Towards Commercial Production of Sponge Medicines
Abstract
:1. Introduction
- To understand metabolite production following steps are needed:
- - Identification of induction factors of metabolite production.
- - Identifying biosynthetic pathways of secondary metabolites.
- - Identification of the location of bioactive compound production.
- Choosing and improving one of the following culture systems:
- - Whole sponge culture.
- - Sponge cell culture.
- - Symbiont culture.
- - Genetic modification.
2. Understanding Metabolite Production in the Sponge
2.1. Induction of Sponge Metabolite Production
2.2. Biosynthesis of Secondary Metabolites
2.3. Location of Secondary Metabolite Production in the Sponge
3. Culture Systems
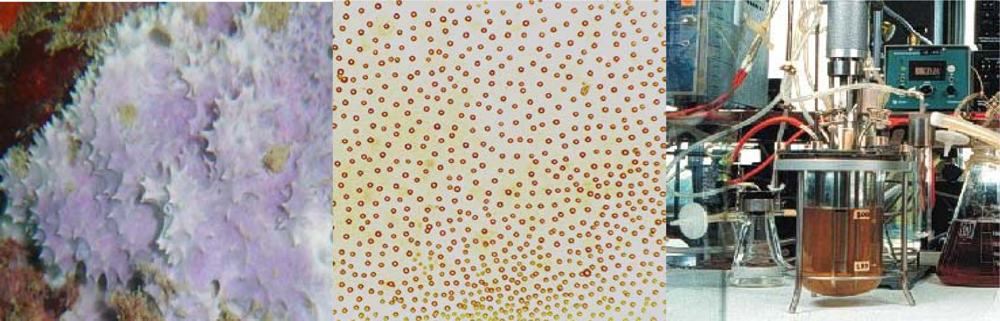
3.1. Sponge Culture
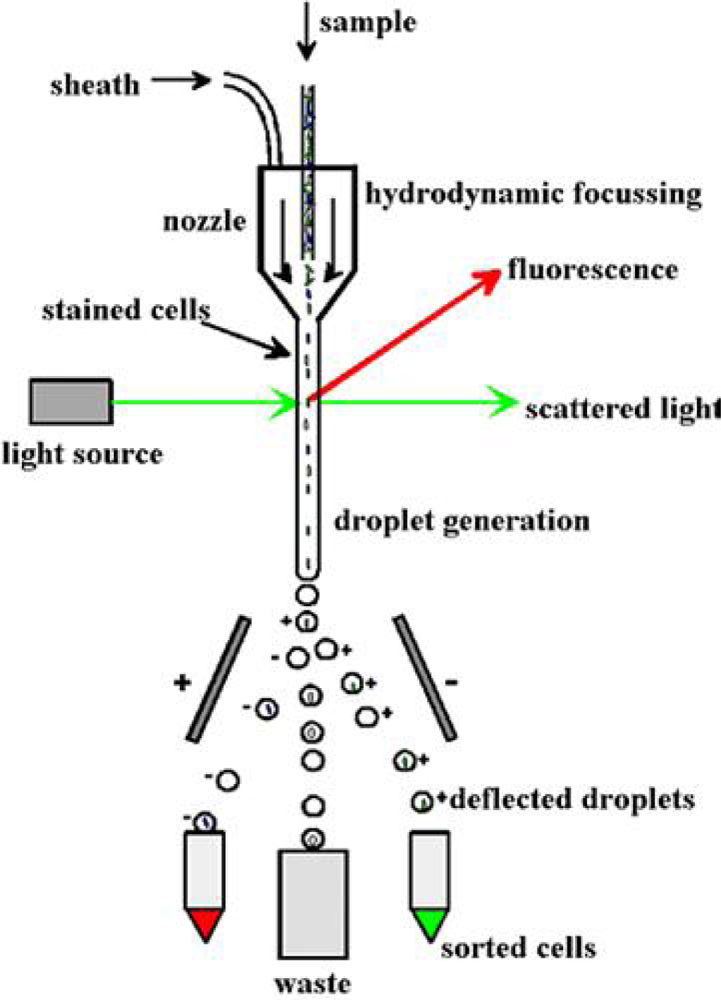
3.2. Sponge Cell Culture
3.3. Symbiont Culture
3.4. Genetic Modification
4. Concluding Summary
- Samples Availability: Available from the authors.
