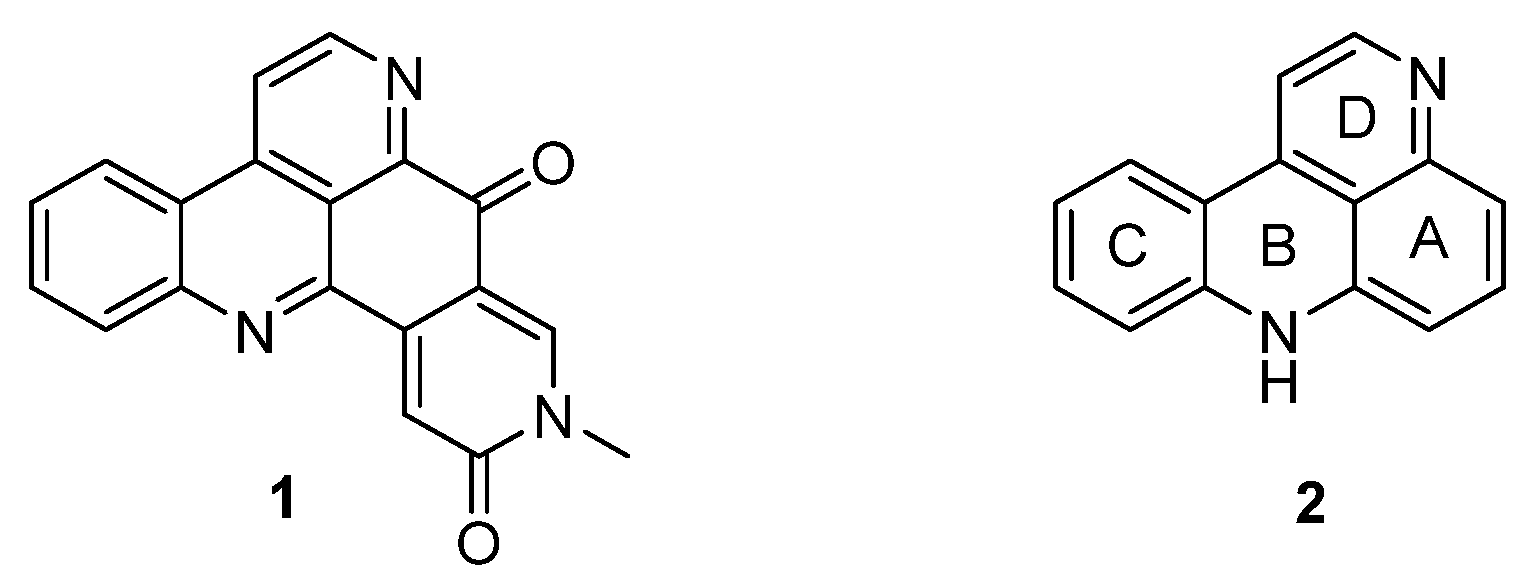
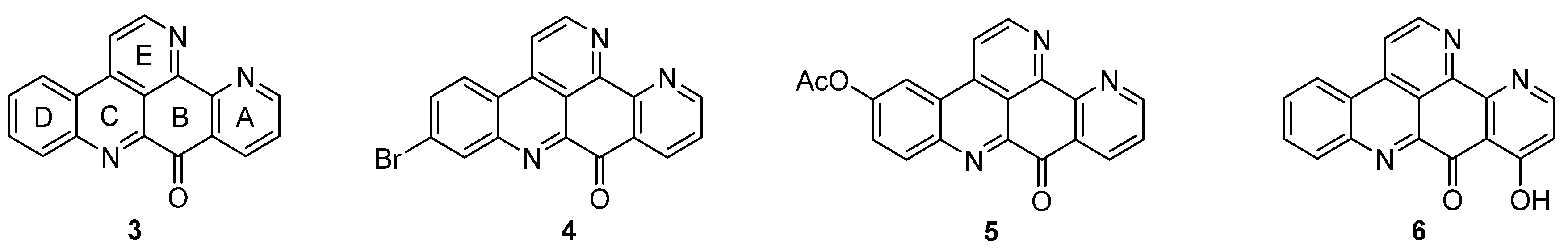
2. Ascididemin-Type Pyridoacridines
Ascididemin (
3) and closely related pyridoacridine alkaloids (bromoleptoclinidinone (
4), neocalliactine acetate (
5), and 10-hydroxyascididemin (
6)) share the same pentacyclic ring system, in which a pyridine ring is annulated to ring A of the 11
H-pyrido[4,3,2-
mn]acridine scaffold (
2) (
Figure 2).
Figure 2.
Structures of the ascididemin-type pyridoacridine alkaloids: ascididemin (3), bromoleptoclinidinone (4), neocalliactine acetate (5), 10-hydroxyascididemin (6).
Figure 2.
Structures of the ascididemin-type pyridoacridine alkaloids: ascididemin (3), bromoleptoclinidinone (4), neocalliactine acetate (5), 10-hydroxyascididemin (6).
