Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review
Abstract
:1. Introduction
2. Marine Algae: Sources, Production Strategies, and Applied Perspectives
3. Biosorption and Its Mechanisms
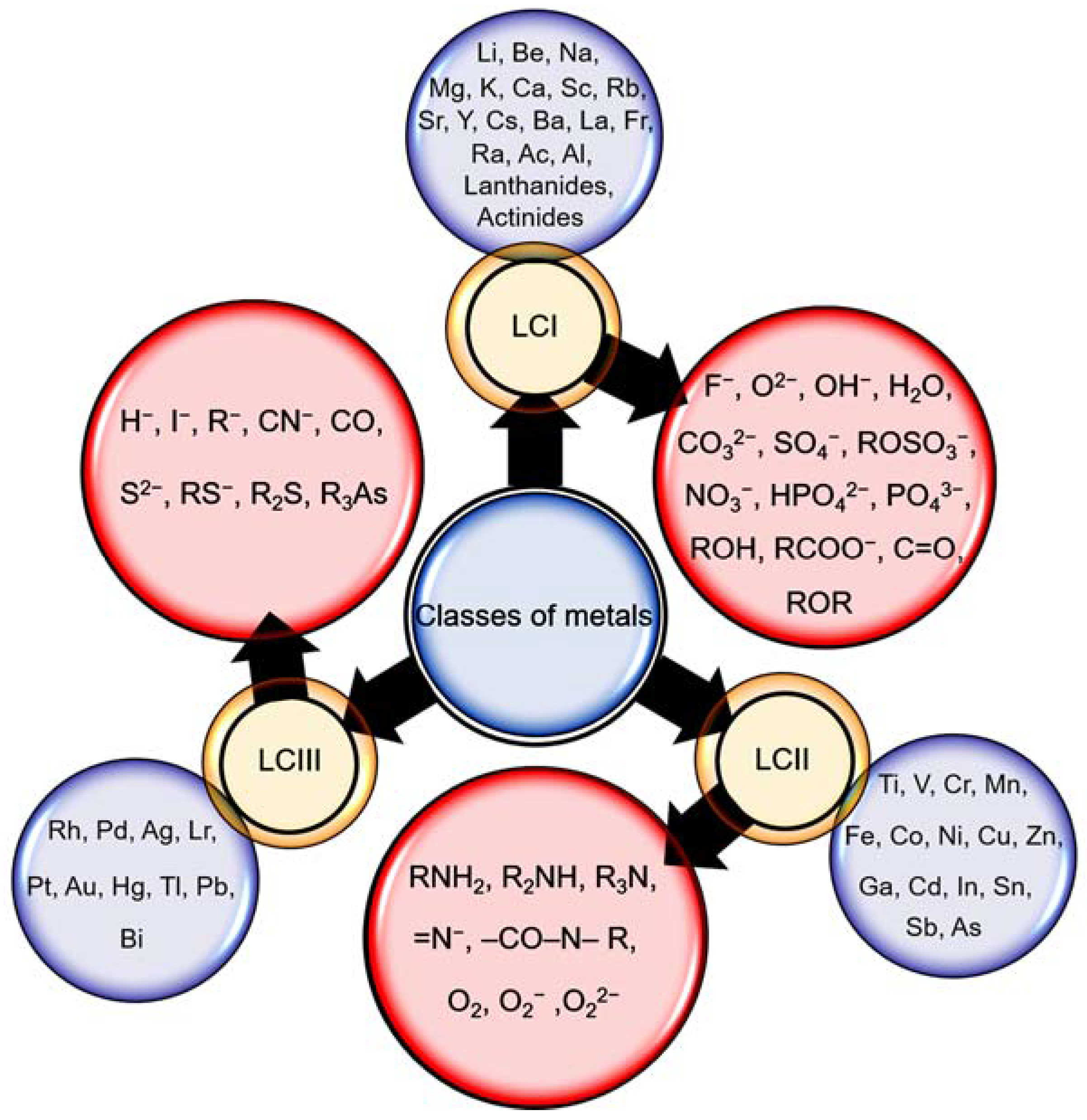
4. Factors Affecting Biosorption
5. Potential Biosorbents
6. Potentially Toxic Elements—Heavy Metals
7. Biosorption of Potentially Toxic Elements
7.1. Biosorption of Cadmium
7.2. Biosorption of Chromium
7.3. Biosorption of Lead
7.4. Biosorption of Zinc
8. Concluding Remarks and Future Considerations
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahmed, I.; Iqbal, H.M.N.; Dhama, K. Enzyme-based biodegradation of hazardous pollutants—An overview. J. Exp. Biol. Agric. Sci. 2017, 5, 402–411. [Google Scholar] [CrossRef]
- Ullah, S.; Zuberi, A.; Alagawany, M.; Farag, M.R.; Dadar, M.; Karthik, K.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Cypermethrin induced toxicities in fish and adverse health outcomes: Its prevention and control measure adaptation. J. Environ. Manag. 2018, 206, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioproc. Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Barrios-Estrada, C.; de Jesús Rostro-Alanis, M.; Muñoz-Gutiérrez, B.D.; Iqbal, H.M.N.; Kannan, S.; Parra-Saldívar, R. Emergent contaminants: Endocrine disruptors and their laccase-assisted degradation―A review. Sci. Total Environ. 2018, 612, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Nabeel, F.; Iqbal, H.M.N.; Li, C.; Zhou, Y. Fluorescent sensor based models for the detection of environmentally-related toxic heavy metals. Sci. Total Environ. 2018, 615, 476–485. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N.; Shah, S.Z.H.; Hu, H.; Wang, W.; Zhang, X. Horseradish peroxidase-assisted approach to decolorize and detoxify dye pollutants in a packed bed bioreactor. J. Environ. Manag. 2016, 183, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Barrios-Estrada, C.; de Jesús Rostro-Alanis, M.; Parra, A.L.; Belleville, M.P.; Sanchez-Marcano, J.; Iqbal, H.M.N.; Parra-Saldívar, R. Potentialities of active membranes with immobilized laccase for Bisphenol A degradation. Int. J. Biol. Macromol. 2017, 108, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Novel characteristics of horseradish peroxidase immobilized onto the polyvinyl alcohol-alginate beads and its methyl orange degradation potential. Int. J. Biol. Macromol. 2017, 105, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Bio-catalytic performance and dye-based industrial pollutants degradation potential of agarose-immobilized MnP using a Packed Bed Reactor System. Int. J. Biol. Macromol. 2017, 102, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Bio-based degradation of emerging endocrine-disrupting and dye-based pollutants using cross-linked enzyme aggregates. Environ. Sci. Pollut. Res. 2017, 24, 7035–7041. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Development of horseradish peroxidase-based cross-linked enzyme aggregates and their environmental exploitation for bioremediation purposes. J. Environ. Manag. 2017, 188, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Asgher, M.; Parra-Saldivar, R.; Hu, H.; Wang, W.; Zhang, X.; Iqbal, H.M.N. Immobilized ligninolytic enzymes: An innovative and environmental responsive technology to tackle dye-based industrial pollutants―A review. Sci. Total Environ. 2017, 576, 646–659. [Google Scholar] [CrossRef] [PubMed]
- Belkhamssa, N.; Rocha-Santos, T.; Ksibi, M. Development of Electrochemical Immunosensors for Endocrine Disrupting Detection. In Euro-Mediterranean Conference for Environmental Integration; Springer: Cham, Switzerland, 2017; pp. 305–306. [Google Scholar]