References
- Faulkner, DJ. Marine natural products. Nat Prod Rep 2002, 19, 1–48. [Google Scholar]
- Sipkema, D; Franssen, MCR; Osinga, R; Tramper, J; Wijffels, RH. Marine sponges as pharmacy. Mar Biotechnol 2005, 7, 142–162. [Google Scholar]
- Dumdei, EJ; Blunt, JW; Munro, MHG; Battershill, CN; Page, MJ. The Whys and Whats of Sponge Chemistry: Why Chemists Extract Sponges and What Problems Does This Cause? Watanabe, Y, Fusetani, N, Eds.; Springer-Verlag: Tokyo, Japan, 1998; pp. 353–364. [Google Scholar]
- Sipkema, D; Osinga, R; Schatton, W; Mendola, D; Tramper, J; Wijffels, RH. Large-scale production of pharmaceuticals by marine sponges: Sea, cell, or synthesis. Biotechnol Bioeng 2005, 90, 201–222. [Google Scholar]
- Hoover, CA; Slattery, M; Marsh, AG. A functional approach to transcriptome profiling: Linking gene expression patterns to metabolites that matter. Mar Biotechnol 2007, 9, 411–419. [Google Scholar]
- Munro, MHG; Blunt, JW; Lake, RJ; Litaudon, M; Battershill, CN; Page, MJ. From Seabed to Sickbed: What are the prospects? In Sponges in Time and Space: Biology, Chemistry, Paleontology; Soest, RVM, van Kempen, TMG, Braekman, JC, Eds.; Balkema: Rotterdam, The Netherland, 1994; pp. 473–484. [Google Scholar]
- Wulff, JL. Ecological interactions of marine sponges. Can J Zool 2006, 84, 146–166. [Google Scholar]
- Proksch, P. Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon 1994, 32, 639–655. [Google Scholar]
- Ruzicka, R; Gleason, DF. Latitudinal variation in spongivorous fishes and the effectiveness of sponge chemical defenses. Oecologia 2008, 154, 785–794. [Google Scholar]
- Abdo, DA; Motti, CA; Battershill, CN; Harvey, ES. Temperature and spatiotemporal variability of salicylihalamide A in the sponge Haliclona sp. J Chem Ecol 2007, 33, 1635–1645. [Google Scholar]
- Page, MJ; Northcote, PT; Webb, VL; Mackey, S; Handley, SJ. Aquaculture trials for the production of biologically active metabolites in the New Zealand sponge Mycale hentscheli (Demospongiae: Poecilosclerida). Aquaculture 2005, 250, 256–269. [Google Scholar]
- Becerro, MA; Turon, X; Uriz, MJ. Natural variation of toxicity in encrusting sponge Crambe crambe (Schidt) in relation to size and environment. J Chem Ecol 1995, 21, 1931–1946. [Google Scholar]
- Lopez-Legentil, S; Bontemps-Subielos, N; Turon, X; Banaigs, B. Secondary metabolite and inorganic contents in Cystodytes sp. (Ascidiacea): Temporal patterns and association with reproduction and growth. Mar Biol 2007, 151, 293–299. [Google Scholar]
- Turon, X; Becerro, MA; Uriz, MJ; Llopis, J. Small-scale association measures in epibenthic communities as a clue for allelochemical interactions. Oecologia 1996, 108, 351–360. [Google Scholar]
- Thacker, RW; Becerro, MA; Lumbang, WA; Paul, VJ. Allelopathic interaction between sponges on a tropical reef. Ecology 1998, 79, 1740–1750. [Google Scholar]
- Becerro, MA; Thacker, RW; Turon, X; Uriz, MJ; Paul, VJ. Biogeography of sponge chemical ecology: Comparisons of tropical an temperate defenses. Oecologia 2003, 135, 91–101. [Google Scholar]
