Antartin, a Cytotoxic Zizaane-Type Sesquiterpenoid from a Streptomyces sp. Isolated from an Antarctic Marine Sediment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Structure Elucidation
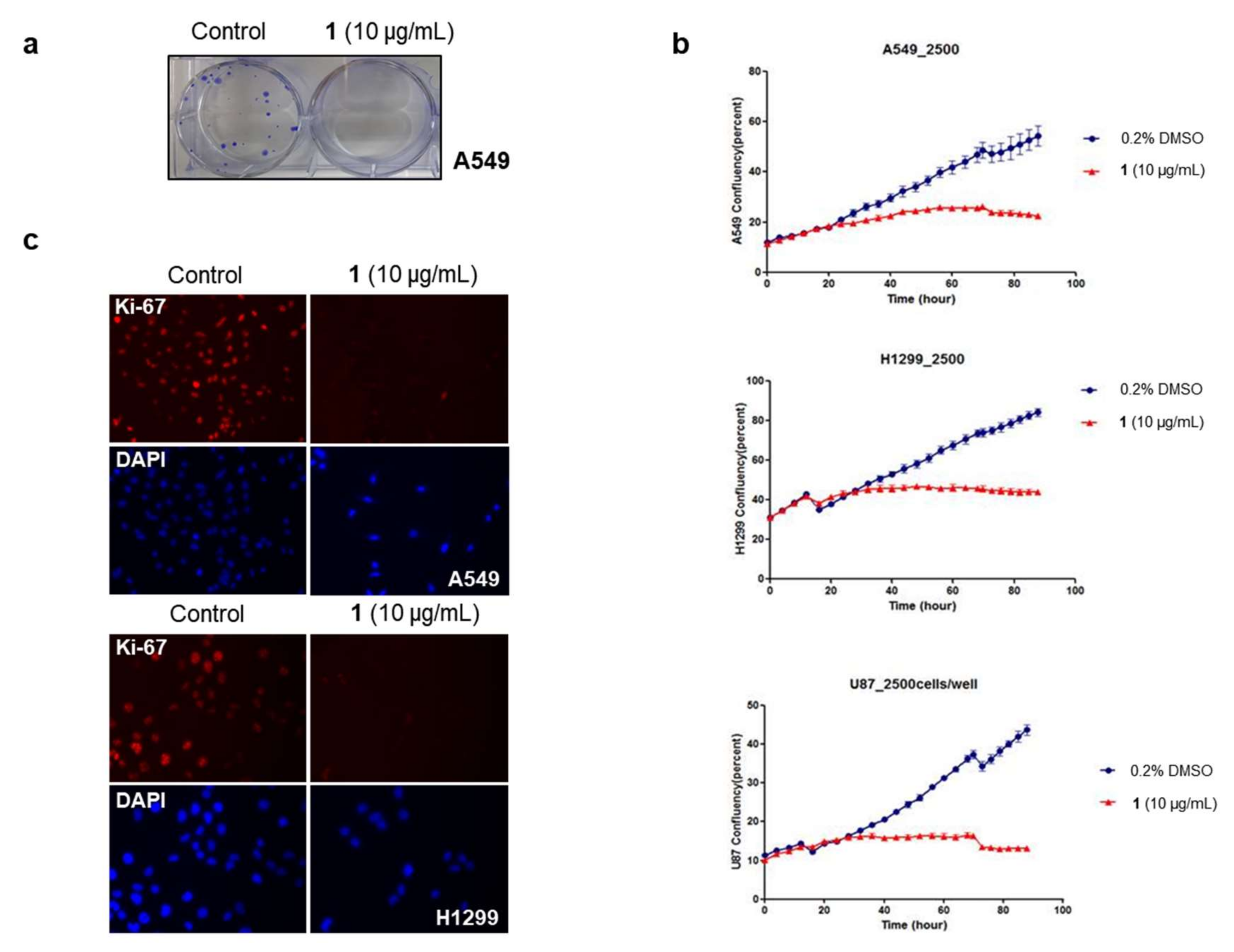
2.2. Bioactivities
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain Isolation and Fermentation
3.3. Extraction and Purification
3.4. Computational Analysis
3.5. Cell Culture and Proliferation Assay
3.6. Focus Formation Assay
3.7. Immunofluorescence Staining
3.8. Cell Cycle Analysis
3.9. Western Blot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spivey, A.C.; Weston, M.; Woodhead, S. Celastraceae sesquiterpenoids: Biological activity and synthesis. Chem. Soc. Rev. 2002, 31, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Kreuger, M.R.O.; Grootjans, S.; Biavatti, M.W.; Vandenabeele, P.; D’Herde, K. Sesquiterpene lactones as drugs with multiple targets in cancer treatment: Focus on parthenolide. Anticancer Drugs 2012, 23, 883–896. [Google Scholar] [PubMed]
- Gliszczyńska, A.; Brodelius, P.E. Sesquiterpene coumarins. Phytochem. Rev. 2012, 11, 77–96. [Google Scholar] [CrossRef]
- Durán-Peña, M.J.; Botubol Ares, J.M.; Hanson, J.R.; Collado, I.G.; Hernández-Galán, R. Biological activity of natural sesquiterpenoids containing a gem-dimethylcyclopropane unit. Nat. Prod. Rep. 2015, 32, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Elissawy, A.M.; El-Shazly, M.; Ebada, S.S.; Singab, A.B.; Proksch, P. Bioactive terpenes from marine-derived fungi. Mar. Drugs 2015, 13, 1966–1992. [Google Scholar] [CrossRef] [PubMed]
