Synthesis of Poly(norbornene-methylamine), a Biomimetic of Chitosan, by Ring-Opening Metathesis Polymerization (ROMP)
Abstract
:1. Introduction
2. Results and Discussion
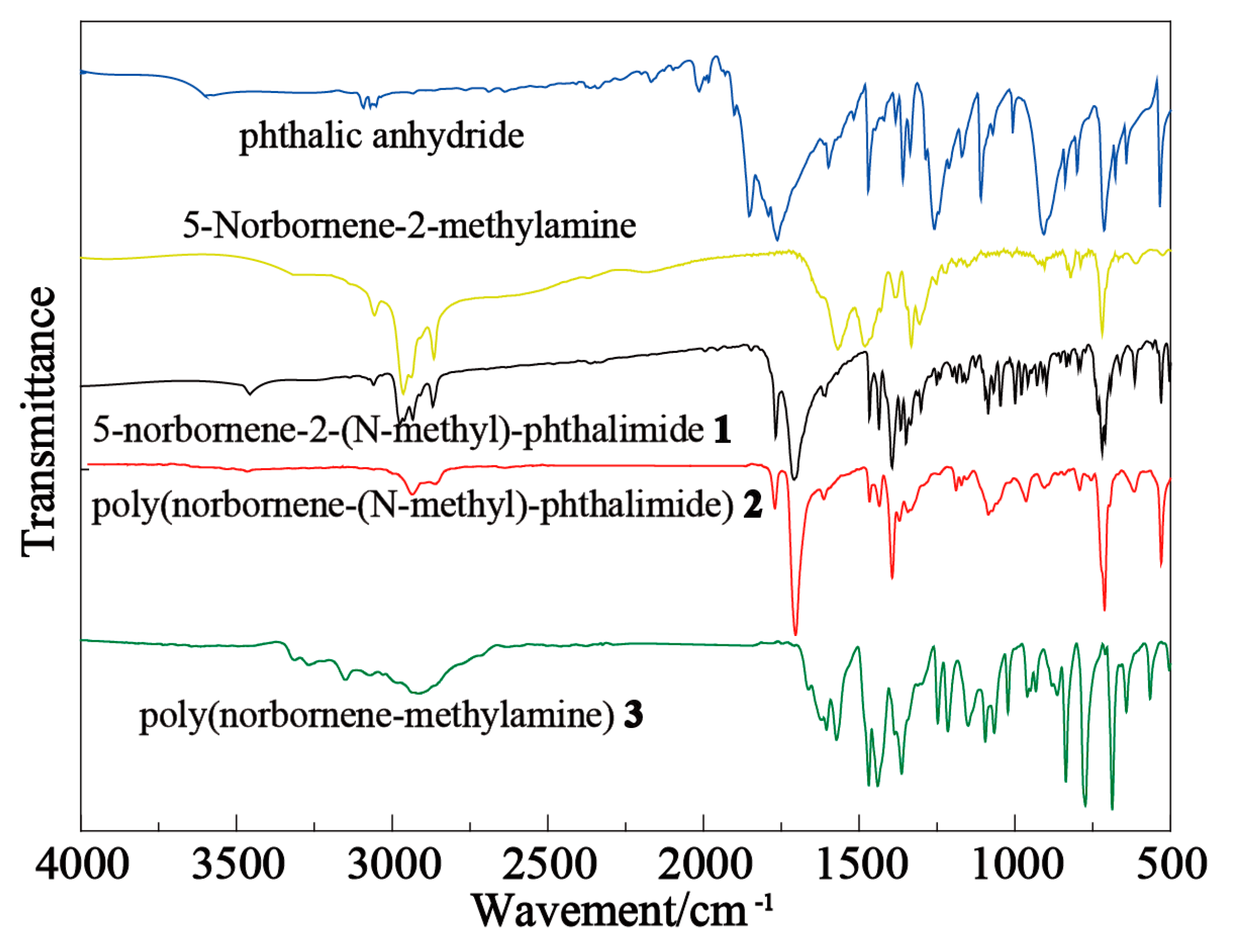
2.1. FTIR Characterization
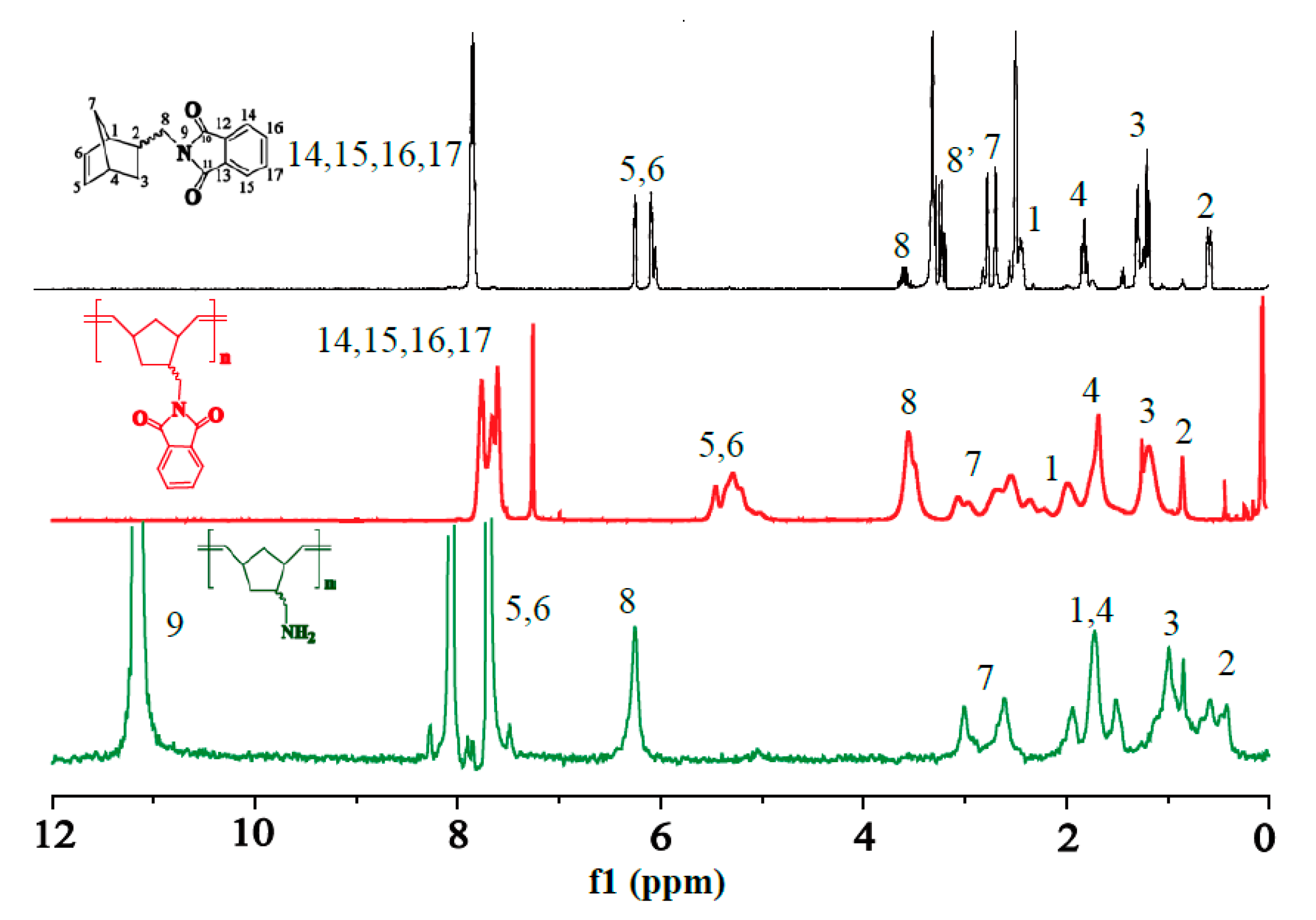
2.2. 1H-NMR Characterization
2.3. TG Characterization
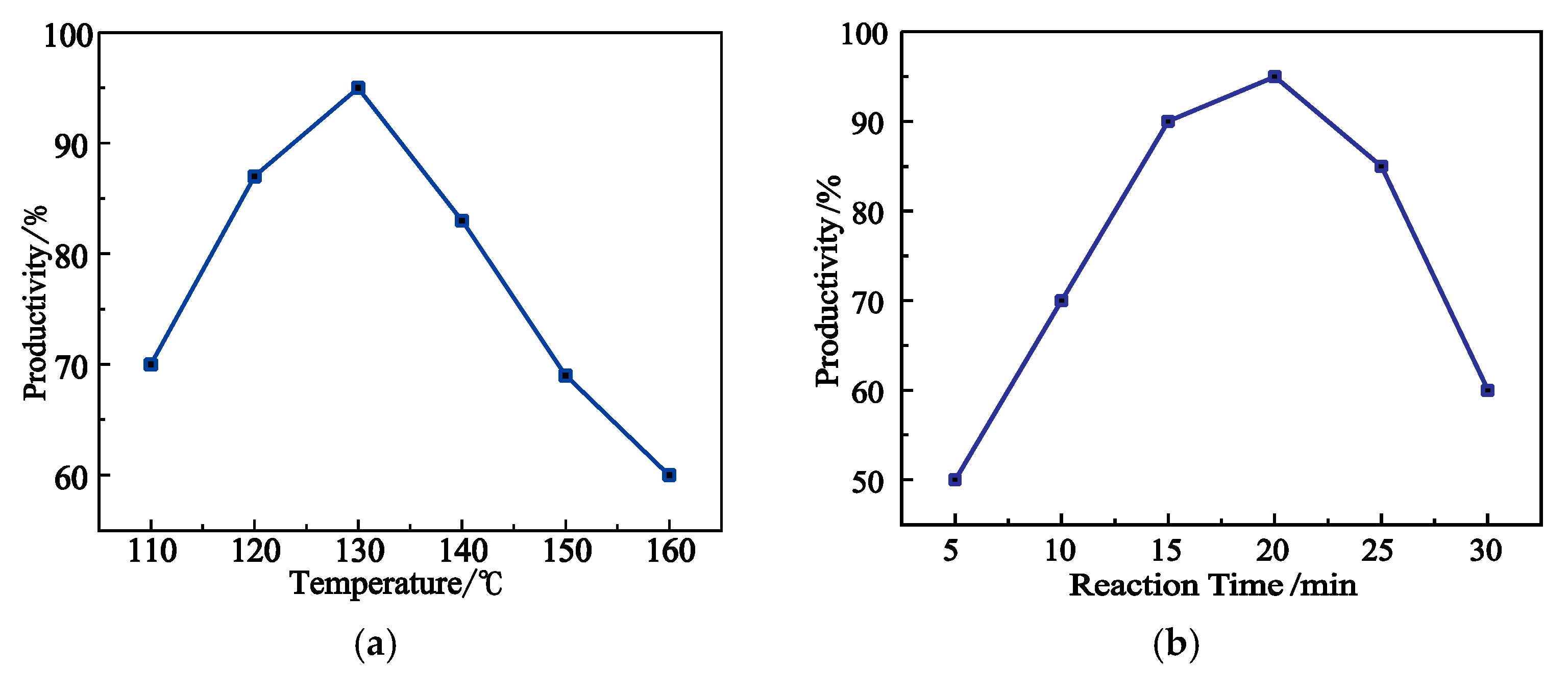
2.4. Optimization of Synthesise Condition for Product 1
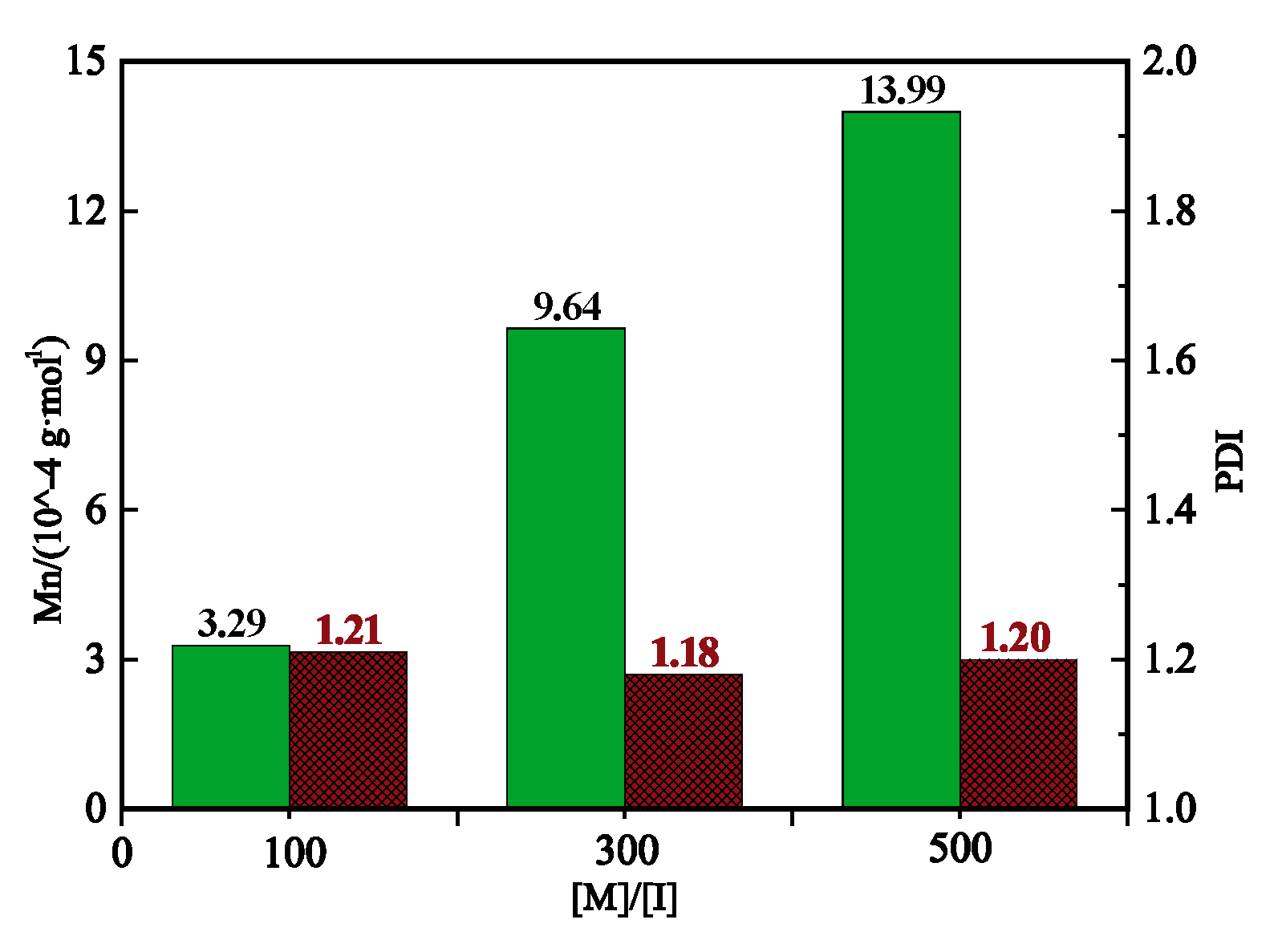
2.5. Molecular Weight and Polydispersity Index
3. Materials and Methods
3.1. Materials
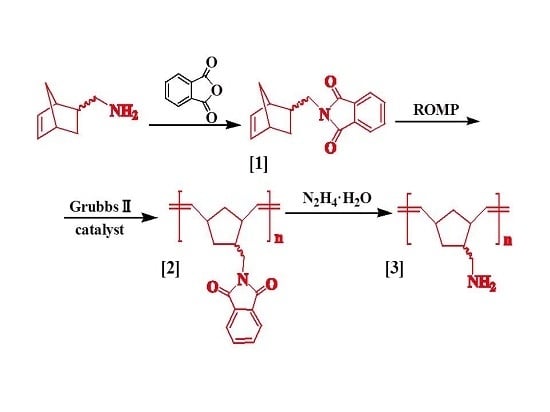
3.2. Synthesis of 5-Norbornene-2-(N-methyl)-phthalimide (Product 1)
3.3. Synthesis of Poly(norbornene-(N-methyl)-phthalimide) (Product 2)
3.4. Synthesis of Poly(norbornene-methylamine) (Product 3)
3.5. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, X.; Mu, C.; Dai, D. Well-Defined Magnetic Responsive Polymers Containing Ammonium FeCl4 from ROMP. Macromol. Chem. Phys. 2016, 217, 2700–2707. [Google Scholar] [CrossRef]
- Nomura, K.; Abdellatif, M.M. Precise synthesis of polymers containing functional end groups by living ring-opening metathesis polymerization (ROMP): Efficient tools for synthesis of block/graft copolymers. Polymer 2010, 51, 1861–1881. [Google Scholar] [CrossRef]