- Pawlik, JR; Chanas, B; Toonen, RJ; Fenical, W. Defenses of Caribean sponges against predatory reef fish. I. Chemical deterrence. Mar Ecol Prog Ser 1995, 127, 183–194. [Google Scholar]
- Schupp, P; Eder, C; Pail, C; Proksch, P. Distribition of secondary metabolites in the sponge Oceanapia sp. and its ecological implications. Mar Biol 1999, 135, 573–580. [Google Scholar]
- Thoms, C; Wolff, M; Padmakumar, K; Ebel, R; Proksch, P. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z Naturforsch 2004, 59c, 113–122. [Google Scholar]
- Baldwin, IT. The alkaloidal responses of wild tobacco to real and simulated herbivory. Oecologia 1988, 77, 378–381. [Google Scholar]
- Thornton, RG; Kerr, RG. Induction of pseuopterosin biosynthesis in the gorgonian Pseudopterogorgia elisabethae. J Chem Ecol 2002, 28, 2083–2090. [Google Scholar]
- Thompson, JE; Murphy, PT; Bergquist, PR; Evans, EA. Environmetally induced variation in diterpene composition of the marine sponge Rhopaloeides odorabile. Biochem Syst Ecol 1987, 15, 595–606. [Google Scholar]
- Webster, NS; Xavier, JR; Freckelton, M; Motti, CA; Cobb, R. Shifts in microbial and chemical patterns within the marine sponge Aplysina aerophoba during a disease outbreak. Environ Microbiol 2008, 10, 3366–3376. [Google Scholar]
- Duckworth, AR; Samples, GA; Wright, AE; Pomponi, SA. In vitro culture of the tropical sponge Axinella corrugata (Demospongia): Effect of food cell concentration on growth, clearance rate and biosynthesis of stevensine. Mar Biotechnol 2003, 5, 519–527. [Google Scholar]
- Thoms, C; Ebel, R; Proksch, P. Activated chemical defense in Aplysina sponges revisited. J Chem Ecol 2006, 32, 97–123. [Google Scholar]
- Ebel, R; Brenzinger, M; Kunze, A; Gross, HJ; Proksch, P. Wound activation of protoxins in marine sponge. Aplysina aerophoba J Chem Ecol 1997, 23, 1451–1462. [Google Scholar]
- Thoms, C; Schupp, PJ. Activated chemical defense in marine sponges-a case study on Aplysinella rhax. J Chem Ecol 2008, 34, 1242–1252. [Google Scholar]
- Walters, KD; Pawlik, JR. Is there a trade-off between wound-healing and chemical defenses among caribbean reef sponges. Integr Comp Biol 2005, 45, 352–358. [Google Scholar]
- Fortman, JL; Sherman, DH. Utilizing the power of microbial genetics to bridge the gap between the promise and the application of matine natural products. ChemBioChem 2005, 6, 960–978. [Google Scholar]
- Grozdanov, L; Hentschel, U. An environmental genomics perspective on the diversity and function of marine sponge-associated microbiota. Curr Opin Microbiol 2007, 10, 215–220. [Google Scholar]
- Kennedy, J; Marchesi, JR; Dobson, ADW. Marine metagenomics: Strategies for the discovery of novel enzymes with biotechnological applications from marine environments. Microb Cell Factories 2008, 7, 27. [Google Scholar]
- Hutchinson, CR. Polyketide and non-ribosomal peptide synthases: Falling together by coming apart. Proc Natl Acad Sci USA 2003, 100, 3010–3012. [Google Scholar]
- Piel, J; Hui, D; Wen, G; Butzke, D; Platzer, M; Fusetani, N; Matsunaga, S. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Proc Natl Acad Sci USA 2004, 101, 16222–16227. [Google Scholar]