- Chatha, S.A.S.; Asgher, M.; Iqbal, H.M.N. Enzyme-based solutions for textile processing and dye contaminant biodegradation―A review. Environ. Sci. Pollut. Res. 2017, 24, 14005–14018. [Google Scholar] [CrossRef] [PubMed]
- Bulgariu, L.; Bulgariu, D. Sustainable Utilization of Marine Algae Biomass for Environmental Bioremediation. In Prospects and Challenges in Algal Biotechnology; Springer: Singapore, 2017; pp. 179–217. [Google Scholar]
- Bulgariu, D.; Bulgariu, L. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresour. Technol. 2012, 103, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.; Duarte, A.C.; Rocha-Santos, T.A. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Lopes, I.; Rocha-Santos, T.A.P.; Gonçalves, F.; Pereira, R. Treatment of real industrial wastewaters through nano-TiO2 and nano-Fe2O3 photocatalysis: Case study of mining and kraft pulp mill effluents. Environ. Technol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T.; Bilal, M.; Iqbal, H.M.N.; Hu, H.; Zhang, X. Reaction mechanism and degradation pathway of rhodamine 6G by photocatalytic treatment. Water Air Soil Pollut. 2017, 228. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.N.; Hu, H.; Wang, W.; Zhang, X. Toxicological Assessment and UV/TiO2-Based Induced Degradation Profile of Reactive Black 5 Dye. Environ. Manag. 2018, 61, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective removal of the heavy metal ions from waters and industrial wastewaters by ion-exchange method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Charerntanyarak, L. Heavy metals removal by chemical coagulation and precipitation. Water Sci. Technol. 1999, 39, 135–138. [Google Scholar]
- Yuan, C.; Weng, C.H. Electrokinetic enhancement removal of heavy metals from industrial wastewater sludge. Chemosphere 2006, 65, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Laldawngliana, C.; Tiwari, D. Iron oxide nano-particles-immobilized-sand material in the treatment of Cu (II), Cd (II) and Pb (II) contaminated waste waters. Chem. Eng. J. 2012, 195, 103–111. [Google Scholar] [CrossRef]
- Qdais, H.A.; Moussa, H. Removal of heavy metals from wastewater by membrane processes: A comparative study. Desalination 2004, 164, 105–110. [Google Scholar] [CrossRef]
- Pohl, P.; Schimmack, W. Adsorption of radionuclides (134Cs, 85Sr, 226Ra, 241Am) by extracted biomasses of cyanobacteria (Nostoc Carneum, N. Insulare, Oscillatoria Geminata and Spirulina Laxis-Sima) and phaeophyceae (Laminaria Digitata and L. Japonica; waste products from alginate production) at different pH. J. Appl. Phycol. 2006, 18, 135–143. [Google Scholar]
- Mata, Y.N.; Torres, E.; Blazquez, M.L.; Ballester, A.; González, F.M.J.A.; Munoz, J.A. Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Singh, A.; Gaur, J.P. Kinetics of adsorption and uptake of Cu2+ by Chlorella vulgaris: Influence of pH, temperature, culture age, and cations. J. Environ. Sci. Heal. A 2002, 37, 399–414. [Google Scholar] [CrossRef]
- Michalak, I.; Chojnacka, K. Interactions of metal cations with anionic groups on the cell wall of the macroalga Vaucheria sp. Eng. Life Sci. 2010, 10, 209–217. [Google Scholar] [CrossRef]
- Dönmez, G.Ç.; Aksu, Z.; Öztürk, A.; Kutsal, T. A comparative study on heavy metal biosorption characteristics of some algae. Process Biochem. 1999, 34, 885–892. [Google Scholar] [CrossRef]
- Davis, T.A.; Volesky, B.; Mucci, A. A review of the biochemistry of heavy metal biosorption by brown algae. Water Res. 2003, 37, 4311–4330. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Munoz, J.A. Comparative study of biosorption of heavy metals using different types of algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Ahmed, I.; Iqbal, H.M.N. High-value compounds from microalgae with industrial exploitability―A review. Front. Biosci. (Sch. Ed.) 2017, 9, 319–342. [Google Scholar] [PubMed]
