The Dynamic Growth Exhibition and Accumulation of Cadmium of Pak Choi (Brassica campestris L. ssp. chinensis) Grown in Contaminated Soils
Abstract
:1. Introduction
2. Experimental Section
2.1. Soil Preparation
2.2. Pot Experiment
2.3. Plant and Soil Analysis
2.4. Quality Control and Statistical Analysis
3. Results and Discussion
3.1. Basic Soil Characteristics

3.2. Dynamic Change of Physiological Characteristics
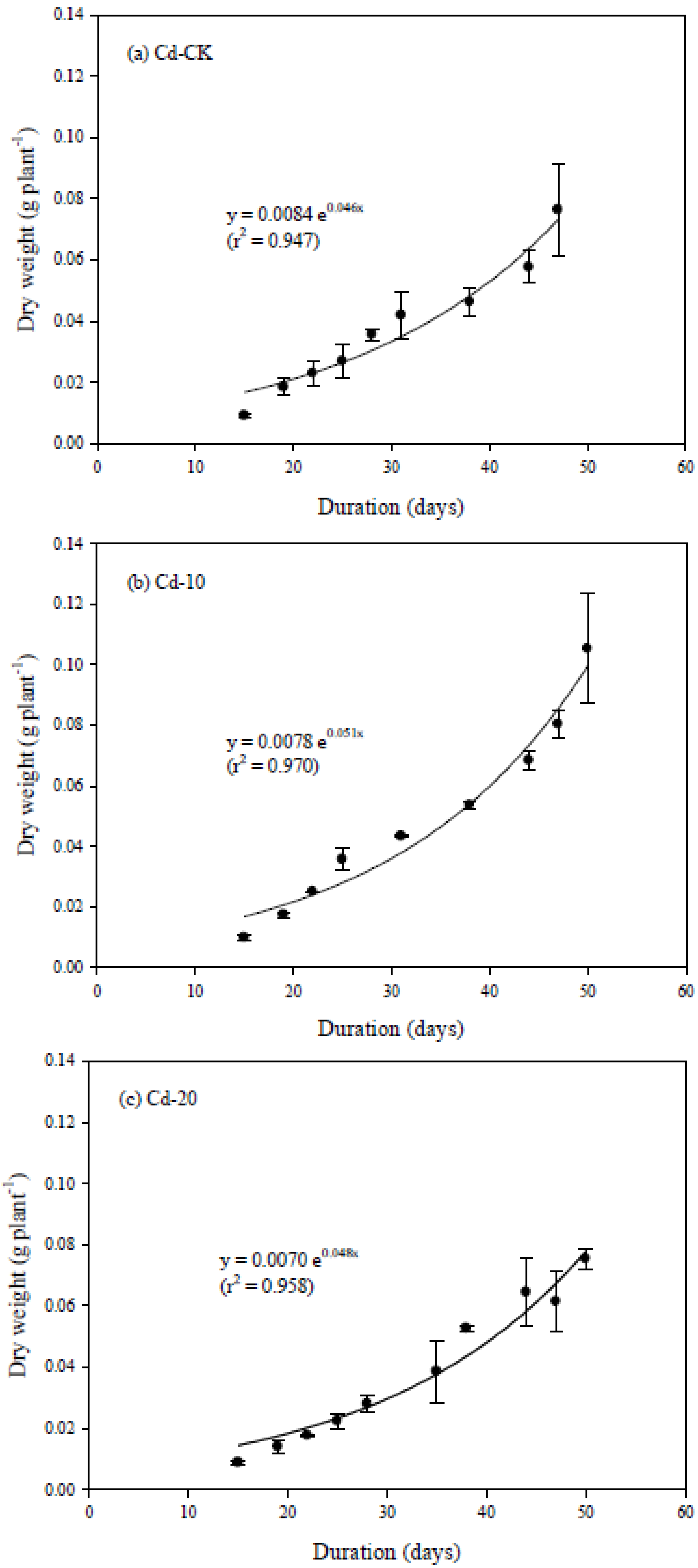
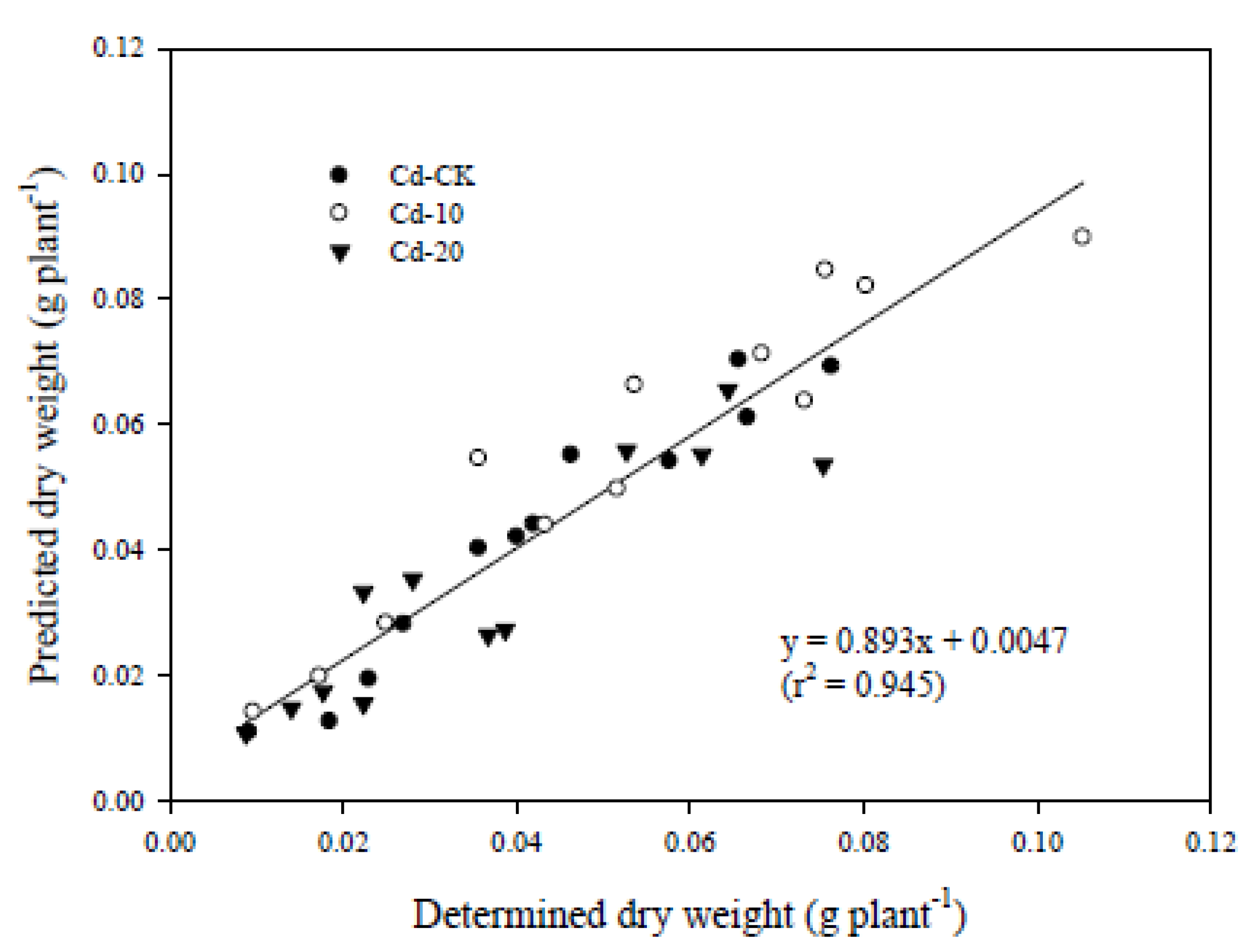
3.3. Relationships between Physiological Characteristics
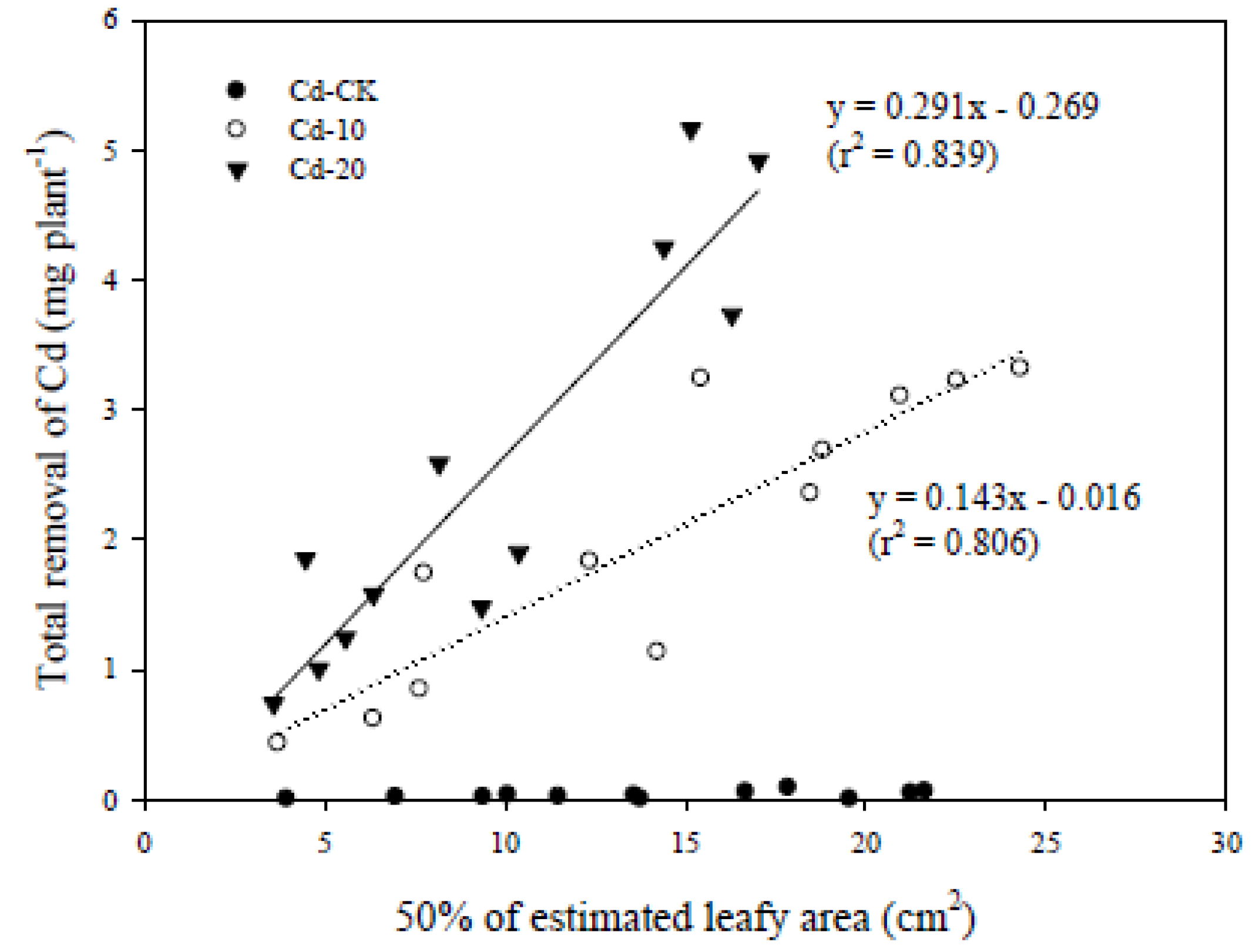
3.4. Bioconcentration and Dynamic Changes in Accumulated Cd Concentration
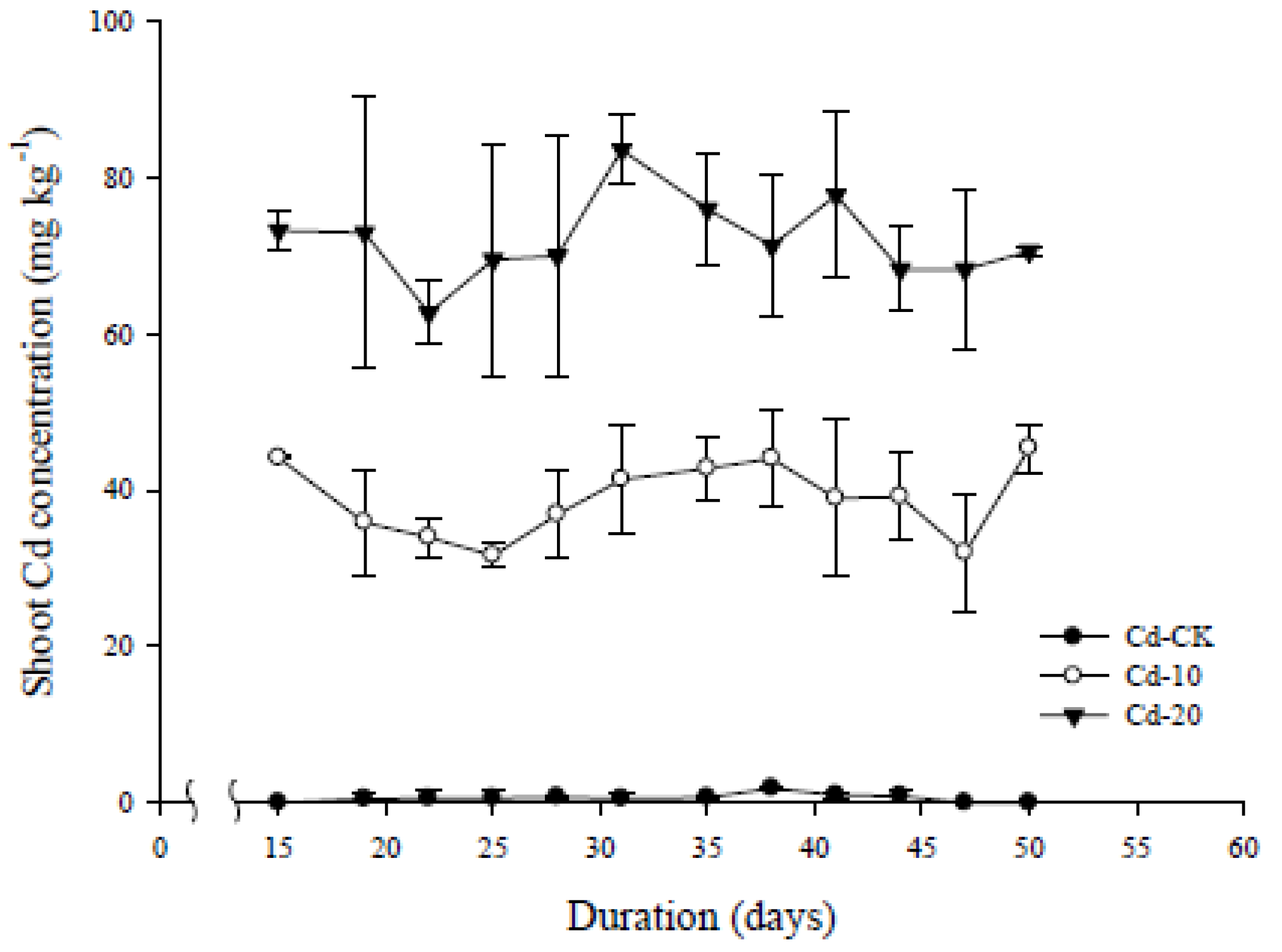
| Treatment | Soil Cd conc. | Shoot Cd conc. | BCFDW * |
|---|---|---|---|
| mg·kg−1 | |||
| Cd-CK | 0.8 ± 0.3 # | 0.7 ± 0.5 | 0.80 |
| Cd-10 | 10.5 ± 0.2 | 38.9 ± 0.8 | 3.96 |
| Cd-20 | 20.1 ± 0.8 | 72.0 ± 0.3 | 3.59 |


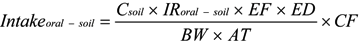
- Intakeoral-soil: exposure dose of oral intake (mg kg−1 day−1)
- Csoil: concentration of concerned pollutant (mg kg−1)
- IRoral-soil: ingestion rate (mg day−1)
- EF: exposure frequency (day year−1)
- ED: exposure period (year−1)
- BW: body weight (kg)
- AT: average time (day)
- CF: conversion factor (kg mg−1)
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kim, S.J.; Chang, A.C.; Page, A.L.; Warneke, J.E. Relative concentrations of cadmium and zinc in tissue of selected food plants grown on sludge-treated soils. J. Environ. Qual. 1988, 17, 568–573. [Google Scholar]