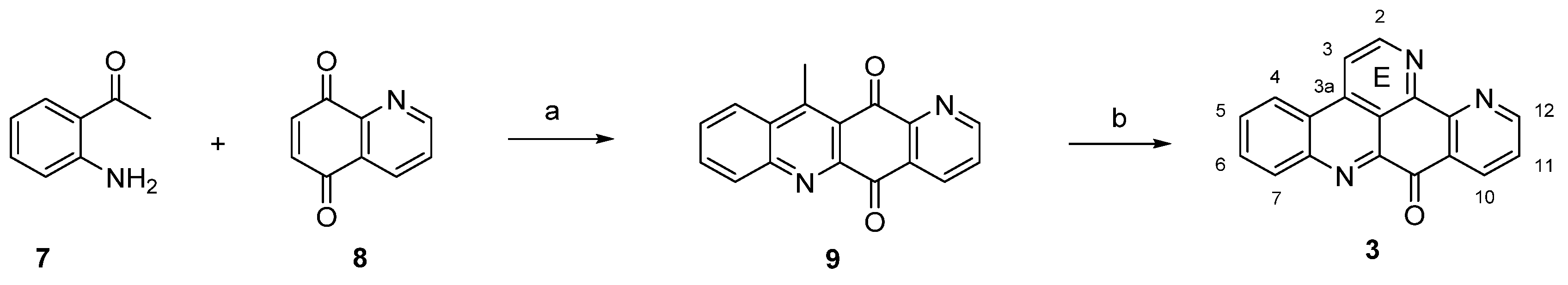
The first and still-leading synthesis of the alkaloid acsididemin (
3) was published by Bracher in 1989 [
7]. Albeit being presented in numerous reviews before, this synthesis is shown here once again, since its crucial steps have found application in several of the more recent approaches to diverse pyridoacridine alkaloids that will be presented below. The synthesis starts with an oxidative amination of 2-aminoacetophenone (
7) and quinoline-5,8-dione (
8), followed by an acid-catalyzed cyclization step to give tetracyclic quinone
9. The final annulation of ring E was performed in a one-pot reaction by condensation of the acidic methyl group with
N,
N-dimethylformamide diethyl acetal, followed by heating with ammonium chloride/glacial acetic acid to give
3 [
7] (
Scheme 1). This high-yielding four-step approach (43% overall yield) has later been applied to the total synthesis of many related pyridoacridines and analogues thereof by simply using pertinent ring-substituted 2-aminoacetophenones as starting materials [
8,
9,
10,
11].
Scheme 1.
First total synthesis of ascididemin (3): (a) CeCl3·7H2O, EtOH, air; then conc. H2SO4/AcOH (73% over two steps); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (59% over two steps).
Scheme 1.
First total synthesis of ascididemin (3): (a) CeCl3·7H2O, EtOH, air; then conc. H2SO4/AcOH (73% over two steps); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (59% over two steps).
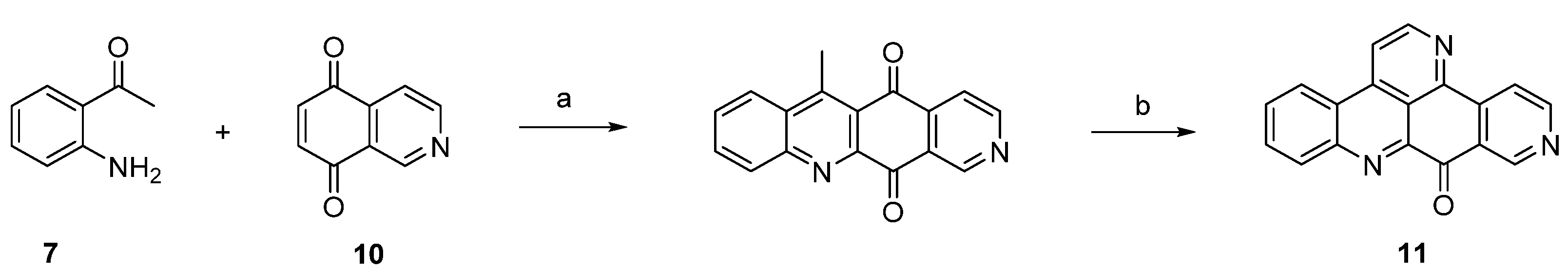
The isomer
11 of ascididemin, in which the nitrogen in ring A is shifted from position 13 to 11, was prepared by the Delfourne group [
12] in full analogy to Bracher’s method, with a surprisingly regioselective oxidative amination (51% yield) of isoquinoline-5,8-dione (
10) and 2-aminoacetophenone (
7) as the key step (
Scheme 2).
Scheme 2.
Synthesis of ascididemin isomer 11. Reagents and conditions: (a) CeCl3·7H2O, EtOH, air; then conc. H2SO4/AcOH (27% over two steps); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (23% over two steps).
Scheme 2.
Synthesis of ascididemin isomer 11. Reagents and conditions: (a) CeCl3·7H2O, EtOH, air; then conc. H2SO4/AcOH (27% over two steps); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (23% over two steps).