- Ferrer, Í.; Rich, J.; Fontrodona, X. Ru(II) complexes containing dmso and pyrazolyl ligands as catalysts for nitrile hydration in environmentally friendly media. Dalton Trans. 2013, 42, 13461–13469. [Google Scholar] [CrossRef] [PubMed]
- Schrock, R.R. Synthesis of Stereoregular Polymers through Ring-Opening Metathesis Polymerization. Acc. Chem. Res. 2014, 47, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- Hyvl, J.; Autenrieth, B.; Schrock, R.R. Proof of Tacticity of Stereoregular ROMP Polymers through Post Polymerization Modification. Macromolecules 2015, 48, 3148–3152. [Google Scholar] [CrossRef]
- Autenrieth, B.; Jeong, H.; Forrest, W.P. Stereospecific Ring-Opening Metathesis Polymerization (ROMP) ofendo-Dicyclopentadiene by Molybdenum and Tungsten Catalysts. Macromolecules 2015, 48, 2480–2492. [Google Scholar] [CrossRef]
- Martinez, H.; Hillmyer, M.A. Ring-Opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 2014, 5, 3507–3532. [Google Scholar] [CrossRef]
- Parker, K.A.; Sampson, N.S. Precision Synthesis of Alternating Copolymers via Ring-Opening Polymerization of 1-Substituted Cyclobutenes. Acc. Chem. Res. 2016, 49, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ciganda, R.; Castel, P. Living ROMP Syntheses and Redox Properties of Triblock Metallocopolymer Redox Cascades. Macromolecules 2016, 49, 4763–4773. [Google Scholar] [CrossRef]
- Wang, Y.; Rapakousiou, A.; Astruc, D. ROMP Synthesis of Cobalticenium–Enamine Polyelectrolytes. Macromolecules 2014, 47, 3767–3774. [Google Scholar] [CrossRef]