- Harrison, R.M.; Chirgawi, M.B. The assessment of air and soil as contributors of some trace-metals to vegetable plants. 3. Experiments with field-grown plants. Sci. Total Environ. 1989, 83, 47–62. [Google Scholar] [CrossRef]
- Jackson, A.P.; Alloway, B.J. The bioavailability of cadmium to lettuce and cabbage in soils previously treated with sewage sludges. Plant Soil 1991, 132, 179–186. [Google Scholar]
- Wagner, G.J. Accumulation of cadmium in crop plants and its consequences to human health. Adv. Agron. 1993, 51, 173–212. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar]
- Mishra, V.K.; Tripathi, B.D. Concurrent removal and accumulation of heavy metals by the three aquatic macrophytes. Bioresour. Technol. 2008, 99, 7091–7097. [Google Scholar] [CrossRef]
- Hernandez, L.E.; Carpena-Ruiz, R.; Garate, A. Alterations in the mineral nutrition of pea seedlings exposed to cadmium. Plant Nutr. 1996, 19, 1581–1598. [Google Scholar] [CrossRef]
- Soylak, M.; Tzen, M.; Souza, A.S.; Korn, M.G.A.; Ferreira, S.L.C. Optimization of microwave assisted digestion procedure for the determination of zinc, copper and nickel in tea samples employing flame atomic spectrometry. J. Hazard. Mater. 2007, 149, 264–268. [Google Scholar] [CrossRef]
- Yang, J.X.; Guo, H.T.; Ma, Y.B.; Wang, L.Q.; Wei, D.P.; Hua, L. Genotypic variations in the accumulation of exhibited by different vegetables. J. Environ. Sci. 2010, 22, 1246–1252. [Google Scholar] [CrossRef]
- Wang, X.L.; Sato, T.; Xing, B.S.; Tao, S. Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ. 2005, 350, 28–37. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.B.; Gou, X.; Su, Y.B.; Wang, G. Risk assessment of heavy metals in soils and vegetables around non-ferrous metals mining and smelting sites, Baiyin, China. J. Environ. Sci. (China) 2006, 18, 1124–1134. [Google Scholar] [CrossRef]