- Bartikova, H.; Hanusova, V.; Skalova, L.; Ambroz, M.; Bousova, I. Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem. 2014, 14, 2478–2494. [Google Scholar] [CrossRef] [PubMed]
- Manivasagan, P.; Kang, K.-H.; Sivakumar, K.; Li-Chan, E.C.Y.; Oh, H.-M.; Kim, S.-K. Marine actinobacteria: An important source of bioactive natural products. Environ. Toxicol. Pharmacol. 2014, 38, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Kornprobst, J.-M. Encyclopedia of Marine Natural Products; Wiley-Blackwell: Oxford, UK, 2010. [Google Scholar]
- Soldatou, S.; Baker, B.J. Cold-water marine natural products, 2006 to 2016. Nat. Prod. Rep. 2017, 34, 585–626. [Google Scholar] [CrossRef] [PubMed]
- Lebar, M.D.; Heimbegner, J.L.; Baker, B.J. Cold-water marine natural products. Nat. Prod. Rep. 2007, 24, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Skropeta, D.; Wei, L. Recent advances in deep-sea natural products. Nat. Prod. Rep. 2014, 31, 999–1025. [Google Scholar] [CrossRef] [PubMed]
- Vettel, P.R.; Coates, R.M. Total synthesis of (-)-prezizaene and (-)-prezizanol. J. Org. Chem. 1980, 45, 5430–5432. [Google Scholar] [CrossRef]
- Kido, F.; Uda, H.; Yoshikoshi, A. Total synthesis of zizaane-type sesquiterpenoids. J. Chem. Soc. D Chem. Commun. 1969, 1335–1336. [Google Scholar] [CrossRef]