Based on Bracher’s synthetic strategy [
7], several modified approaches towards ascididemin-type pyridoacridines have been developed. Most of these syntheses aim at the replacement of the 2-aminoacetophenone building block by more complex 2-substituted anilines, which bear a residue that introduces not only C-3 and C-3a of the final pentacycle (as the acetyl residue does), but contain, in addition, equivalents of the later C-2 an the ring nitrogen of ring E (for numbering of the ring system, see
Scheme 1). In earlier approaches,
N-trifluoroacetamidokynuramine (bearing a protected 3-aminopropanoyl side-chain) [
13] and 2-aminocinnamaldehyde-
N,
N-dimethylhydrazone [
14] were used for this purpose.
In a very recent syntheses of various ascididemin-type alkaloids, consisting of the natural products ascididemin (
3), bromoleptoclinidinone (
4), neocalliactine acetate (
5), 10-hydroxyascididemin (
6), as well as two synthetic analogues 5-methoxyascididemin (
17) and deazaascididemin (
18), Yin
et al. introduced a novel C
3N unit, the Boc-protected propargylamine group, for this purpose [
15].
The required alkyne building blocks
12 were synthesized via Sonogashira reaction of the alkyne
N-Boc-propargylamine and a variety of 2-iodoanilines. In analogy to the original synthesis [
7], this approach started with an oxidative amination step of alkynylanilines
12 with quinones
13 and
14. The so-obtained arylaminoquinones
15 and
16 were transferred to alkaloids
3–
6, and analogues
17 and
18 via a Brønsted acid-promoted tandem annulation in very good yields (
Scheme 3) [
15].
Scheme 3.
Synthesis of pyridoacridine natural products 3–6 and synthetic ascididemin analogues 5-methoxyascididemin (17) and deazaascididemin (18): (a) CeCl3·7H2O, MeOH, O2 (50%–80%); (b) Fe2(SO4)3, conc. H2SO4/AcOH, O2 (44%–86%).
Scheme 3.
Synthesis of pyridoacridine natural products 3–6 and synthetic ascididemin analogues 5-methoxyascididemin (17) and deazaascididemin (18): (a) CeCl3·7H2O, MeOH, O2 (50%–80%); (b) Fe2(SO4)3, conc. H2SO4/AcOH, O2 (44%–86%).
As a number of investigations on the biological activities of ascididemin-type pyridoacridines indicated that cytotoxic activity and selectivity (e.g., against human and protozoal cells) is strongly dependent on the shape of ring A (especially the presence and position of heteroatoms in this ring) [
5,
16,
17,
18,
19], numerous investigations aimed at working out novel and flexible approaches to ring A analogues of ascididemin (
3) were undertaken in the past decade.
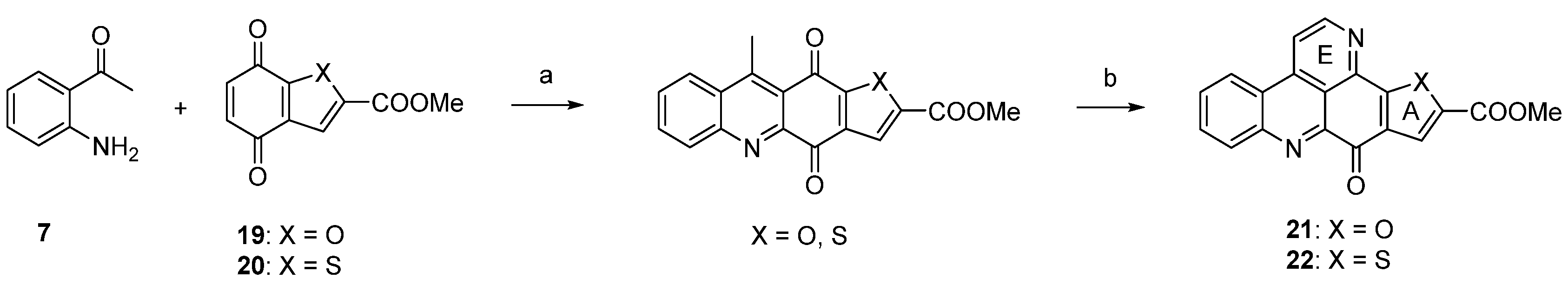
The approach described by Copp in 2010 [
18] in general follows Bracher’s strategy [
7], but the authors utilized thiophene and furan quinones
19 and
20 instead of quinoline-5,8-dione (
8) in order to introduce heteroatom-containing five-membered ring A substitutes. The final annulation of ring E (under concomitant rearomatization) was performed with paraformaldehyde and ammonium chloride to give ascididemin-type pyridoacridines
21 and
22 (
Scheme 4) [
18].
Scheme 4.
Synthesis of ascididemin-type pyridoacridines 21 and 22 by Copp: (a) CeCl3·7H2O, MeOH, air (22%–92%); then conc. H2SO4/AcOH (81%–94%); (b) NH4Cl, (CH2O)n, AcOH (76%–83%).
Scheme 4.
Synthesis of ascididemin-type pyridoacridines 21 and 22 by Copp: (a) CeCl3·7H2O, MeOH, air (22%–92%); then conc. H2SO4/AcOH (81%–94%); (b) NH4Cl, (CH2O)n, AcOH (76%–83%).