- Martinez, H.; Hillmyer, M.A. Carboxy-Telechelic Polyolefins in Cross-Linked Elastomers. Macromolecules 2014, 47, 479–485. [Google Scholar] [CrossRef]
- Annunziata, L.; Fouquay, S.; Michaud, G. Mono- and di-cyclocarbonate telechelic polyolefins synthesized from ROMP using glycerol carbonate derivatives as chain-transfer agents. Polym. Chem. 2013, 4, 1313–1316. [Google Scholar] [CrossRef]
- Vanbiervliet, E.; Fouquay, S.; Jean-Francois, C. From Epoxide to Cyclodithiocarbonate Telechelic Polycyclooctene through Chain-Transfer Ring-Opening Metathesis Polymerization (ROMP): Precursors to Non-Isocyanate Polyurethanes (NIPUs). Macromolecules 2017, 50, 69–82. [Google Scholar] [CrossRef]
- Bingöl, B.; Kroeger, A.; Jannasch, P. Well-defined phosphonated homo- and copolymers via direct ring opening metathesis polymerization. Polymer 2013, 54, 6676–6688. [Google Scholar] [CrossRef]
- Ferraz, C.P.; Fonseca, L.R.; Tomazetti, V. Copolymers from norbornene and norbornadiene with organized morphologies and high Tg values obtained via ROMP with a highly reactive [RuCl3(PCy3)2] complex. New J. Chem. 2016, 40, 9424–9431. [Google Scholar] [CrossRef]
- Suga, T.; Sakata, M.; Aoki, K. Synthesis of Pendant Radical- and Ion-Containing Block Copolymers via Ring-Opening Metathesis Polymerization for Organic Resistive Memory. ACS Macro Lett. 2014, 3, 703–707. [Google Scholar] [CrossRef]