- Chen, C.M.; Liu, M.C. Ecological risk assessment on a cadmium contaminated soil landfill—A preliminary evaluation based on toxicity tests on local species and site-specific information. Sci. Total Environ. 2006, 359, 120–129. [Google Scholar] [CrossRef]
- Römkens, P.F.; Guo, H.Y.; Chu, C.L.; Liu, T.S.; Chiang, C.F.; Koopmans, G.F. Prediction of cadmium uptake by brown rice and derivation of soil-plant transfer models to improve soil protection guidelines. Environ. Pollut. 2009, 157, 2435–2444. [Google Scholar] [CrossRef]
- Alexander, P.D.; Alloway, B.J.; Dourado, A.M. Genotypic variations in the accumulation of Cd, Cu, Pb, and Zn exhibited by six commonly grown vegetables. Environ. Pollut. 2006, 144, 736–745. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.S.; Li, H.F.; Jiang, R.F. Accumulation of cadmium in the edible parts of six vegetable species grown in Cd-contaminated soils. J. Environ. Manag. 2009, 90, 1117–1122. [Google Scholar] [CrossRef]
- Chen, H.L.; Lai, H.Y.; Wang, S.M.; Kuo, Y.C.; Lu, C.J. Effect of biosolids and Cd/Pb interaction on the growth and Cd accumulation of Brassica rapa grown in Cd-contaminated soils. Water Air Soil Poll. 2010, 206, 385–394. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis, Part 3, Chemical Methods; Page, A.L., Sparks, D.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA. Inc. and SSSA. Inc.: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis, Part 3, Chemical Methods; Page, A.L., Sparks, D.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA. Inc. and SSSA. Inc.: Madison, WI, USA, 1996; pp. 417–435. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-Size Analysis. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed.; Klute, A., Campbell, G.S., Nielsen, D.R., Jackson, R.D., Mortland, M.M., Eds.; ASA. Inc. and SSSA. Inc.: Madison, WI, USA, 1986; pp. 383–412. [Google Scholar]
- Gardner, W.H. Water Content. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed.; Klute, A., Campbell, G.S., Nielsen, D.R., Jackson, R.D., Mortland, M.M., Eds.; ASA. Inc. and SSSA. Inc.: Madison, WI, USA, 1986; pp. 493–544. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Methods of Soil Analysis, Part 3, Chemical Methods; Page, A.L., Sparks, D.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; ASA. Inc. and SSSA. Inc.: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Blaylock, M.J.; Salt, D.E.; Dushenkov, S.; Zakharova, O.; Gussman, C.; Kapulnik, Y.; Ensley, B.D.; Raskin, I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997, 31, 860–865. [Google Scholar] [CrossRef]
- Chen, H.S.; Huang, Q.Y.; Liu, L.N.; Cai, P.; Liang, W.; Li, M. Poultry manure compost alleviates the phytotoxicity of soil cadmium: Influence on growth of pakchoi (Brassica chinensis L.). Pedosphere 2010, 20, 63–70. [Google Scholar] [CrossRef]
- Hawkins, T.S.; Gardiner, E.S.; Comer, G.S. Modeling the relationship between extractable chlorophyll and SPAD-502 readings for endangered plant species research. J. Nat. Conserv. 2009, 17, 123–127. [Google Scholar] [CrossRef]
- EPA/Taiwan. Flame Atomic Absorption Spectrometer. Method code No: NIEA M111.00C; Environmental Analysis Laboratory, Environmental Protection Administration of Taiwan: Taipei, Taiwan, 2002.