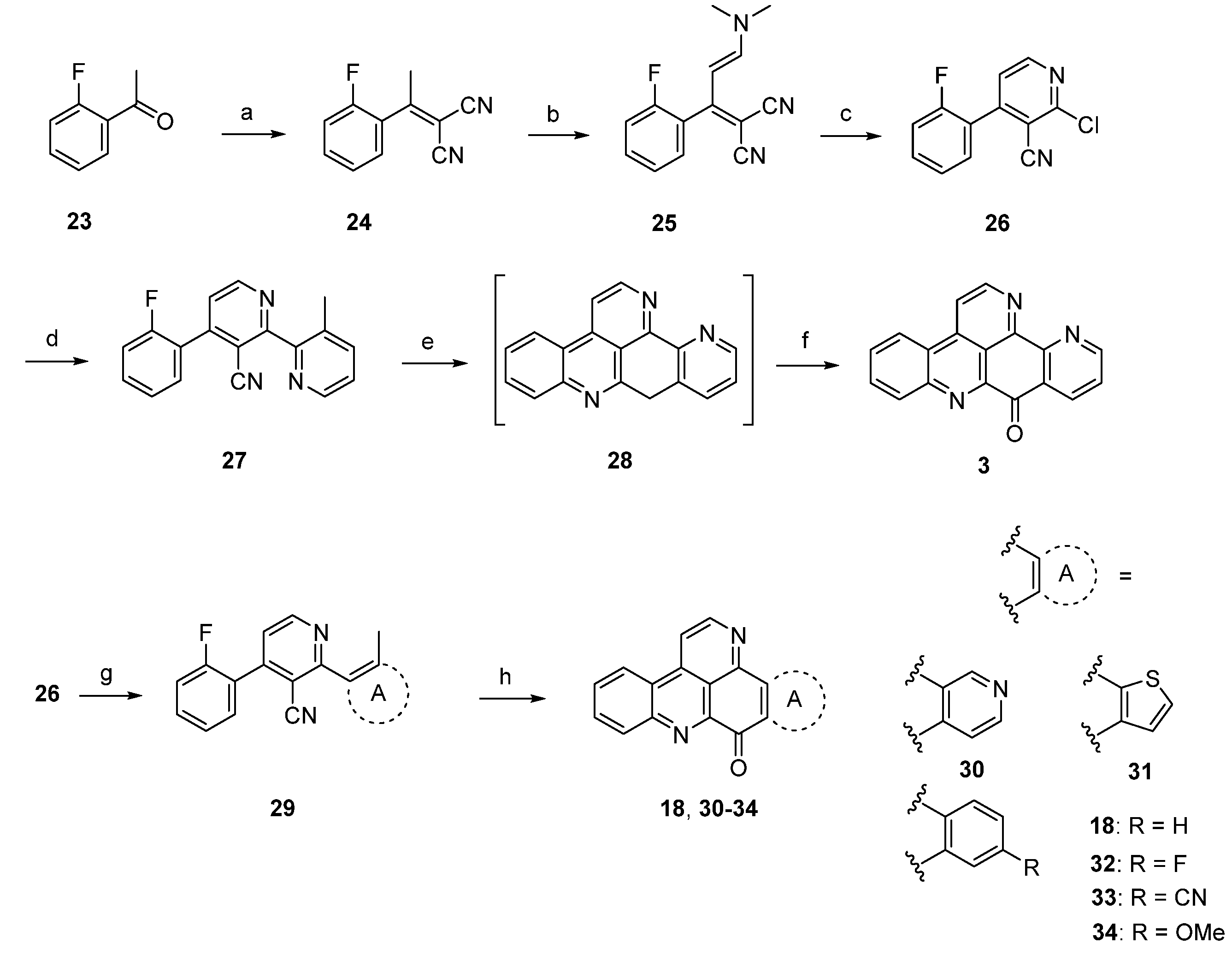
A completely different approach towards ascididemin (
3) and its analogues using an anionic ring closure as the key step was published by Kristensen in 2012 [
20]. Synthesis of ascididemin (
3) started with a Knoevenagel condensation of 2’-fluoroacetophenone (
23) and malononitrile. The CH-acidic methyl group in the resulting product
24 was condensed with
N,
N-dimethylformamide dimethyl acetal to give enamine
25. Upon exposure of
25 to HCl in glacial acetic acid cyclization to the 2-chloropyridine,
26 was accomplished. Negishi cross-coupling with 3-methylpyridin-2-yl zinc bromide under PEPPSI-iPr catalysis gave the bipyridine
27, the central precursor for the anionic cascade. Treating
27 with NaH in DMF under microwave irradiation furnished 9-deoxyascididemin (
28) via an anionic ring closure cascade. As
28 proved to be very difficult to handle, the crude intermediate was directly oxidized by bubbling oxygen through the solution. The desired alkaloid
3 was finally isolated in 45% overall yield (
Scheme 5). An isomer of the alkaloid, prepared by using 4-methylpyridin-3-yl zinc bromide was obtained in the same manner in comparable overall yield.
Scheme 5.
