Polosa, R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: A systematic review. Ther. Adv. Drug Safety 2014, 5, 67–86. [Google Scholar] [CrossRef]
- Romagna, G.; Allifranchini, E.; Bocchietto, E.; Todeschi, S.; Esposito, M.; Farsalinos, K.E. Cytotoxicity evaluation of electronic cigarette vapor extract on cultured mammalian fibroblasts (clearstream-life): Comparison with tobacco cigarette smoke extract. Inhal. Toxicol. 2013, 25, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Romagna, G.; Allifranchini, E.; Ripamonti, E.; Bocchietto, E.; Todeschi, S.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int. J. Environ. Res. Public Health 2013, 10, 5146–5162. [Google Scholar] [CrossRef] [PubMed]
- Nutt, D.J.; Phillips, L.D.; Balfour, D.; Curran, V.; Dockrell, M.; Foulds, J.; Fagerstrom, K.; Letlape, K.; Milton, A.; Polosa, R.; et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur. Addict. Res. 2014, 20, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Arimilli, S.; Damratoski, B.E.; Bombick, B.; Borgerding, M.F.; Prasad, G.L. Evaluation of cytotoxicity of different tobacco product preparations. Regul. Toxicol. Pharmacol. 2012, 64, 350–360. [Google Scholar] [CrossRef] [PubMed]
- The Rationale and Strategy for Conducting In Vitro Toxicology Testing of Tobacco Smoke. Available online: http://www.coresta.org/Reports/IVT_TF_Rationale-IVT-Testing-Tob.-Smoke_Report_Jun04.pdf (accessed on 27 October 2014).
- Determination of “Tar,” Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke-Official Method. Available online: http://laws.justice.gc.ca/en/T-11.5/SOR-2000-272/182471.html. (accessed on 8 January 2006).
- Rickert, W.S.; Wright, W.G.; Trivedi, A.H.; Momin, R.A.; Lauterbach, J.H. A comparative study of the mutagenicity of various types of tobacco products. Regul. Toxicol. Pharmacol. 2007, 48, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Official Method T-115. Determination of “Tar”, Nicotine and Carbon Monoxide in Mainstream Tobacco Smoke. Available online: http://laws-lois.justice.gc.ca/eng/regulations/SOR-2000-273/page-14.html (accessed on 29 October 2014).
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Letters 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Bombick, D.W.; Doolittle, D.J. The role of chemical structure and cell type in the cytotoxicity of low molecular weight aldehydes and pyridines. In Vitro Toxicol. 1995, 8, 349–356. [Google Scholar]
- Human IL-8 ELISA Kit. For the Quantitative Determination of Human IL-8 Concentrations in Serum, Plasma, Cell Culture Supernatant and Other Biological Fluids. Available online: http://www.abazyme.com/inserts/EL10008.pdf (accessed on 20 October 2014).
- Aufderheide, M.; Gressmann, H. A modified ames assay reveals the mutagenicity of native cigarette mainstream smoke and its gas vapour phase. Exp. Toxicol. Pathol. 2007, 58, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Maron, D.M.; Ames, B.N. Revised methods for the salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.; Scott, A.; Carmichael, P.; Shi, W.; Costales, C. Evaluation of an automated in vitro micronucleus assay in CHO-K1 cells. Mutat. Res. 2007, 630, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, D.; Reeve, L.; Gatehouse, D.; Vanparys, P. A core in vitro genotoxicity battery comprising the AMES test plus the in vitro micronucleus test is sufficient to detect rodent carcinogens and in vivo genotoxins. Mutat. Res. 2011, 721, 27–73. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S.; Sandur, S.K.; Pandey, M.K.; Sethi, G. Inflammation and cancer: How hot is the link? Biochem. Pharmacol. 