Impact of Chlorine Dioxide Gas Sterilization on Nosocomial Organism Viability in a Hospital Room
Abstract
:1. Introduction
2. Experimental Section
2.1. Setting
2.2. Organisms
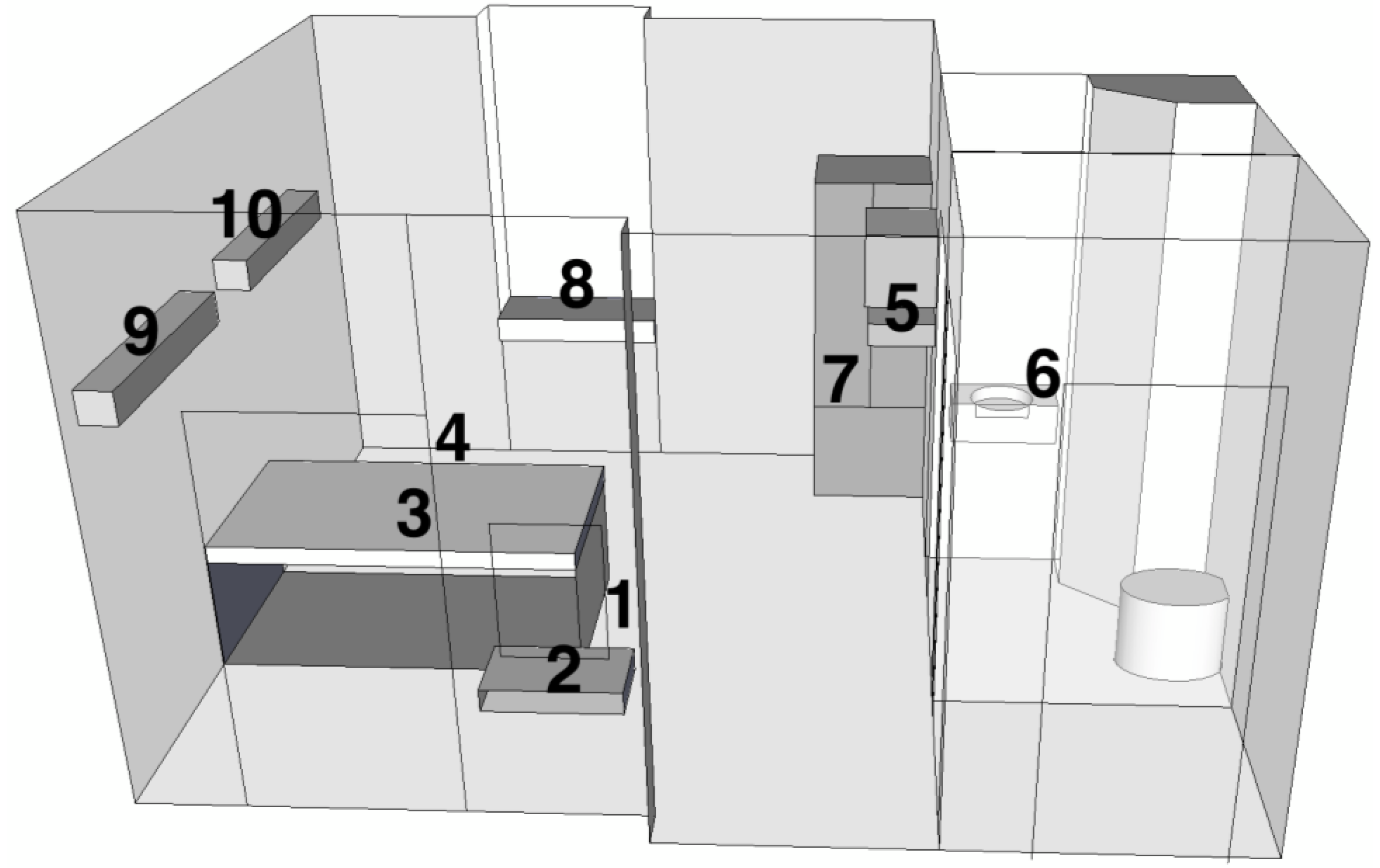
| Site # | Description | Meters from: | ||
|---|---|---|---|---|
| Injection Site | Floor | Ceiling | ||
| 1 | Floor next to injection tubing | 0.3 | 0.0 | 3.3 |
| 2 | Small wall mounted countertop | 1.3 | 1.0 | 2.3 |
| 3 | On top of bed mattress | 1.6 | 1.3 | 2.0 |
| 4 | Floor | 2.7 | 0.0 | 3.3 |
| 5 | Between wall mounted TV and VCR | 3.0 | 2.3 | 1.0 |
| 6 | Top of sink in bathroom | 3.3 | 1.3 | 2.0 |
| 7 | Inside metal cabinet | 3.7 | 1.0 | 2.3 |
| 8 | On window countertop | 4.0 | 1.3 | 2.0 |
| 9 | Top of wall mounted light fixture | 4.3 | 2.7 | 0.7 |
| 10 | Top of wall mounted light fixture | 5.3 | 2.7 | 0.7 |
2.3. Chlorine Dioxide Sterilization
2.4. Safety Considerations
2.5. Sterilization Parameters
2.6. Organism Processing
| ClO2 Conc. (ppm) | Exposure (ppm-h) | % RH | Mean Log Reduction (% inactivation) of all 10 placement sites † | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. baumannii HDR BC 9782 | E. coli ATCC 25922 | E. coli ATCC 51446 | E. faecalis ATCC 29212 | E. faecalis ATCC 51295 | M. smegmatis ATCC 14468 | S. aureus ATCC 25323 | S. aureus ATCC 43300 | |||
| 351 | 677 | 50 | 9.03 (100%) | 9.96 (100%) | 10.00 (100%) | 10.1 (100%) | 9.96 (100%) | 10.0 (100%) | 8.48 (100%) | 9.04 (100%) |
| 377 | 890 | 65 | 7.65 (100%) | 8.66 (100%) | 9.13 (100%) | 8.28 (100%) | 9.06 (100%) | 8.91 (100%) | 7.41 (100%) | 8.27 (100%) |
| 379 | 767 | 65 | 9.33 (100%) | 9.02 (100%) | 9.35 (100%) | 9.40 (100%) | 9.21 (100%) | 9.22 (100%) | 8.41 (100%) | 8.69 (100%) |
| 385 | 770 | 65 | 8.24 (100%) | 8.93 (100%) | 9.03 (100%) | 8.03 (100%) | 9.04 (100%) | 9.08 (100%) | 8.37 (100%) | 8.54 (100%) |
| 376 | 788 | 64 | 8.04 (98%) | 8.93 (98%) | 9.01 (98%) | 8.24 (99%) | 9.24 (97%) | 8.97 (99%) | 8.42 (99%) | 8.06 (98%) |
| 378 | 781 | 66 | 9.02 (98%) | 9.75 (97%) | 9.71 (97%) | 10.07 (100%) | 9.82 (98%) | 9.76 (97%) | 9.90 (99%) | 9.93 (99%) |
3. Results and Discussion
| ClO2 Conc. (ppm) | Exposure (ppm-h) | % RH | B. atrophaeus spores | |
|---|---|---|---|---|
| No. of sites with growth | No. of sites without growth | |||
| 351 | 677 | 50 | 0 | 10 |
| 377 | 890 | 65 | 0 | 10 |
| 379 | 767 | 65 | 0 | 10 |
| 385 | 770 | 65 | 0 | 10 |
| 376 | 788 | 64 | 1 | 9 |
| 378 | 781 | 66 | 1 | 9 |
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating health care-associated infections and deaths in US hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar]