- Cho, Y.Y.; Oh, S.B.; Oh, M.M.; Son, J.E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumber (Cucumis sativus L.) using leaf length, width, and SPAD value. Sci. Hortic. 2007, 111, 330–334. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Plants. In Trace Element in Soils and Plants, 3rd ed.; Kabata-Pendias, A., Pendias, H., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 73–98. [Google Scholar]
- Japenga, J.; Koopmans, G.F.; Song, J.; Römkens, P.F. A Feasibility test to estimate the duration of phytoextraction of heavy metals from polluted soils. Int. J. Phytoremediat. 2007, 9, 115–132. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. Soil Colloids: Seat of Soil Chemical and Physical Acidity. In The Nature and Properties of Soils; Brady, N.C., Weil, R.R., Eds.; Pearson Education Inc.: Upper Saddle River, NJ, USA, 2008; pp. 311–358. [Google Scholar]
- Le Bail, M.; Jeuffroy, M.H.; Bouchard, C.; Barbottin, A. It is possible to forecast the grain quality and yield of different varieties of winter wheat from Minolta SPAD meter measurements. Eur. J. Agron. 2005, 23, 379–391. [Google Scholar] [CrossRef]
- Montero, F.J.; de Juan, J.A.; Cuesta, A.; Brasa, A. Nondestructive methods to estimate leaf area in vitis vinifera L. HortScience 2000, 35, 696–698. [Google Scholar]
- Huang, J.L.; He, F.; Cui, K.H.; Buresh, R.J.; Xu, B.; Gong, W.H.; Peng, S.B. Determination of optimal nitrogen rate for rice varieties using a chlorophyll meter. Field Crop. Res. 2008, 105, 70–80. [Google Scholar] [CrossRef]