- Blunt, JW; Copp, BR; Hu, WP; Munro, MHG; Northcote, PT; Prinsep, MR. Marine natural products. Nat Prod Rep 2008, 26, 170–244. [Google Scholar]
- Moco, S. Metabolomics technologies applied to the identification of compounds in plants; Wageningen University: Wageningen, The Netherland, 2007. [Google Scholar]
- Hahn, S; Stoilov, TB; Ha, TBT; Readerstorff, D; Doss, GA; Li, H; Djerassi, C. Biosyntethic studies of marine lipids. 17. The course of chain elongation and desaturation in long-chain fatty acids. J Am Chem Soc 1988, 110, 8117–8124. [Google Scholar]
- Raederstorff, D; Shu, AYL; Thompson, JE; Djerassi, C. Biosyntethic studies of marine lipids. 11 1 Synthesis, biosynthesis, and absolute configuration of the internally branched demospongic acid 22-Methyl-5,9-octacosadienoic acid. J Org Chem 1987, 52, 2337–2346. [Google Scholar]
- Byrne, KM; Smith, SK; Ondeyka, JG. Biosynthesis of nodulisporic acid a: Precursor studies. J Am Chem Soc 2002, 124, 7055–7060. [Google Scholar]
- Sara, M; Bavestrello, G; Cattanea-vietti, R; Cerrano, C. Endosymbiosis in sponges: Relevance for epigenesist and evolution. Symbiosis 1998, 25, 57–70. [Google Scholar]
- Taylor, MW; Radax, R; Steger, D; Wagner, M. Sponge-associated microorganisms: Evolution, Ecology and Biotechnological potential. Microbiol Mol Biol Rev 2007, 71, 295–347. [Google Scholar]
- Wijffels, RH. Potential of sponges and microalgae for marine biotechnology. Trends Biotechnol 2008, 26, 26–31. [Google Scholar]
- Becerro, MA; Paul, VJ; Starmer, J. Intracolonial variation in chemical defenses of the sponge Cacospongia sp. and its consequences on generalist fish predators and the specialist nudibranch predator Glossodoris pallida. Mar Ecol Prog Ser 1998, 168, 187–196. [Google Scholar]
- Kubanek, J; Whalen, KE; Engel, S; Kelly, SR; Henkel, TP; Fenical, W; Pawlik, JR. Multiple defensive roles for triterpene glycosides from two Caribbean sponges. Oecologia 2002, 131, 125–136. [Google Scholar]
- Unson, MD; Faulkner, DJ. Cyanobacterial symbiont biosynthesis of chlorinated metabolites from Dysidea herbacea (Porifera). Experientia 1993, 49, 349–353. [Google Scholar]
- Garson, MJ; Flowers, AE; Webb, RI; Charan, RD; McCaffrey, EJ. A sponge/dinoflagellate association in the haplosclerid sponge Haliclona sp.: Cellular origin of cytotoxic alkaloids by Percoll density gradient fractionation. Cell Tissue Res 1998, 293, 365–373. [Google Scholar]
- Uriz, MJ; Turon, X; Galera, J; Tur, JM. New light on the cell location of avarol within the sponge Dysidea avara (Dendroceratida). Cell Tissue Res 1996, 285, 519–527. [Google Scholar]
- Pomponi, SA; Willoughby, R. Sponge Cell Culture for the Production of Bioactive Metabolites; van Soest, R, van Kempen, TMG, Braekman, JC, Eds.; Balkema: Rotterdam, The Netherlands, 1994; pp. 395–400. [Google Scholar]
- Lee, KL; Lee, JH; Lee, HK. Microbial symbiosis in marine sponges. J Microbiol 2001, 39, 254–264. [Google Scholar]
- Proksch, P; Edrada, RA; Ebel, R. Drugs from the seas-current status and microbiological implications. Appl Microbiol Biotechnol 2002, 59, 125–134. [Google Scholar]
- Hill, RT. Manzamine-producing actinomycetes. United States Patent Application 20050244938.