- Leroux, F.; Montembault, V.; Piogé, S. High Molar Mass Poly(1,4-butadiene)-graft-poly(ε-caprolactone) Copolymers by ROMP: Synthesis via the Grafting-From Route and Self-Assembling Properties. Macromolecules 2016, 49, 4739–4745. [Google Scholar] [CrossRef]
- Xu, G.; Wang, D.; Buchmeiser, M.R. Functional Polyolefins: Poly(ethylene)-graft-Poly(tert-butyl acrylate) via Atom Transfer Radical Polymerization From a Polybrominated Alkane. Macromol. Rapid Commun. 2012, 33, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.M.; Khosravi, E. Comb-Like Graft Copolymers of Poly(oxa)norbornene: Efficient Synthesis Using a Combination of ROMP and Click Chemistry. Macromol. Chem. Phys. 2015, 216, 964–976. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Liao, P.L.; Shen, Z.H. Precise size control of sub-10 nm structures of cholesteryl-containing mesogen-jacketed liquid crystalline polymers. Polymer 2016. [Google Scholar] [CrossRef]
- Wang, L.Y.; Li, Y.F.; Zhu, F.M. Homo- and copolymerizaton of norbornene and norbornene derivative with Ni- and Pd-based β-ketoiminato complexes and MAO. Eur. Polym. J. 2006, 42, 322–327. [Google Scholar] [CrossRef]
- Chung, T.C. Synthesis of functional polymers via borane monomers and metathesis catalysts. J. Mol. Catal. 1992, 76, 15–31. [Google Scholar] [CrossRef]