- Zhao, B.; Lei, L.; Vassylyev, D.G.; Lin, X.; Cane, D.E.; Kelly, S.L.; Yuan, H.; Lamb, D.C.; Waterman, M.R. Crystal structure of albaflavenone monooxygenase containing a moonlighting terpene synthase active site. J. Biol. Chem. 2009, 284, 36711–36719. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-W.; Peng, K.; Liu, Z.; Zhang, G.-Y.; Li, J.; Wang, N.; Steinmetz, A.; Liu, Y. Strepsesquitriol, a rearranged zizaane-type sesquiterpenoid from the deep-sea-derived actinomycete Streptomyces sp. SCSIO 10355. J. Nat. Prod. 2013, 76, 2360–2363. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chou, W.K.W.; Himmelberger, J.A.; Litwin, K.M.; Harris, G.G.; Cane, D.E.; Christianson, D.W. Reprogramming the chemodiversity of terpenoid cyclization by remolding the active site contour of epi-isozizaene synthase. Biochemistry 2014, 53, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, S.; Lin, X.; Nara, A.; Komatsu, M.; Cane, D.E.; Ikeda, H. Characterization of a silent sesquiterpenoid biosynthetic pathway in Streptomyces avermitilis controlling epi-isozizaene albaflavenone biosynthesis and isolation of a new oxidized epi-isozizaene metabolite. Microb. Biotechnol. 2011, 4, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Cane, D.E. Biosynthesis of the sesquiterpene antibiotic albaflavenone in Streptomyces coelicolor. Mechanism and stereochemistry of the enzymatic formation of epi-isozizaene. J. Am. Chem. Soc. 2009, 131, 6332–6333. [Google Scholar] [CrossRef] [PubMed]