- Martinez, J.A.; Ruthazer, R.; Hansjosten, K.; Barefoot, L.; Snydman, D.R. Role of environmental contamination as a risk factor for acquisition of vancomycin-resistant enterococci in patients treated in a medical intensive care unit. Arch. Intern. Med. 2003, 163, 1905–1912. [Google Scholar] [CrossRef]
- Ray, A.J.; Hoyen, C.K.; Taub, T.F.; Eckstein, E.C.; Donskey, C.J. Nosocomial transmission of vancomycin-resistant enterococci from surfaces. JAMA 2002, 287, 1400–1401. [Google Scholar] [CrossRef]
- Boyce, J.M.; Potter-Bynoe, G.; Chenevert, C.; King, T. Environmental contamination due to methicillin-resistant Staphylococcus aureus: Possible infection control implications. Infect. Control. Hosp. Epidemiol. 1997, 18, 622–627. [Google Scholar]
- Hardy, K.J.; Oppenheim, B.A.; Gossain, S.; Gao, F.; Hawkey, P.M. A study of the relationship between environmental contamination with methicillin-resistant Staphylococcus aureus (MRSA) and patients’ acquisition of MRSA. Infect. Control. Hosp. Epidemiol. 2006, 27, 127–132. [Google Scholar] [CrossRef]
- Huang, S.S.; Datta, R.; Platt, R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch. Intern. Med. 2006, 166, 1945–1951. [Google Scholar] [CrossRef]
- Falk, P.S.; Winnike, J.; Woodmansee, C.; Desai, M.; Mayhall, C.G. Outbreak of vancomycin-resistant enterococci in a burn unit. Infect. Control Hosp. Epidemiol. 2000, 21, 575–582. [Google Scholar]
- Rampling, A.; Wiseman, S.; Davis, L.; Hyett, A.P.; Walbridge, A.N.; Payne, G.C.; Cornaby, A.J. Evidence that hospital hygiene is important in the control of methicillin-resistant Staphylococcus aureus. J. Hosp. Infect. 2001, 49, 109–116. [Google Scholar]
- Aygun, G.; Demirkiran, O.; Utku, T.; Mete, B.; Urkmez, S.; Yilmaz, M.; Yasar, H.; Dikmen, Y.; Ozturk, R. Environmental contamination during a carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit. J. Hosp. Infect. 2002, 52, 259–262. [Google Scholar] [CrossRef]
- Boyce, J.M.; Havill, N.L.; Otter, J.A.; McDonald, L.C.; Adams, N.M.; Cooper, T.; Thompson, A.; Wiggs, L.; Killgore, G.; Tauman, A.; Noble-Wang, J. Impact of hydrogen peroxide vapor room decontamination on Clostridium difficile environmental contamination and transmission in a healthcare setting. Infect. Control Hosp. Epidemiol. 2008, 29, 723–729. [Google Scholar] [CrossRef]
- French, G.L.; Otter, J.A.; Shannon, K.P.; Adams, N.M.; Watling, D.; Parks, M.J. Tackling contamination of the hospital environment by methicillin-resistant Staphylococcus aureus (MRSA): A comparison between conventional terminal cleaning and hydrogen peroxide vapour decontamination. J. Hosp. Infect. 2004, 57, 31–37. [Google Scholar] [CrossRef]