- Lin, F.F.; Qiu, L.F.; Deng, J.S.; Shi, Y.Y.; Chen, L.S.; Wang, K. Investigation of SPAD meter-based indices for estimating rice nitrogen status. Comput. Electron. Agric. 2010, 71, S60–S65. [Google Scholar] [CrossRef]
- Yu, H.; Wu, H.S.; Wang, Z.J. Evaluation of SPAD and dualex for in-season corn nitrogen status estimation. Acta Agron. Sin. 2010, 36, 840–847. [Google Scholar]
- Hussain, F.; Bronson, K.F.; Yadvinder, S.; Bijay, S.; Peng, S. Use of chlorophyll meter sufficiency indies for nitrogen management of irrigated rice in Asia. Agron. J. 2000, 92, 875–879. [Google Scholar]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Liu, X.; Peng, K.; Wang, A.; Lian, C.L.; Shen, Z.G. Cadmium accumulation and distribution in populations of Phytolacca Americana L. and the role of transpiration. Chemosphere 2010, 78, 1136–1141. [Google Scholar] [CrossRef]
- WHO. Fifty-Fifth Report of the Joint FAO/WHO Expert Committee on Food Additives. In WHO Technical Report Series 901; World Health Organization: Geneva, Switzerland, 2001; pp. 61–69, 97–98. [Google Scholar]
- Martinez, C.E.; Motto, H.L. Solubility of lead, zinc and copper added to mineral soils. Environ. Pollut. 2000, 107, 153–158. [Google Scholar] [CrossRef]
- Wang, C.X.; Mo, Z.; Wang, H.; Wang, Z.J.; Cao, Z.H. The transportation, time-dependent distribution of heavy metals in paddy crops. Chemosphere 2003, 50, 717–723. [Google Scholar] [CrossRef]
- Wallace, W.G.; Lee, B.G.; Luoma, S.N. Subcellular compartmentalization of Cd and Zn in two bivalves. I. Significance of metal-sensitive fractions (MSF) and biologically detoxified metal (BDM). Mar. Ecol. Prog. Ser. 2003, 249, 183–197. [Google Scholar] [CrossRef]