- Centella, M.H.; Arévalo-Gallegos, A.; Parra-Saldivar, R.; Iqbal, H.M.N. Marine-derived bioactive compounds for value-added applications in bio-and non-bio sectors. J. Clean. Prod. 2017, 168, 1559–1565. [Google Scholar] [CrossRef]
- Parsaeimehr, A.; Mancera-Andrade, E.I.; Robledo-Padilla, F.; Iqbal, H.M.N.; Parra-Saldivar, R. A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res. 2017, 26, 312–322. [Google Scholar] [CrossRef]
- Bule, M.H.; Ahmed, I.; Maqbool, F.; Bilal, M.; Iqbal, H.M.N. Microalgae as a source of high-value bioactive compounds. Front. Biosci. (Sch. Ed.) 2018, 10, 197–216. [Google Scholar] [PubMed]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbial. Rev. 2012, 36, 761–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.D.; Chen, C.C.; Huynh, P.; Chang, J.S. Exploring the potential of using algae in cosmetics. Bioresour. Technol. 2015, 184, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Parra, R.; Iqbal, H.M.N. Phycobiliproteins: A Novel Green Tool from Marine Origin Blue-Green Algae and Red Algae. Protein Peptide Lett. 2017, 24, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dionisio-Sese, M.L. Aquatic microalgae as potential sources of UV-screening compounds. Philippine J. Sci. 2010, 139, 5–16. [Google Scholar]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Kiuru, P.; D’Auria, M.V.; Muller, C.D.; Tammela, P.; Vuorela, H.; Yli-Kauhaluoma, J. Exploring marine resources for bioactive compounds. Planta Med. 2014, 80, 1234–1246. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Y.; Wan, M.; Yan, Y.; Feng, F.; Qu, X.; Wang, J.; Shen, G.; Li, W.; Fan, J.; Wang, W. Novel flat-plate photobioreactors for microalgae cultivation with special mixers to promote mixing along the light gradient. Bioresour. Technol. 2014, 159, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ariede, M.B.; Candido, T.M.; Jacome, A.L.M.; Velasco, M.V.R.; de Carvalho, J.C.M.; Baby, A.R. Cosmetic attributes of algae-A review. Algal Res. 2017, 25, 483–487. [Google Scholar] [CrossRef]
- Sanghvi, A.; Martin Lo, Y. Present and potential industrial applications of macro-and microalgae. Recent Pat. Food Nutr. Agric. 2010, 2, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Choi, I.G.; Kim, K.H. Red macroalgae as a sustainable resource for bio-based products. Trends Biotechnol. 2015, 33, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and microalgae as a potential source for commercial applications along with biofuels production: A biorefinery approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Veglio, F.; Beolchini, F. Removal of metals by biosorption: A review. Hydrometallurgay 1997, 44, 301–316. [Google Scholar] [CrossRef]
- Volesky, B. Biosorption and me. Water Res. 2007, 41, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, K. Biosorption and bioaccumulation―The prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Flouty, R.; Estephane, G. Bioaccumulation and biosorption of copper and lead by a unicellular algae Chlamydomonas reinhardtii in single and binary metal systems: A comparative study. J. Environ. Manag. 2012, 111, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents―A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- El Hameed, A.H.A.; Eweda, W.E.; Abou-Taleb, K.A.; Mira, H.I. Biosorption of uranium and heavy metals using some local fungi isolated from phosphatic fertilizers. Ann. Agric. Sci. 2015, 60, 345–351. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Balasubramanian, R. Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J. Environ. Manag. 2015, 160, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Yu, H.Q. Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour. Technol. 