- Andersen, B.; Rasch, M.; Hochlin, K.; Jensen, F.H.; Wismar, P.; Fredriksen, J.E. Decontamination of rooms, medical equipment and ambulances using an aerosol of hydrogen peroxide disinfectant. J. Hosp. Infect. 2006, 62, 149–155. [Google Scholar] [CrossRef]
- Umezawa, K.; Asai, S.; Inokuchi, S.; Miyachi, H. A comparative study of the bactericidal activity and daily disinfection housekeeping surfaces by a new portable pulsed UV radiation device. Curr. Microbiol. 2012, 64, 581–587. [Google Scholar] [CrossRef]
- Clark, J.; Barrett, S.P.; Rogers, M.; Stapleton, R. Efficacy of super-oxidized water fogging in environmental decontamination. J. Hosp. Infect. 2006, 64, 386–390. [Google Scholar] [CrossRef]
- Rastogi, V.K.; Wallace, L.; Smith, L.S. Disinfection of Acinetobacter baumannii-contaminated surfaces relevant to medical treatment facilities with ultraviolet C light. Mil. Med. 2007, 172, 1166–1169. [Google Scholar]
- Canter, D.A.; Gunning, D.; Rodgers, P.; O’Connor, L.; Traunero, C.; Kempter, C.J. Remediation of Bacillus anthracis contamination in the US Department of Justice mail facility. Biosecur. Bioterror. 2005, 3, 119–127. [Google Scholar] [CrossRef]
- Barth, E.; Rupert, R.; Stroud, F.; Rice, E.; Potoka, B. Environmental response to intentional dissemination of Bacillus anthracis spores in the United States—2001. Remediat. J. 2003, 13, 99–111. [Google Scholar] [CrossRef]
- Smith, P.W.; Anderson, A.O.; Christopher, G.W.; Cieslak, T.J.; Devreede, G.J.; Fosdick, G.A.; Greiner, C.B.; Hauser, J.M.; Hinrichs, S.H.; Huebner, K.D.; et al. Designing a biocontainment unit to care for patients with serious communicable diseases: A consensus statement. Biosecur. Bioterror. 2006, 4, 351–365. [Google Scholar] [CrossRef]
- Brown, B.A.; Springer, B.; Steingrube, V.A.; Wilson, R.W.; Pfyffer, G.E.; Garcia, M.J.; Menendez, M.C.; Rodriguez-Salgado, B.; Jost, K.C., Jr.; Chiu, S.H. Mycobacterium wolinskyi sp. nov. and Mycobacterium goodii sp. nov., two new rapidly growing species related to Mycobacterium smegmatis and associated with human wound infections: A cooperative study from the International Working Group on Mycobacterial Taxonomy. Int. J. Syst. Evol. Microbiol. 1999, 49, 1493–1511. [Google Scholar]
- Obee, P.; Griffith, C.; Cooper, R.; Bennion, N. An evaluation of different methods for the recovery of meticillin-resistant Staphylococcus aureus from environmental surfaces. J. Hosp. Infect. 2007, 65, 35–41. [Google Scholar] [CrossRef]
- Wright, J.B.; Lam, K.; Burrell, R.E. Wound management in an era of increasing bacterial antibiotic resistance: A role for topical silver treatment. Am. J. Infect. Control 1998, 26, 572–577. [Google Scholar] [CrossRef]