2006, 72, 1605–1621. [Google Scholar]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Bahl, V.; Lin, S.; Xu, N.; Davis, B.; Wang, Y.H.; Talbot, P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012, 34, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Balharry, D.; Sexton, K.; BeruBe, K.A. An in vitro approach to assess the toxicity of inhaled tobacco smoke components: Nicotine, cadmium, formaldehyde and urethane. Toxicology 2008, 244, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Saul, J.; Crooks, I.; Camacho, O.M.; Dillon, D.; Meredith, C. The resolving power of in vitro genotoxicity assays for cigarette smoke particulate matter. Toxicol. in Vitro 2013, 27, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Rosefort, C.; Fauth, E.; Zankl, H. Micronuclei induced by aneugens and clastogens in mononucleate and binucleate cells using cytokinesis block assay. Mutagenesis 2004, 19, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fuke, S.; Betsuyaku, T.; Nasuhara, Y.; Morikawa, T.; Katoh, H.; Nishimura, M. Chemokines in bronchiolar epithelium in the development of chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2004, 31, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Moretto, N.; Facchinetti, F.; Southworth, T.; Civelli, M.; Singh, D.; Patacchini, R. α,β-Unsaturated aldehydes contained in cigarette smoke elicit IL-8 release in pulmonary cells through mitogen-activated protein kinases. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 296, L839–L848. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.R.; Massey, E.D.; Smith, G. An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem. Toxicol. 2004, 42, S53–S83. [Google Scholar] [CrossRef] [PubMed]
- Misra, M.; Leverette, R.D.; Hamm, J.T.; Vulimiri, S.V. In vitro toxicological evaluation of cigarette smoke particulate matter: Effect of Dimethyl Sulfoxide (DMSO) as solvent. Beiträge zur Tabakforschung International 2010, 24, 2–9. [Google Scholar]
- Jianhua, Y.; Gao, Q.; Mi, Q.; Li, X.; Miao, M.; Cheng, P.; Luo, Y. In vitro micronucleus assay for the analysis of total particulate matter in cigarette smoke: Comparison of flow cytometry and laser scanning cytometry with microscopy. Mutat. Res. 2013, 755, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Rickert, W.S.; Trivedi, A.H.; Momin, R.A.; Wright, W.G.; Lauterbach, J.H. Effect of smoking conditions and methods of collection on the mutagenicity and cytotoxicity of cigarette mainstream smoke. Toxicol. Sci. 2007, 96, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Fields, W.R.; Leonard, R.M.; Odom, P.S.; Nordskog, B.K.; Ogden, M.W.; Doolittle, D.J. Gene expression in Normal Human Bronchial Epithelial (NHBE) cells following in vitro exposure to cigarette smoke condensate. Toxicol. Sci. 2005, 86, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Pryor, W.A.; Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 1993, 686, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Kalra, R.; Singh, S.P.; Pena-Philippides, J.C.; Langley, R.J.; Razani-Boroujerdi, S.; Sopori, M.L. Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in an animal model. Clin. Vaccine Immunol. 2004, 11, 563–568. [Google Scholar] [CrossRef]
- Tayyarah, R.; Long, G.A. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Reg. Toxicol. Pharmacol. 2014, 2014. [Google Scholar] [CrossRef]
- Finch, G.L.; Nikula, K.J.; Chen, B.T.; Barr, E.B.; Chang, I.Y.; Hobbs, C.H. Effect of chronic cigarette smoke exposure on lung clearance of tracer particles inhaled by rats. Fundam. Appl. Toxicol. 1995, 24, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Rodu, B. The scientific foundation for tobacco harm reduction, 2006–2011. Harm Reduct. J. 2011, 8. [Google Scholar] [CrossRef] [PubMed]
- McNeill, A.; Munafò, M.R. Reducing harm from tobacco use. J. Psychopharmacol. 2012, 2012. [Google Scholar] [CrossRef]
- Fagerström, K.O.; Bridgmanb, K. Tobacco harm reduction: The need for new products that can compete with cigarettes. Addict. Behav. 2014, 39, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Gartner, C.E.; Hall, W.D. Should Australia lift its ban on low nitrosamine 576 smokeless tobacco products? Med. J. Aust. 2008, 188, 44–46. [Google Scholar]
- Phillips, C.V.; Rodu, B. Tobacco harm reduction: Opportunity and opposition. Drugs Alcohol Today 2013, 13, 73–78. [Google Scholar] [CrossRef]
- The Use of Nicotine Replacement Therapy to Reduce Harm in Smokers. Available online: htpp://mhra.gsi.gov.uk (accessed on 27 October 2014).