- Santiago, A.A.; Cruz-Morales, J.A.; Vargas, J.; Tlenkopatchev, M.A.; Gavino, R.; Malkanduev, Y.A.; Sivov, N.A. Synthesis of New Polymer Ionomers via Ring-Opening Metathesis Polymerization. Open J. Org. Polym. Mater. 2014, 4, 84–91. [Google Scholar] [CrossRef]
- Pawar, G.M.; Weckesser, J.; Blechert, S.; Buchmeiser, M.R. Ring opening metathesis polymerization-derived block copolymers bearing chelating ligands: Synthesis, metal immobilization and use in hydroformylation under micellar conditions. Beilstein J. Org. Chem. 2010, 6, 28. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, J.; Bai, X. Preparation of cationic cobaltoceniumpolymers and block copolymers by “living” ring-opening metathesispolymerization. Chem. Sci. 2012, 3, 580–583. [Google Scholar] [CrossRef]
- Ashok Kothapalli, V.; Shetty, M.; de Los Santos, C. Thio-bromo “Click,” post-polymerization strategy for functionalizing ring opening metathesis polymerization (ROMP)-derived materials. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 179–185. [Google Scholar] [CrossRef]
- Alfred, S.F.; Lienkamp, K.; Madkour, A.E. Water-soluble ROMP polymers from amine-functionalized norbornenes. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 6672–6676. [Google Scholar] [CrossRef]
- Li, P.; Zhao, J.; Chen, Y. Preparation and characterization of chitosan physical hydrogels with enhanced mechanical and antibacterial properties. Carbohydr. Polym. 