2014, 160, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Tewari, S.; Rai, J.P.N. Ion exchange during heavy metal bio-sorption from aqueous solution by dried biomass of macrophytes. Bioresour. Technol. 2008, 99, 1932–1938. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Bhat, A.H.; Buang, A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: Kinetic and equilibrium modeling. J. Clean. Prod. 2018, 171, 1361–1375. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.M. Biosorption of heavy metal ions from aqueous solution by red macroalgae. J. Hazard. Mater. 2011, 192, 1827–1835. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.P.; King, P.; Prasad, V.S.R.K. Removal of copper from aqueous solution using Ulva fasciata sp.—A marine green algae. J. Hazard. Mater. 2006, 137, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, W.; Liu, D.; Liu, T.; Wang, Z. Biosorption isotherm study of Cd2+, Pb2+ and Zn2+ biosorption onto marine bacterium Pseudoalteromonas sp. SCSE709-6 in multiple systems. J. Mol. Liq. 2017, 247, 230–237. [Google Scholar] [CrossRef]
- Brouers, F.; Al-Musawi, T.J. On the optimal use of isotherm models for the characterization of biosorption of lead onto algae. J. Mol. Liq. 2015, 212, 46–51. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Hassan, A.F.; Azab, Y.A. Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt. J. Basic Appl. Sci. 2016, 3, 241–249. [Google Scholar] [CrossRef]
- Vendruscolo, F.; da Rocha Ferreira, G.L.; Antoniosi Filho, N.R. Biosorption of hexavalent chromium by microorganisms. Int. Biodeter. Biodegr. 2017, 119, 87–95. [Google Scholar] [CrossRef]
- Sivaprakash, K.; Adlin Blessi, T.L.; Madhavan, J. Biosorption of Nickel from Industrial Wastewater using Zygnema sp. J. Inst. Eng. (India) Ser. A 2015, 96, 319–326. [Google Scholar] [CrossRef]
- Torab-Mostaedi, M.; Asadollahzadeh, M.; Hemmati, A.; Khosravi, A. Biosorption of lanthanum and cerium from aqueous solutions by grapefruit peel: Equilibrium, kinetic and thermodynamic studies. Res. Chem. Int. 2015, 41, 559–573. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Kyzas, G.Z. Progress in batch biosorption of heavy metals onto algae. J. Mol. Liq. 2015, 209, 77–86. [Google Scholar] [CrossRef]
- Demey, H.; Vincent, T.; Guibal, E. A novel algal-based sorbent for heavy metal removal. Chem. Eng. J. 2018, 332, 582–595. [Google Scholar] [CrossRef]
- Herrera-Estrella, L.R.; Guevara-Garcia, A.A. Heavy metal adaptation. In Encyclopedia of Life Sciences (ELS); John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 1–9. [Google Scholar]
- Gaur, A.; Adholeya, A. Prospects of arbuscular mycorrhizal fungi in phytoremediation of heavy metal contaminated soils. Curr. Sci. 2004, 86, 528–534. [Google Scholar]
- Lasat, M.M. Phytoextraction of metals from contaminated soil: A review of plant/soil/metal interaction and assessment of pertinent agronomic issues. J. Hazard. Subst. Res. 1999, 2. [Google Scholar] [CrossRef]
- Torres, M.A.; Barros, M.P.; Campos, S.C.; Pinto, E.; Rajamani, S.; Sayre, R.T.; Colepicolo, P. Biochemical biomarkers in algae and marine pollution: A review. Ecotoxicol. Environ. Saf. 2008, 71, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Brower, J.B.; Ryan, R.L.; Pazirandeh, M. Comparison of ion-exchange resins and biosorbents for the removal of heavy metals from plating factory wastewater. Environ. Sci. Technol. 1997, 31, 2910–2914. [Google Scholar] [CrossRef]
- Duffus, J.H. “Heavy metals” a meaningless term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Hong, K.S.; Lee, H.M.; Bae, J.S.; Ha, M.G.; Jin, J.S.; Hong, T.E.; Kim, J.P.; Jeong, E.D. Removal of heavy metal ions by using calcium carbonate extracted from starfish treated by protease and amylase. J. Anal. Sci. Technol. 2011, 2, 75–82. [Google Scholar] [CrossRef]