- Moore, D.; Aveyard, P.; Connock, M.; Wang, D.; Fry-Smith, A.; Barton, P. Effectiveness and safety of nicotine replacement therapy assisted reduction to stop smoking: Systematic review and meta-analysis. BMJ 2009, 338. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Spyrou, A.; Voudris, V. Impact of flavour variability on electronic cigarette use experience: An internet survey. Int. J. Environ. Res. Public Health 2013, 10, 7272–7282. [Google Scholar] [CrossRef] [PubMed]
- Farsalinos, K.E.; Romagna, G.; Tsiapras, D.; Kyrzopoulos, S.; Voudris, V. Characteristics, perceived side effects and benefits of electronic cigarette use: A worldwide survey of more than 19,000 consumers. Int. J. Environ. Res. Public Health 2014, 11, 4356–4373. [Google Scholar] [CrossRef] [PubMed]
- Hajek, P.; Etter, J.-F.; Benowitz, B.; Eissenberg, T.; McRobbie, H. Electronic cigarettes: Review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014, 2014. [Google Scholar] [CrossRef]
- Ruyan®E-cigarette Bench-Top Tests. Available online: http://www.healthnz.co.nz/DublinEcigBenchtopHandout.pdf (accessed 20 November 2013).
- Evaluation of e-Cigarettes. Available online: http://www.fda.gov/downloads/drugs/Scienceresearch/UCM173250.pdf (accessed on 10 November 2013).
- Hadwiger, M.; Trehy, M.; Ye, W.; Moore, T.; Allgire, J.; Westenberger, B. Identification of amino-tadalafil and rimonabant in electronic cigarette products using high pressure liquid chromatography with diode array and tandem mass spectrometric detection. J. Chromatogr. A. 2010, 1217, 7547–7555. [Google Scholar] [CrossRef] [PubMed]
- Cahn, Z.; Siegel, M. Electronic cigarettes as a harm reduction strategy for tobacco control: A step forward or a repeat of past mistakes? J. Public Health Policy 2011, 32, 16–31. [Google Scholar] [CrossRef]
- Pellegrino, R.M.; Tinghino, B.; Mangiaracina, G.; Marani, A.; Vitali, M.; Protano, C.; Osborn, J.F.; Cattaruzza, M.S. Electronic cigarettes: An evaluation of exposure to chemicals and fine Particulate Matter (PM). Ann. Ig. 2012, 24, 279–288. [Google Scholar] [PubMed]
- Kim, H.; Shin, H. Determination of tobacco-specific nitrosamines in replacement liquids of electronic cigarettes by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A. 2013, 1291, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Etter, J.; Zäther, E.; Svensson, S. Analysis of refill liquids for electronic cigarettes. Addiction 2013, 108, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Villarreal, A.; Bozhilov, K.; Lin, S.; Talbot, P. Metal and silicate particles including nanoparticles are present in electronic cigarette cartomizer fluid and aerosol. PLoS One 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Burstyn, I. Peering through the mist: Systematic review of what the chemistry of contaminants in electronic cigarettes tells us about health risks. BMC Public Health 2014, 14. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Misra, M.; Leverette, R.D.; Cooper, B.T.; Bennett, M.B.; Brown, S.E. Comparative In Vitro Toxicity Profile of Electronic and Tobacco Cigarettes, Smokeless Tobacco and Nicotine Replacement Therapy Products: E-Liquids, Extracts and Collected Aerosols. Int. J. Environ. Res. Public Health 2014, 11, 11325-11347. https://doi.org/10.3390/ijerph111111325
Misra M, Leverette RD, Cooper BT, Bennett MB, Brown SE. Comparative In Vitro Toxicity Profile of Electronic and Tobacco Cigarettes, Smokeless Tobacco and Nicotine Replacement Therapy Products: E-Liquids, Extracts and Collected Aerosols. International Journal of Environmental Research and Public Health. 2014; 11(11):11325-11347. https://doi.org/10.3390/ijerph111111325
Chicago/Turabian StyleMisra, Manoj, Robert D. Leverette, Bethany T. Cooper, Melanee B. Bennett, and Steven E. Brown. 2014. "Comparative In Vitro Toxicity Profile of Electronic and Tobacco Cigarettes, Smokeless Tobacco and Nicotine Replacement Therapy Products: E-Liquids, Extracts and Collected Aerosols" International Journal of Environmental Research and Public Health 11, no. 11: 11325-11347. https://doi.org/10.3390/ijerph111111325