- Mei, C.; Zhou, S.; Zhu, L.; Ming, J.; Zeng, F.; Xu, R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar. Drugs 2017, 15. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Cheng, X.; Gao, Z.; Zhao, R.; Zhang, S. Inhibition of tumor cell growth by adenine is mediated by apoptosis induction and cell cycle S phase arrest. Oncotarget 2017, 8, 94286–94296. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, F.; Kousara, M.; Chen, L.; Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef] [PubMed]
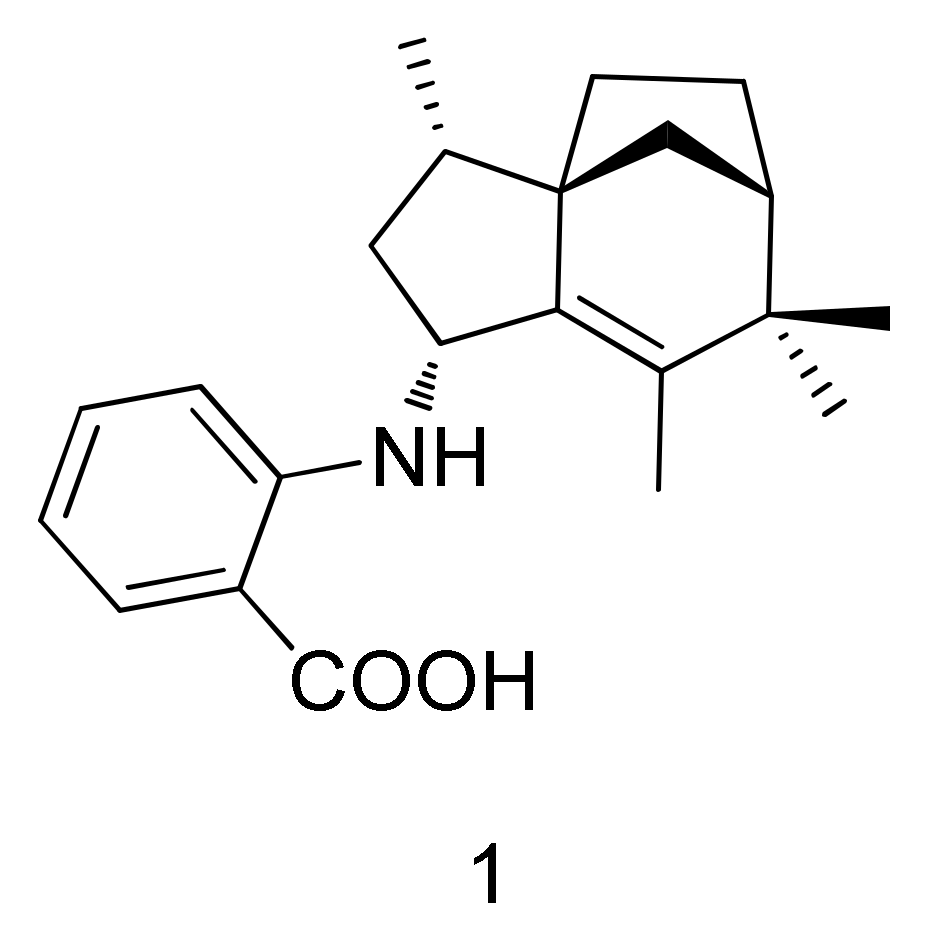
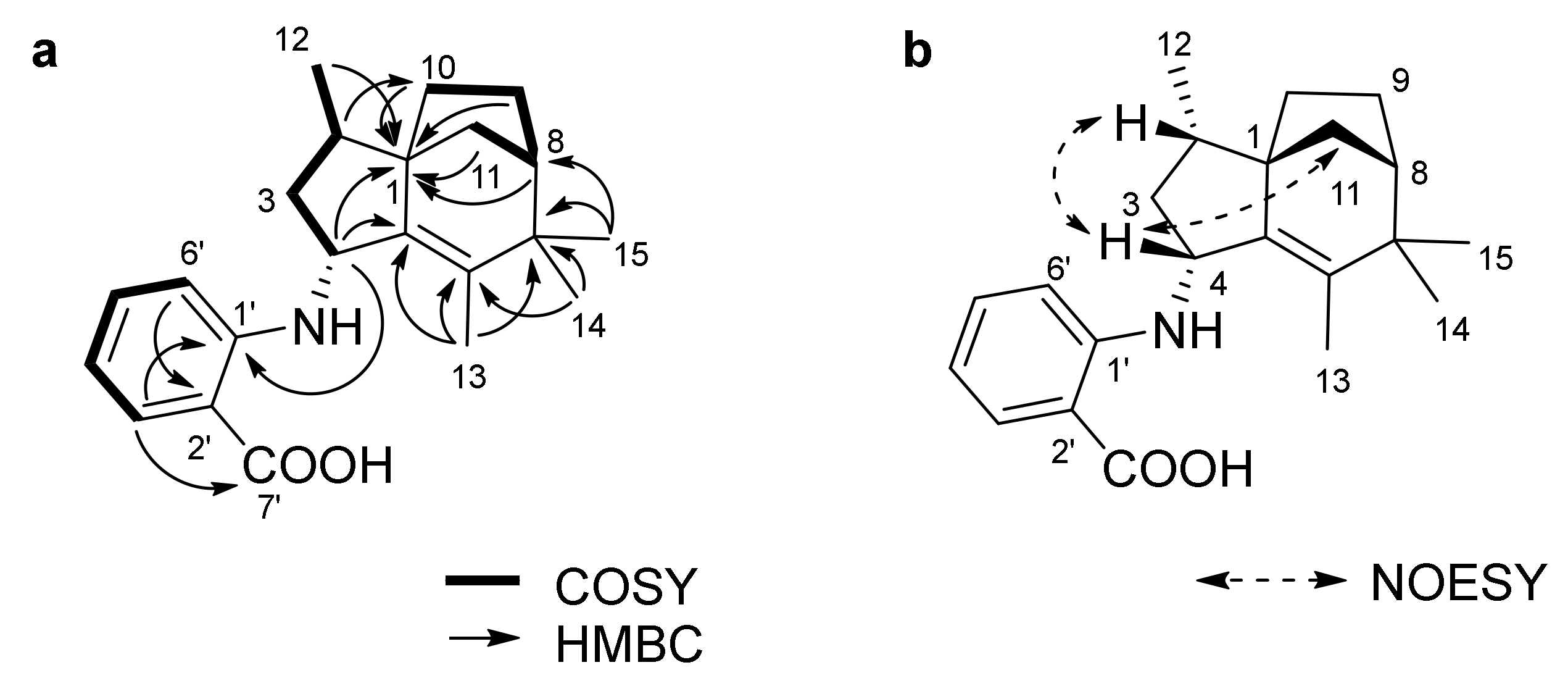


| Position | δc, Type 2 | δH, (J in Hz) | COSY | HMBC |
|---|---|---|---|---|
| 1 | 54.2, qC | |||
| 2 | 38.7, CH | 2.07, m | 3a, 12 | 1, 3, 10, 11, 12 |
| 3a | 41.7, CH2 | 1.49, m | 2, 4 | 2, 12 |
| 3b | 1.83, m | 4, 5 | ||
| 4 | 53.9, CH | 4.24, d (6.1) | 3a | 1, 2, 5, 6, 1′ |
| 5 | 146.8, qC | |||
| 6 | 136.4, qC | |||
| 7 | 42.4, qC | |||
| 8 | 49.1, CH | 1.90, m | 9a, 9b, 10, 11 | 1, 6 |
| 9a | 25.8, CH2 | 1.67, m | 8, 10a | 1, 8, 11 |
| 9b | 1.84, m | 8, 10b | ||
| 10a | 30.8, CH2 | 1.28, br | 9a | |
| 10b | 1.48, m | 9b | 1, 5, 8 | |
| 11 | 38.6, CH2 | 1.60, d (3.0) | 8 | 1, 5, 7, 8, 9, 10 |
| 12 | 14.3, CH3 | 0.94, d (6.8) | 2 | 1, 2, 3 |
| 13 | 14.0, CH3 | 1.52, s | 5, 6, 7 | |
| 14 | 25.7, CH3 | 1.04, s | 6, 7, 8, 15 | |
| 15 | 29.6, CH3 | 1.10, s | 6, 7, 8, 14 | |
| 1′ | 152.1, qC | |||
| 2′ | 111.9, qC | |||
| 3′ | 133.7, CH | 7.85, dd (6.9, 2.0) | 4′ | 1′, 5′, 6′, 7′ |
| 4′ | 115.3, CH | 6.51, dd (6.9, 6.9) | 3′, 5′ | 2′, 3′, 5′, 6′ |
| 5′ | 135.5, CH | 7.31, dd (6.9, 6.9) | 4′, 6′ | 1′, 3′, 6′ |
| 6′ | 113.0, CH | 6.69, dd (6.9, 2.0) | 5′ | 2′, 4′, 7′ |
| 7′ | 172.5, qC |