- Sakai, R; Yoshida, K; Kimura, A; Koike, K; Jimbo, M; Kobiyama, A; Kamiya, H. Cellular origin of dysiherbaine, an excitory amino acid derived from a marine sponge. ChemBioChem 2008, 9, 543–551. [Google Scholar]
- Holler, U; Wright, AD; Matthee, GF; Konig, GM; Draeger, S; Aust, HJ; Schulz, B. Fungi from marine sponges: Diversity, biological activity and secondary metabolites. Mycol Res 2000, 104, 1354–1365. [Google Scholar]
- Newman, DJ; Hill, RT. New drugs from marine microbes: The tide is turning. JIndMicrobiolBiotechnol 2006, 33, 539–544. [Google Scholar]
- Muller, WEG; Zahn, RK; Gasic, MJ; Dogovi, N; Maidhof, PA; Becker, C; Diehl-Seifert, B; Eich, E. Avarol, a cytostatically active compound from the marin sponge Dysidea avara. Comput Biochem Physiol 1985, 80C, 47–52. [Google Scholar]
- Dayton, PK. Observations of growth, dispersal and population dynamics of some sponges in McMurdo Sound, Antarctica. Colloques Internationaux du CNRS 1979, 291, 271–282. [Google Scholar]
- de Caralt, S; Uriz, MJ; Wijffels, RH. Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Mar Ecol Prog Ser 2008, 360, 97–106. [Google Scholar]
- de Voogd, N. The mariculture potential of the indonesian reef-dweeling sponge Callyspongia (Euplacella) biru: Growth, survival and bioactive compounds. Aquaculture 2007, 262, 54–64. [Google Scholar]
- Duckworth, AR; Pomponi, SA. Relative importance of bacteria, microalgae and yeast for growth of the sponge Halichondria melanadocia (De Laubenfels, 1936): A laboratory study. J Exp Mar Biol Ecol 2005 323, 151–195.
- Garrabou, J; Zabala, M. Growth dynamics in four Mediterranean Demosponges. Estuarine Coastal Shelf Sci 2001, 52, 293–303. [Google Scholar]
- Koopmans, M; Wijffels, RH. Seasonal growth rate of the sponge Haliclona oculata (demospongiae: Haplosclerida). Mar Biotechnol 2008, 10, 502–510. [Google Scholar]
- Barthel, D; Theede, H. A new method for the culture of marine sponges and its application for experimental studies. Ophelia 1986, 25(2), 75–82. [Google Scholar]
- Belarbi, EH; Ramirez Dominguez, M; Ceron Garcia, MC; Contreras Gomez, A; Garcia Camacho, F; Molina Grima, E. Cultivation of explants of the marine sponge Crambe crambe in closed systems. Biomol Eng 2003, 20, 333–337. [Google Scholar]
- De Caralt, S; Agell, G; Uriz, MJ. Long-term culture of sponge explants: Conditions enhancing survival and growth, and assessment of bioactivity. Biomol Eng 2003, 20, 339–347. [Google Scholar]
- Duckworth, AR; Battershill, CN. Sponge aquaculture for the production of biolocigally active metabolites: The influence of farming protocols and environment. Aquaculture 2003, 221, 311–329. [Google Scholar]
- Mendola, D. Aquaculture of three phyla of marine invertebrates to yield bioactive metabolites: Process developments and economics. Biomol Eng 2003, 20, 441–458. [Google Scholar]
- Osinga, R; Belarbi, EH; Molina Grima, E; Tramper, J; Wijffels, RH. Progress towards a controlled culture of the marine sponge Pseadosuberites andrewsi in a bioreactor. J Biotechnol 2003, 100, 141–146. [Google Scholar]
- Sipkema, D; Yosef, NAM; Adamczewski, M; Osinga, R; Mendola, D; Tramper, J; Wijffels, RH. Hypothesized kinetic models for describing the growthof globular and encrusting demosponges. Mar Biotechnol 2005, 8, 40–51. [Google Scholar]
- Hadas, E; Shpigel, M; Ilan, M. Sea ranching of the marine sponge Negombata magnifica (Demospongiae, Latrunculiidae) as a first step for latrunculin B mass production. Aquaculture 2005, 244, 159–169. [Google Scholar]
- Osinga, R; De Beukelaer, PB; Meijer, EM; Tramper, J; Wijffels, RH. Growth of the sponge Pseudosuberites (aff.) andrewsi in a closed system. J Biotechnol 1999, 70, 155–161. [Google Scholar]