- Volesky, B.; Holan, Z.R. Biosorption of heavy metals. Biotechnol. Prog. 1995, 11, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Rao, R.A.K.; Ajmal, M. Heavy metal pollution and its control through nonconventional adsorbents (1998–2007): A review. J. Int. Environ. Appl. Sci. 2008, 3, 101–141. [Google Scholar]
- Dietert, R.R.; Piepenbrink, M.S. Lead and immune function. Crit. Rev. Toxicol. 2006, 36, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, Y.; Liang, J.; Shan, G.; Wang, Y.; Shi, Q. Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin. Chim. Acta 2006, 370, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, N.D. Mechanisms of lead-induced hypertension and cardiovascular disease. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H454–H465. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.H.; Harp, J.P.; Han, D.Y. Pb neurotoxicity: Neuropsychological effects of lead toxicity. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Pant, N.; Kumar, G.; Upadhyay, A.D.; Patel, D.K.; Gupta, Y.K.; Chaturvedi, P.K. Reproductive toxicity of lead, cadmium, and phthalate exposure in men. Environ. Sci. Pollut. Res. 2014, 21, 11066–11074. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, F.; Arato, I.; Lilli, C.; Bellucci, C.; Bodo, M.; Calvitti, M.; Luca, G. Acute effects of lead on porcine neonatal Sertoli cells in vitro. Toxicol. In Vitro 2018, 48, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Bae, O.N.; Chung, S.M.; Kang, K.T.; Lee, J.Y.; Chung, J.H. Enhancement of platelet aggregation and thrombus formation by arsenic in drinking water: A contributing factor to cardiovascular disease. Toxicol. Appl. Pharmacol. 2002, 179, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Kapaj, S.; Peterson, H.; Liber, K.; Bhattacharya, P. Human health effects from chronic arsenic poisoning―A review. J. Environ. Sci. Health Part A 2006, 41, 2399–2428. [Google Scholar] [CrossRef] [PubMed]
- States, J.C.; Barchowsky, A.; Cartwright, I.L.; Reichard, J.F.; Futscher, B.W.; Lantz, R.C. Arsenic toxicology: Translating between experimental models and human pathology. Environ. Health Perspect. 2011, 119, 1356–1363. [Google Scholar] [CrossRef] [PubMed]
- Argos, M.; Ahsan, H.; Graziano, J.H. Arsenic and human health: Epidemiologic progress and public health implications. Rev. Environ. Health 2012, 27, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Naujokas, M.F.; Anderson, B.; Ahsan, H.; Aposhian, H.V.; Graziano, J.H.; Thompson, C.; Suk, W.A. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ. Health Perspect. 2013, 121, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Duruibe, J.O.; Ogwuegbu, M.O.C.; Egwurugwu, J.N. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Martin, S.; Griswold, W. Human health effects of heavy metals. Environ. Sci. Technol. Br. Citizens 2009, 15, 1–6. [Google Scholar]
- Bernard, A. Cadmium & its adverse effects on human health. Indian J. Med. Res. 2008, 128, 557–564. [Google Scholar] [PubMed]
- Karri, V.; Kumar, V.; Ramos, D.; Oliveira, E.; Schuhmacher, M. Comparative In Vitro Toxicity Evaluation of Heavy Metals (Lead, Cadmium, Arsenic, and Methylmercury) on HT-22 Hippocampal Cell Line. Biol. Trace Elem. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Klein, C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.K. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicol. 2003, 189, 147–163. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Gosens, I.; Cassee, F.R.; Zanella, M.; Manodori, L.; Brunelli, A.; Costa, A.L.; Stone, V. Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology 2016, 10, 1084–1095. [Google Scholar] [CrossRef] [PubMed]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M.R. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef] [PubMed]