Synthesis of ascididemin (
3) through anionic ring closure: (
a) Malononitrile, NH
4OAc, toluene/AcOH; (
b) dimethylformamide dimethyl acetal, CH
2Cl
2; (
c) HCl gas, AcOH (81% over three steps); (
d) 3-methylpyridin-2-ylzinc bromide, PEPPSI-iPr, THF (80%); (
e) NaH, DMF; (
f) O
2 (69% over two steps) [
20]; Synthesis of the ring A analogues
18,
30–
34; (
g) Negishi or Suzuki cross-coupling reactions (44%–88%); (
h) NaH, DMPU (19%–29%).
Scheme 5.
Synthesis of ascididemin (
3) through anionic ring closure: (
a) Malononitrile, NH
4OAc, toluene/AcOH; (
b) dimethylformamide dimethyl acetal, CH
2Cl
2; (
c) HCl gas, AcOH (81% over three steps); (
d) 3-methylpyridin-2-ylzinc bromide, PEPPSI-iPr, THF (80%); (
e) NaH, DMF; (
f) O
2 (69% over two steps) [
20]; Synthesis of the ring A analogues
18,
30–
34; (
g) Negishi or Suzuki cross-coupling reactions (44%–88%); (
h) NaH, DMPU (19%–29%).
Based on this methodology, several ring A analogues of ascididemin
18,
30–
34 were prepared (
Scheme 5) [
21]. Different ring A equivalents were introduced via Neghishi or Suzuki cross-coupling reactions to give biaryls
29. The following anionic ring closure was achieved here by using NaH in
N,
N’-dimethylpropylene urea (DMPU). However, the yields of the final cyclization cascade were rather low (12%–29%) here, and several target compounds bearing heteroarenes as ring E equivalents (among them the annulated thiazole kuanoniamine A) could not be obtained at all.
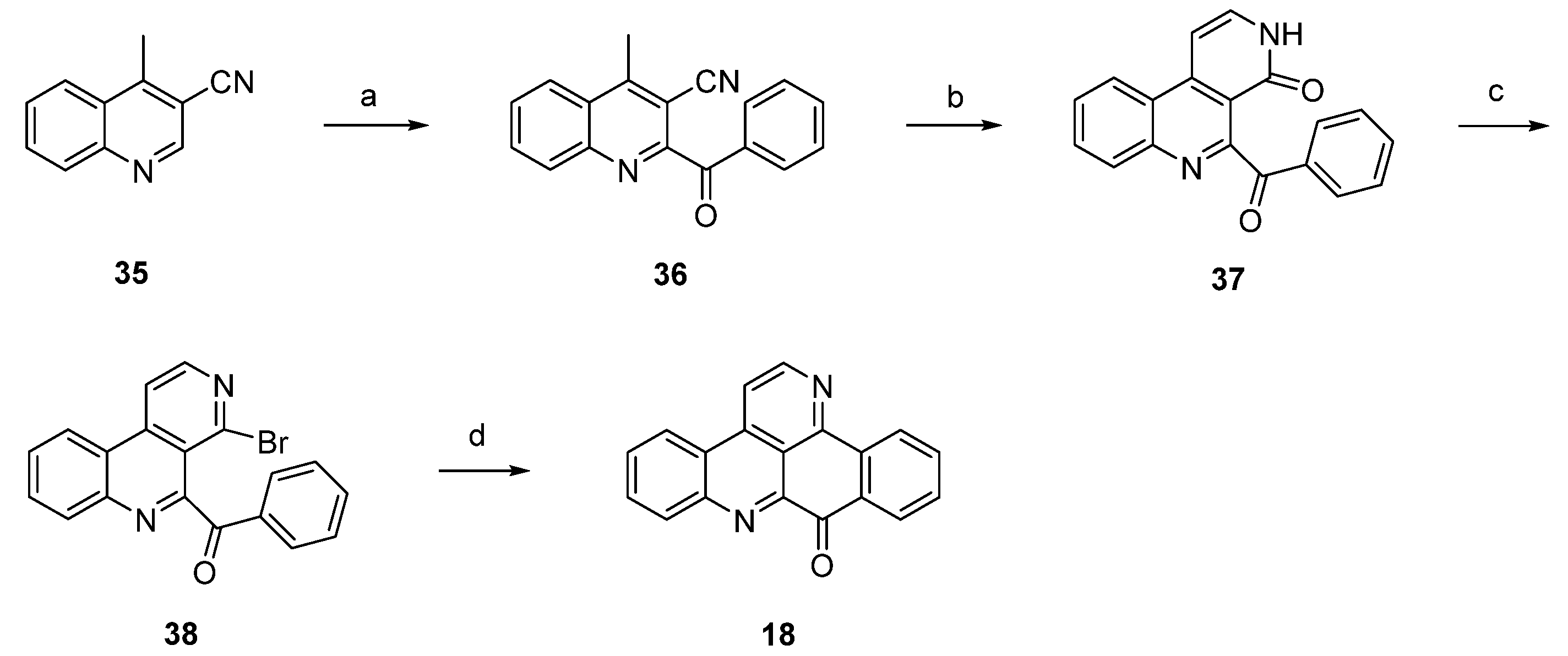
In 2012, Raeder and Bracher published a new synthetic approach to the pyridoacridine ring system involving two radical reactions as the key steps [
22]. Readily available quinoline
35 was subjected to Minisci benzoylation with a benzoyl radical generated from benzaldehyde to furnish ketone
36. The initially low yield of this step was increased by replacing sulfuric acid by trifluoroacetic acid and by adding additional amounts of radical starters (iron(II) sulfate,
tert-butyl hydroperoxide) after intervals of 45 min. The annulation of the bromopyridine ring, which later forms ring E of the target compound, was accomplished in a two-step protocol. First,
36 was condensed with dimethylformamide diethyl acetal, followed by heating with concentrated sulfuric acid/glacial acetic acid to give pyridone
37. In the second step,
37 was converted to bromo compound
38 by heating with phosphoryl bromide. The final intramolecular cyclization was performed through conversion of the bromopyridine moiety to an aryl radical with tributyltin hydride and azobis(isobutyronitrile) (AIBN) to give deazaascididemin (
18) (
Scheme 6). Unfortunately, this approach gives only a very low overall yield, and the scope of this route has not been explored.