- Pomponi, SA; Willoughby, R; Kaighn, ME; Wright, AE. Development of techniques for in vitro production of bioactive natural products from marine sponges. In Invertebrate Cell Culture: Novel Directions and Biotechnology Applications; Maramorosch, K, Mitsuhashi, J, Eds.; Science Publishers: Enfield, NH, USA, 1997; pp. 231–237. [Google Scholar]
- Thompson, JE; Barrow, KD; Faulkner, DJ. Localization of two brominated metabolites, aerothionen and homoaerothionin, in spherulous cells of the marine sponge Aplysina fistularis. Acta Zool Stockholm 1983, 64, 199–210. [Google Scholar]
- Muller, WEG; Wimmer, W; Schatton, W; Bohm, M; Batel, R; Filic, Z. Initiation of an aquaculture of sponges for the sustainable production of bioactive metabolites in open systems: Example, Geodia cydonium. Mar Biotechnol 1999, 1, 569–579. [Google Scholar]
- De Caralt, S; Uriz, MJ; Wijffels, RH. Cell culture from sponges: Pluripotency and immortality. Trends Biotechnol 2008, 25, 467–471. [Google Scholar]
- Mendola, D. The importance of water flow for culture of Dysidea avara sponges; Wageningen University: Wageningen, The Netherland, 2008. [Google Scholar]
- Sipkema, D; Van Wielink, R; Van Lammeren, AAM; Tramper, J; Osinga, R; Wijffels, RH. Primmorphs from seven marine sponges: Formation and structure. J Biotechnol 2003, 100, 127–139. [Google Scholar]
- Li, Z; He, L; Miao, X. Cultivable bacterial community from south China sea sponge as revealed by DGGE fingerprinting and 16S rDNA phylogenetic analysis. Curr Microbiol 2007, 55, 465–472. [Google Scholar]
- Muscholl-Silberhorn, A; Thiel, V; Imhoff, JF. Abundance and bioactivity of cultured sponge-associated bacteria from the Mediterranean Sea. Microb Ecol 2007, 55, 94–106. [Google Scholar]
- Kaeberlein, T; Lewis, K; Epstein, SS. Isolating “uncultivable” microorganisms in pure culture in a simulated natural environment. Science 2006, 296, 1127–1129. [Google Scholar]
- Kennedy, J; Baker, P; Piper, C; Cotter, PD; Walsh, M; Mooij, MJ; Bourke, MB; Rea, MC; O’Connor, PM; Ross, RP; Gill, C; O’Gara, F; Machesi, JR; Dobson, ADW. Isolation and analysis of bacteria with antimicrobial activities from the marine sponge Haliclona simulans collected from Irish waters. Mar Biotechnol 2009, 11, 384–396. [Google Scholar]
- Piel, J. Bacterial symbionts: Prospects for the sustainable production of invertebrate-derived pharmaceuticals. Curr Med Chem 2006, 13, 39–50. [Google Scholar]
- Anthony, JR; Anthony, LC; Nowroozi, F; Kwon, G; Newman, JD; Keasling, JD. Optimization of the mevalonate-based isoprenoid biosynthetic pathway in Escherichia coli for production of the anti-malarial drug presursor amorpha-4,11-diene. Metab Eng 2009, 11, 13–19. [Google Scholar]
- Engels, B; Dahm, P; Jennewein, S. Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (Paclitaxel) production. Metab Eng 2008, 10, 201–206. [Google Scholar]
- Ro, DK; Ouellet, M; Paradise, EM; Burd, H; Eng, D; Paddon, CJ; Newman, JD; Keasling, JD. Induction of multiple pleiotropic drug resistance genes in yeast engineered to produce an increased level of anti-malarial drug precursor, artemisinic acid. BMC Biotechnol 2008, 8, 83. [Google Scholar]
- Chemler, JA; Koffas, MAG. Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin Biotechnol 2008, 19, 597–605. [Google Scholar]


© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koopmans, M.; Martens, D.; Wijffels, R.H. Towards Commercial Production of Sponge Medicines. Mar. Drugs 2009, 7, 787-802. https://doi.org/10.3390/md7040787
Koopmans M, Martens D, Wijffels RH. Towards Commercial Production of Sponge Medicines. Marine Drugs. 2009; 7(4):787-802. https://doi.org/10.3390/md7040787
Chicago/Turabian StyleKoopmans, Marieke, Dirk Martens, and Rene H. Wijffels. 2009. "Towards Commercial Production of Sponge Medicines" Marine Drugs 7, no. 4: 787-802. https://doi.org/10.3390/md7040787