- Lamaia, C.; Kruatrachuea, M.; Pokethitiyooka, P.; Upathamb, E.S.; Soonthornsarathoola, V. Toxicity and accumulation of lead and cadmium in the filamentous green alga Cladophora fracta (OF Muller ex Vahl) Kutzing: A laboratory study. Sci. Asia 2005, 31, 121–127. [Google Scholar] [CrossRef]
- Godlewska-Żyłkiewicz, B. Analytical applications of living organisms for preconcentration of trace metals and their speciation. Crit. Rev. Anal.Chem. 2001, 31, 175–189. [Google Scholar] [CrossRef]
- Arief, V.O.; Trilestari, K.; Sunarso, J.; Indraswati, N.; Ismadji, S. Recent progress on biosorption of heavy metals from liquids using low cost biosorbents: Characterization, biosorption parameters and mechanism studies. CLEAN–Soil Air Water 2008, 36, 937–962. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Wilde, K.L.; Stauber, J.L.; Markich, S.J.; Franklin, N.M.; Brown, P.L. The effect of pH on the uptake and toxicity of copper and zinc in a tropical freshwater alga (Chlorella sp.). Arch. Environ. Contam. Toxicol. 2006, 51, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D. Absorption and adsorption of heavy metals by microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 2013; Chapter 32; pp. 602–611. [Google Scholar]
- Gupta, V.K.; Rastogi, A. Equilibrium and kinetic modelling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J. Hazard. Mater. 2008, 153, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.K.; Gaur, J.P. Use of algae for removing heavy metal ions from wastewater: Progress and prospects. Crit. Rev. Biotechnol. 2005, 25, 113–152. [Google Scholar] [CrossRef] [PubMed]
- Perales-Vela, H.V.; Pena-Castro, J.M.; Canizares-Villanueva, R.O. Heavy metal detoxification in eukaryotic microalgae. Chemosphere 2006, 64, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, S.; Volesky, B. Biosorption by marine algae. In Bioremediation; Springer: Dordrecht, The~Netherlands, 2000; pp. 139–169. [Google Scholar]
- Romera, E.; Gonzalez, F.; Ballester, A.; Blazquez, M.L.; Munoz, J.A. Biosorption with algae: A statistical review. Crit. Rev. Biotechnol. 2006, 26, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Sillanpää, M. Applications of chitin-and chitosan-derivatives for the detoxification of water and wastewater—A short review. Adv. Colloid Int. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Filipič, M. Mechanisms of cadmium induced genomic instability. Mutat. Res./Fund. Mol. Mech. Mutagen. 2012, 733, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Demim, S.; Drouiche, N.; Aouabed, A.; Benayad, T.; Dendene-Badache, O.; Semsari, S. Cadmium and nickel: Assessment of the physiological effects and heavy metal removal using a response surface approach by L. gibba. Ecol. Eng. 2013, 61, 426–435. [Google Scholar] [CrossRef]
- Sbihi, K.; Cherifi, O.; El Gharmali, A.; Oudra, B.; Aziz, F. Accumulation and toxicological effects of cadmium, copper and zinc on the growth and photosynthesis of the freshwater diatom Planothidium lanceolatum (Brébisson) Lange-Bertalot: A laboratory study. J. Mater. Environ. Sci. 2012, 3, 497–506. [Google Scholar]
- Bădescu, I.S.; Bulgariu, D.; Bulgariu, L. Alternative utilization of algal biomass (Ulva sp.) loaded with Zn (II) ions for improving of soil quality. J. Appl. Phycol. 2017, 29, 1069–1079. [Google Scholar] [CrossRef]
- Cheng, J.; Yin, W.; Chang, Z.; Lundholm, N.; Jiang, Z. Biosorption capacity and kinetics of cadmium (II) on live and dead Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 211–221. [Google Scholar] [CrossRef]
- Mirghaffari, N.; Moeini, E.; Farhadian, O. Biosorption of Cd and Pb ions from aqueous solutions by biomass of the green microalga, Scenedesmus quadricauda. J. Appl. Phycol. 2015, 27, 311–320. [Google Scholar] [CrossRef]