- Lai, H.Y.; Hseu, Z.Y.; Chen, T.C.; Chen, B.C.; Guo, H.Y.; Chen, Z.S. Health risk-based assessment and management of heavy metals-contaminated soil sites in Taiwan. Int. J. Environ. Res. Public Health 2010a, 7, 3595–3614. [Google Scholar] [CrossRef]
- Lai, H.Y.; Juang, K.W.; Chen, Z.S. Large-area experiment on uptake of metals by twelve plant species growing in soils contaminated with multiple metals. Int. J. Phytoremediat. 2010, 12, 785–797. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lai, H.-Y.; Chen, B.-C. The Dynamic Growth Exhibition and Accumulation of Cadmium of Pak Choi (Brassica campestris L. ssp. chinensis) Grown in Contaminated Soils. Int. J. Environ. Res. Public Health 2013, 10, 5284-5298. https://doi.org/10.3390/ijerph10115284
Lai H-Y, Chen B-C. The Dynamic Growth Exhibition and Accumulation of Cadmium of Pak Choi (Brassica campestris L. ssp. chinensis) Grown in Contaminated Soils. International Journal of Environmental Research and Public Health. 2013; 10(11):5284-5298. https://doi.org/10.3390/ijerph10115284
Chicago/Turabian StyleLai, Hung-Yu, and Bo-Ching Chen. 2013. "The Dynamic Growth Exhibition and Accumulation of Cadmium of Pak Choi (Brassica campestris L. ssp. chinensis) Grown in Contaminated Soils" International Journal of Environmental Research and Public Health 10, no. 11: 5284-5298. https://doi.org/10.3390/ijerph10115284
APA StyleLai, H.-Y., & Chen, B.-C. (2013). The Dynamic Growth Exhibition and Accumulation of Cadmium of Pak Choi (Brassica campestris L. ssp. chinensis) Grown in Contaminated Soils. International Journal of Environmental Research and Public Health, 10(11), 5284-5298. https://doi.org/10.3390/ijerph10115284