2017, 157, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Zvonimir, B.M.; Borislav, K. Spatial and electronic structure of highly basic organic molecules: Cyclopropeneimines and some related systems. J. Phys. Chem. A 1999, 103, 6678–6684. [Google Scholar]
- Lv, A.; Cui, Y.; Du, F.-S.; Li, Z.-C. Thermally Degradable Polyesters with Tunable Degradation Temperatures via Postpolymerization Modification and Intramolecular Cyclization. Macromolecules 2016, 49, 8449–8458. [Google Scholar] [CrossRef]
- Lv, A.; Li, Z.-L.; Du, F.-S.; Li, Z.C. Synthesis, Functionalization, and Controlled Degradation of High Molecular Weight Polyester from Itaconic Acid via ADMET Polymerization. Macromolecules 2014, 47, 7707–7716. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Wang, H.; Qu, X.; Chen, Y. Synthesis of Poly(norbornene-methylamine), a Biomimetic of Chitosan, by Ring-Opening Metathesis Polymerization (ROMP). Mar. Drugs 2017, 15, 223. https://doi.org/10.3390/md15070223
Li N, Wang H, Qu X, Chen Y. Synthesis of Poly(norbornene-methylamine), a Biomimetic of Chitosan, by Ring-Opening Metathesis Polymerization (ROMP). Marine Drugs. 2017; 15(7):223. https://doi.org/10.3390/md15070223
Chicago/Turabian StyleLi, Na, Huanhuan Wang, Xiaosai Qu, and Yu Chen. 2017. "Synthesis of Poly(norbornene-methylamine), a Biomimetic of Chitosan, by Ring-Opening Metathesis Polymerization (ROMP)" Marine Drugs 15, no. 7: 223. https://doi.org/10.3390/md15070223