Scheme 6.
Synthetic approach towards deazaascididemin (18): (a) Benzaldehyde, AcOH, Et3N, H2O, FeSO4, tert-BuOOH (51%); (b) dimethylformamide diethyl acetal, DMF; then H2SO4, AcOH (39%); (c) POBr3, anisole (27%); (d) Bu3SnH, AIBN, toluene (5%).
Scheme 6.
Synthetic approach towards deazaascididemin (18): (a) Benzaldehyde, AcOH, Et3N, H2O, FeSO4, tert-BuOOH (51%); (b) dimethylformamide diethyl acetal, DMF; then H2SO4, AcOH (39%); (c) POBr3, anisole (27%); (d) Bu3SnH, AIBN, toluene (5%).
In a related approach, Bracher [
23] intended to utilize the Minisci reaction as a final cyclization step for the synthesis of ascididemin (
3). However, attempted acidic hydrolysis of dioxolane
39 (to give aldehyde
40 as an acyl radical precursor) resulted in unexpected cyclization to the benzo[
f]pyrido[2′,3′:3,4]pyrrolo[2,1-
a][2,7]naphthyridine
41 (
Scheme 7).
Scheme 7.
Unsuccessful approach to the ascididemin precursor 40 giving benzo[f]pyrido[2′,3′:3,4]pyrrolo[2,1-a][2,7]naphthyridine (41): (a) H2SO4, H2O (43%).
Scheme 7.
Unsuccessful approach to the ascididemin precursor 40 giving benzo[f]pyrido[2′,3′:3,4]pyrrolo[2,1-a][2,7]naphthyridine (41): (a) H2SO4, H2O (43%).
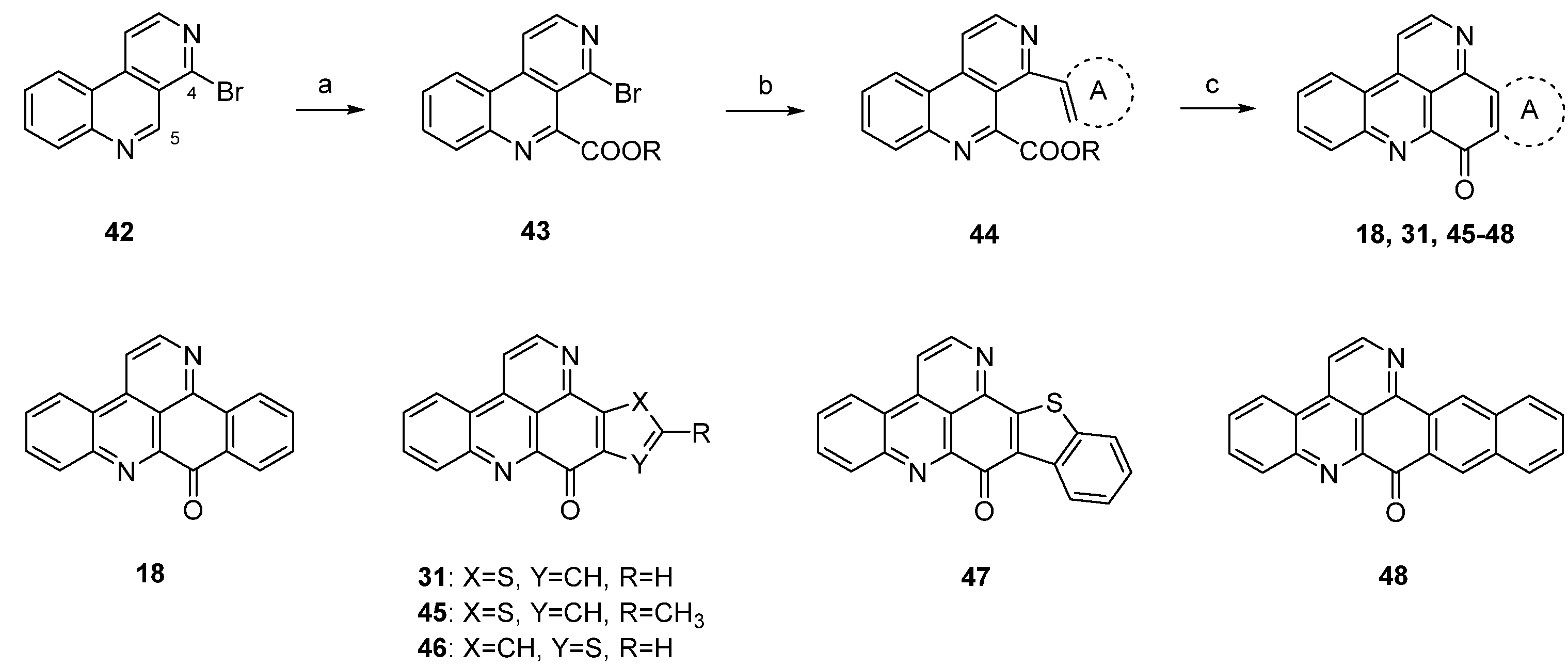
A different synthetic approach to ring A analogues of ascididemin from the Bracher group [
24] started with a high-yielding, regioselective Minsici-type homolytic methoxycarbonylation at C-5 of readily available 4-brombenzo[
c][2,7]naphthyridine (
42), followed by an introduction of ring A equivalents through Suzuki cross-coupling with appropriate (hetero)areneboronic acids. The final intramolecular ring closure was achieved by treating the 4,5-disubstituted benzo[
c][2,7]naphthyridines
44 with trifluoromethanesulfonic acid under microwave irradiation. This final superacid-aided Friedel-Crafts-type acylation step furnished pyridoacridines
18,
31,
45–
48 in 63%–92% yields (