| Lung (NSCLC2) | Colon | Prostate | Pancreatic | |||
| H1299 | A549 | HCT116 | PC3 | Mia-paca2 | ASPC1 | |
| DMSO | 100 ± 4 | 100 ± 6.4 | 100 ± 1.6 | 100 ± 3.8 | 100 ± 3.8 | 100 ± 3.5 |
| 1 | 24.8 ± 4.5 | 12.2 ± 0.8 | 1.6 ± 0.4 | 13.9 ± 4.1 | 14.2 ± 4 | 63.2 ± 4.9 |
| Liver | Cervix | Brain | Thyroid | Skin | ||
| HepG2 | HeLa | U87 | Cal62 | CHL-1 | SK-Mel28 | |
| DMSO | 100 ± 5.1 | 100 ± 12.9 | 100 ± 4.0 | 100 ± 2.6 | 100 ± 2.6 | 100 ± 2.4 |
| 1 | 20.5 ± 5.1 | 4.1 ± 3.6 | 0.8 ± 1.4 | 0.2 ± 0.0 | 0.4 ± 0.1 | 14.1 ± 2.8 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Lee, E.J.; Lee, J.; Leutou, A.S.; Shin, Y.-H.; Choi, B.; Hwang, J.S.; Hahn, D.; Choi, H.; Chin, J.; et al. Antartin, a Cytotoxic Zizaane-Type Sesquiterpenoid from a Streptomyces sp. Isolated from an Antarctic Marine Sediment. Mar. Drugs 2018, 16, 130. https://doi.org/10.3390/md16040130
Kim D, Lee EJ, Lee J, Leutou AS, Shin Y-H, Choi B, Hwang JS, Hahn D, Choi H, Chin J, et al. Antartin, a Cytotoxic Zizaane-Type Sesquiterpenoid from a Streptomyces sp. Isolated from an Antarctic Marine Sediment. Marine Drugs. 2018; 16(4):130. https://doi.org/10.3390/md16040130
Chicago/Turabian StyleKim, Dayoung, Eun Ju Lee, Jihye Lee, Alain S. Leutou, Yern-Hyerk Shin, Bomi Choi, Ji Sun Hwang, Dongyup Hahn, Hyukjae Choi, Jungwook Chin, and et al. 2018. "Antartin, a Cytotoxic Zizaane-Type Sesquiterpenoid from a Streptomyces sp. Isolated from an Antarctic Marine Sediment" Marine Drugs 16, no. 4: 130. https://doi.org/10.3390/md16040130