- Lupea, M.; Bulgariu, L.; Macoveanu, M. Biosorption of Cd(II) from aqueous solution on marine green algae biomass. Environ. Eng. Manag. J. 2012, 11, 607–615. [Google Scholar]
- Sarı, A.; Tuzen, M. Biosorption of Pb (II) and Cd (II) from aqueous solution using green alga (Ulva lactuca) biomass. J. Hazard. Mater. 2008, 152, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Sarı, A.; Tuzen, M. Biosorption of cadmium (II) from aqueous solution by red algae (Ceramium virgatum): Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2008, 157, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Balasubramanian, R.; Iyer, C.S.P. Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 2007, 98, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Bayramoğlu, G.; Tuzun, I.; Celik, G.; Yilmaz, M.; Arica, M.Y. Biosorption of mercury (II), cadmium (II) and lead (II) ions from aqueous system by microalgae Chlamydomonas reinhardtii immobilized in alginate beads. Int. J. Miner. Process. 2006, 81, 35–43. [Google Scholar] [CrossRef]
- Gupta, V.K.; Shrivastava, A.K.; Jain, N. Biosorption of chromium (VI) from aqueous solutions by green algae Spirogyra species. Water Res. 2001, 35, 4079–4085. [Google Scholar] [CrossRef]
- Kaewsarn, P.; Yu, Q. Cadmium (II) removal from aqueous solutions by pre-treated biomass of marine alga Padina sp. Environ. Pollut. 2001, 112, 209–213. [Google Scholar] [CrossRef]
- Matheickal, J.T.; Yu, Q.; Woodburn, G.M. Biosorption of cadmium (II) from aqueous solutions by pre-treated biomass of marine alga Durvillaea potatorum. Water Res. 1999, 33, 335–342. [Google Scholar] [CrossRef]
- Atieh, M.A.; Bakather, O.Y.; Tawabini, B.S.; Bukhari, A.A.; Khaled, M.; Alharthi, M.; Fettouhi, M.; Abuilaiwi, F.A. Removal of chromium (III) from water by using modified and nonmodified carbon nanotubes. J. Nanomat. 2010. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Vaiopoulou, E.; Gikas, P. Effects of chromium on activated sludge and on the performance of wastewater treatment plants: A review. Water Res. 2012, 46, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Cirelli, A.F. Cr (VI) and Cr (III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, C.; Zhu, X.; Wang, X. Removal of chromium from aqueous solution by using oxidized multiwalled carbon nanotubes. J. Hazard. Mater. 2009, 162, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of lead (II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Cechinel, M.A.P.; de Souza, A.A.U. Study of lead (II) adsorption onto activated carbon originating from cow bone. J. Clean. Prod. 2014, 65, 342–349. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Cristian, P.; Violeta, P.; Anita-Laura, R.; Raluca, I.; Alexandrescu, E.; Andrei, S.; Daniela, I.E.; Raluca, M.A.; Cristina, M.; Ioana, C.A. Removal of zinc ions from model wastewater system using bicopolymer membranes with fumed silica. J. Water Process Eng. 2015, 8, 1–10. [Google Scholar] [CrossRef]


| Heavy Metal | Major Uses/Sources | Toxic Effects | Mechanism of Toxicity | References |
|---|---|---|---|---|
| Lead (Pb) | Lead batteries, lead paint, devices to shield from X-rays. | Nervous system, male reproductive system, microvascular endothelium, immune system, impairs mammalian spermatogenesis and sperm quality in vivo, inhibits sperm functions in vitro. | Lead has no biological functions. Oxidative stress (reactive oxygen species, ROS), with a reduction in the effects of antioxidants, is the principal mechanism. Lead ions also replace other ions such as Ca2+, Mg2+, and Na+ and disturb normal cell functions such as cellular adhesion, apoptosis, and neurotransmitter release. | [83,84,85,86,87,88] |