Scheme 8). Significantly lower yields were obtained with the corresponding ethyl esters. However, these cyclization reactions were successful only with electron-rich aryl residues (phenyl, naphthyl, thiophenes) in position 4. Electron-withdrawing substituents (acetyl, chloro, bromo) on a benzene or thiophene ring led to the complete failure of this cyclization step [
24].
Scheme 8.
Synthesis of ring A analogues of ascididemin 18, 31, 45–48 through an intramolecular trifluoromethanesulfonic acid-aided Friedel-Crafts-type cyclization step: (a) Methyl pyruvate, H2O2, FeSO4, H2SO4, AcOH; then MnO2, CH2Cl2 (93% over two steps); (b) (hetero)areneboronic acid, Pd(Ph3P)4 (cat.), K2CO3, THF, H2O (41%–75%); (c) CF3-SO3H, microwave irradiation (63%–92%).
Scheme 8.
Synthesis of ring A analogues of ascididemin 18, 31, 45–48 through an intramolecular trifluoromethanesulfonic acid-aided Friedel-Crafts-type cyclization step: (a) Methyl pyruvate, H2O2, FeSO4, H2SO4, AcOH; then MnO2, CH2Cl2 (93% over two steps); (b) (hetero)areneboronic acid, Pd(Ph3P)4 (cat.), K2CO3, THF, H2O (41%–75%); (c) CF3-SO3H, microwave irradiation (63%–92%).
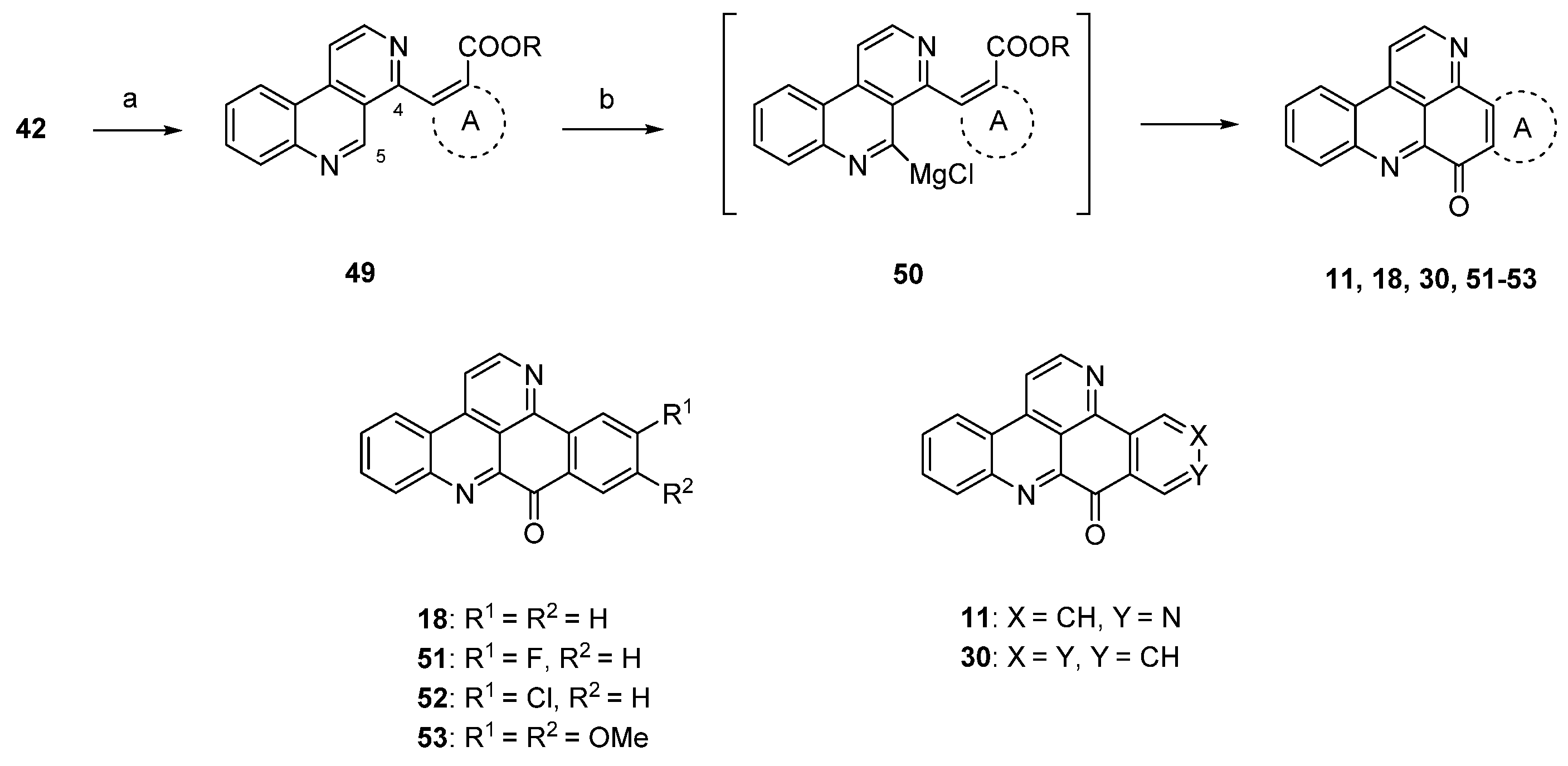
Due to the fact that the above-mentioned protocol [
24] only allows for the introduction of electron-rich carbocyclic and heterocyclic ring A substitutes, Plodek
et al. developed a new approach to the pyridoacridine ring system in which electron-deficient (hetero)arenes also serve as sources for ring A [
25]. The introduction of the ring A scaffolds was achieved through Suzuki cross-coupling of 4-brombenzo[
c][2,7]naphthyridine (
42) with (hetero)areneboronic acids bearing an ester moiety in the
ortho position or through Negishi cross-coupling with pyridylzinc compounds which were obtained by regioselective ring metalation of ethyl nicotinate or ethyl isonicotinate. The resulting 5-substituted benzo[
c][2,7]naphthyridines
49 were metalated regioselectively at the
peri position (C-5) with Knochel’s TMPMgCl·LiCl (2.2 equivalents), and intramolecular nucleophilic attack of the resulting arylmagnesium species
50 at the ester group furnished ring A analogues
18,
31,
45–
48 and isomers
11 and
30 of ascididemin in poor-to-modest yields (
Scheme 9) [