- Smith, P.W.; Gibbs, S.; Sayles, H.; Hewlett, A.; Rupp, M.E.; Iwen, P.C. Observations on hospital room contamination testing. Healthc. Infect. 2013, 18, 10–13. [Google Scholar] [CrossRef]
- Murray, P.; Baron, E.; Jorgensen, J.; Landry, M.; Pfaller, M. Manual of Clinical Microbiology; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- Moat, J.; Cargill, J.; Shone, J.; Upton, M. Application of a novel decontamination process using gaseous ozone. Can. J. Microbio. 2009, 55, 928–933. [Google Scholar] [CrossRef]
- Berrington, A.W.; Pedler, S.J. Investigation of gaseous ozone for MRSA decontamination of hospital side-rooms. J. Hosp. Infect. 1998, 40, 61–65. [Google Scholar] [CrossRef]
- Chan, P.C.; Huang, L.M.; Lin, H.C.; Chang, L.Y.; Chen, M.L.; Lu, C.Y.; Lee, P.I.; Chen, J.M.; Lee, C.Y.; Pan, H.J.; Wang, J.T.; Chang, S.C.; Chen, Y.C. Control of an outbreak of pandrug-resistant Acinetobacter baumannii colonization and infection in a neonatal intensive care unit. Infect. Control. Hosp. Epidemiol. 2007, 28, 423–429. [Google Scholar]
- Frieden, T.R.; Munsiff, S.S.; Low, D.E.; Willey, B.M.; Williams, G.; Faur, Y.; Eisner, W.; Warren, S.; Kreiswirth, B. Emergence of vancomycin-resistant enterococci in New York City. Lancet 1993, 342, 76–79. [Google Scholar] [CrossRef]
- Lai, K.K.; Kelley, A.L.; Melvin, Z.S.; Belliveau, P.P.; Fontecchio, S.A. Failure to eradicate vancomycin-resistant enterococci in a university hospital and the cost of barrier precautions. Infect. Control. Hosp. Epidemiol. 1998, 19, 647–652. [Google Scholar]
- Noble, M.A.; Isaac-Renton, J.L.; Bryce, E.A.; Roscoe, D.L.; Roberts, F.J.; Walker, M.; Scharf, S.; Walsh, A.; Altamirano-Dimas, M.; Gribble, M. The toilet as a transmission vector of vancomycin-resistant enterococci. J. Hosp. Infect. 1998, 40, 237–241. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lowe, J.J.; Gibbs, S.G.; Iwen, P.C.; Smith, P.W.; Hewlett, A.L. Impact of Chlorine Dioxide Gas Sterilization on Nosocomial Organism Viability in a Hospital Room. Int. J. Environ. Res. Public Health 2013, 10, 2596-2605. https://doi.org/10.3390/ijerph10062596
Lowe JJ, Gibbs SG, Iwen PC, Smith PW, Hewlett AL. Impact of Chlorine Dioxide Gas Sterilization on Nosocomial Organism Viability in a Hospital Room. International Journal of Environmental Research and Public Health. 2013; 10(6):2596-2605. https://doi.org/10.3390/ijerph10062596
Chicago/Turabian StyleLowe, John J., Shawn G. Gibbs, Peter C. Iwen, Philip W. Smith, and Angela L. Hewlett. 2013. "Impact of Chlorine Dioxide Gas Sterilization on Nosocomial Organism Viability in a Hospital Room" International Journal of Environmental Research and Public Health 10, no. 6: 2596-2605. https://doi.org/10.3390/ijerph10062596