| Arsenic(Ar) | Agricultural chemicals (pesticides, fungicides, herbicides). | Cardiovascular/peripheral vascular disease, developmental abnormalities, immunological, and neurological disorders, carcinogenesis, diabetes, portal fibrosis. | Oxidative stress, genotoxicity, alteration in DNA repair, and p53 suppression (major contributor to carcinogenesis). | [89,90,91,92,93] |
| Cadmium (Cd) | Metal industry, paint pigments, fertilizers, cigarette smoke, food. | Pulmonary and gastrointestinal irritation, carcinogenesis (development of adenocarcinomas), Kidneys, liver and bones are also effected by cadmium exposure. | Competition with other ions (zinc, iron, copper), genotoxicity, lipid peroxidation, oxidative stress. | [94,95,96,97] |
| Chromium Cr(III)/Cr (VI) | Anticorrosive, industrial welding, chrome plating, leather industry, wood preservation. | Carcinogenic, gastric and intestinal ulcers, sperm damage, male reproductive system problems, anemia. | Cr (VI) is more potent than Cr (III); Oxidative stress, genotoxicity, alteration in cellular signaling pathway | [94,98] |
| Mercury (Hg) | Natural processes involved oceanic emissions and biomass burning. Anthropogenic sources included power plants, metal industry and gold mining. | Alzheimer’s disease, Parkinsonism, respiratory depression | Binding of mercury with sulfhydryl (–SH) groups disrupts normal cellular enzymatic processes. Increase in free radical concentration due to blockage of GSH by Hg is responsible for cell-damaging effects. | [94,95,97] |
| Copper (Cu) | Agriculture (fertilizers), leather industry (tanning), and photo-voltaic cells. | Carcinogenic, neurodegenerative disorders, responsible for complications in diabetes, promotes atherosclerosis. | Oxidative stress, enzyme inhibition, replaces normal ions of the body. | [94,99,100,101] |
| Zinc (Zn) | Oil refinery, mining, brass manufacturing, plumbing. | Ataxia, depression, gastrointestinal irritation, hematuria, icterus, impotence, kidney and liver failure, lethargy, macular degeneration, metal fume fever, prostate cancer, seizures, vomiting. | [94,102] |
| Potentially Toxic Elements | Algae Used | Adsorption Capacity | References |
|---|---|---|---|
| Zn(II) | Ulva sp. | 29.63 mg/g | [118] |
| Cd(II) | Chlorella vulgaris (dead) | 96.8% | [119] |
| Cd(II) | Chlorella vulgaris (live) | 95.2% | [119] |
| Cd | Scenedesmus quadricauda | 66% | [120] |
| Pb | Scenedesmus quadricauda | 82% | [120] |
| Cd(II) | Ulva lactuca | 85% | [121] |
| Cd(II) | Ulva lactuca | 29.2 mg/g | [122] |
| Pb(II) | Ulva lactuca | 34.7 mg/g | [122] |
| Cd(II) | Ceramium virgatum | 39.7 mg/g | [123] |
| Cu(II) | Ulva fasciata | 73.5 mg/g | [124] |
| Cu(II) | Sargassum sp. | 72.5 mg/g | [124] |
| Hg(II) | Chlamydomonas reinhardtii | 89.5 mg/g | [125] |
| Cd(II) | Chlamydomonas reinhardtii | 66.5 mg/g | [125] |
| Pb(II) | Chlamydomonas reinhardtii | 253.6 mg/g | [125] |
| Cr(VI) | Spirogyra sp. | 14.7 × 103 mg metal/kg | [126] |
| Cd(II) | Padina sp. | 90% | [127] |
| Cd(II) | Durvillaea potatorum | 90% | [128] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilal, M.; Rasheed, T.; Sosa-Hernández, J.E.; Raza, A.; Nabeel, F.; Iqbal, H.M.N. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Mar. Drugs 2018, 16, 65. https://doi.org/10.3390/md16020065
Bilal M, Rasheed T, Sosa-Hernández JE, Raza A, Nabeel F, Iqbal HMN. Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review. Marine Drugs. 2018; 16(2):65. https://doi.org/10.3390/md16020065
Chicago/Turabian StyleBilal, Muhammad, Tahir Rasheed, Juan Eduardo Sosa-Hernández, Ali Raza, Faran Nabeel, and Hafiz M. N. Iqbal. 2018. "Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—A Review" Marine Drugs 16, no. 2: 65. https://doi.org/10.3390/md16020065