25]. This key step was inspired by the synthesis of demethyldeoxyamphimedine published by the Bracher group in 2014 [
26] (see below).
Scheme 9.
TMPMgCl·LiCl-mediated synthesis of ring A analogues 18, 31, 45–48 and isomers 11 and 30 of ascididemin (3): (a) For Suzuki cross-coupling reactions: areneboronic acid, Pd2(dba)3, P(tBu)3, KF, THF (73%–80%); for Negishi cross-coupling reactions: pyridylzinc compounds, Pd(dba)2, P(2-furyl)3, THF (71%–76%); (b) TMPMgCl·LiCl, THF (27%–39%).
Scheme 9.
TMPMgCl·LiCl-mediated synthesis of ring A analogues 18, 31, 45–48 and isomers 11 and 30 of ascididemin (3): (a) For Suzuki cross-coupling reactions: areneboronic acid, Pd2(dba)3, P(tBu)3, KF, THF (73%–80%); for Negishi cross-coupling reactions: pyridylzinc compounds, Pd(dba)2, P(2-furyl)3, THF (71%–76%); (b) TMPMgCl·LiCl, THF (27%–39%).
The protocol shown in
Scheme 9 is the most flexible one for the preparation of ring A analogues of ascididemin (
3), which are of special interest for the development of anticancer compounds.
3. Amphimedine-Type Pyridoacridines
Amphimedine-type pyridoacridines consist of the pentacyclic alkaloids amphimedine (
1), neoamphimedine (
54), deoxyamphimedine (
55), and demethyldeoxyamphimedine (
56) (
Figure 3) [
26]. Compared to the ascididemin subclass, rings A and B are connected in a different manner here. While most of the published syntheses of ascididemin-type pyridoacridines are more or less based on Bracher’s synthetic methodology [
7], many different and versatile strategies towards the amphimedine scaffold have been developed [
6]. During the last 15 years, three approaches towards neoamphimedine (
54) and two syntheses of demethyldeoxyamphimedine (
56) have been reported.
Figure 3.
Structures of the amphimedine-type pyridoacridine alkaloids: amphimedine (1), neoamphimedine (54), deoxyamphimedine (55) and demethyldeoxyamphimedine (56).
Figure 3.
Structures of the amphimedine-type pyridoacridine alkaloids: amphimedine (1), neoamphimedine (54), deoxyamphimedine (55) and demethyldeoxyamphimedine (56).
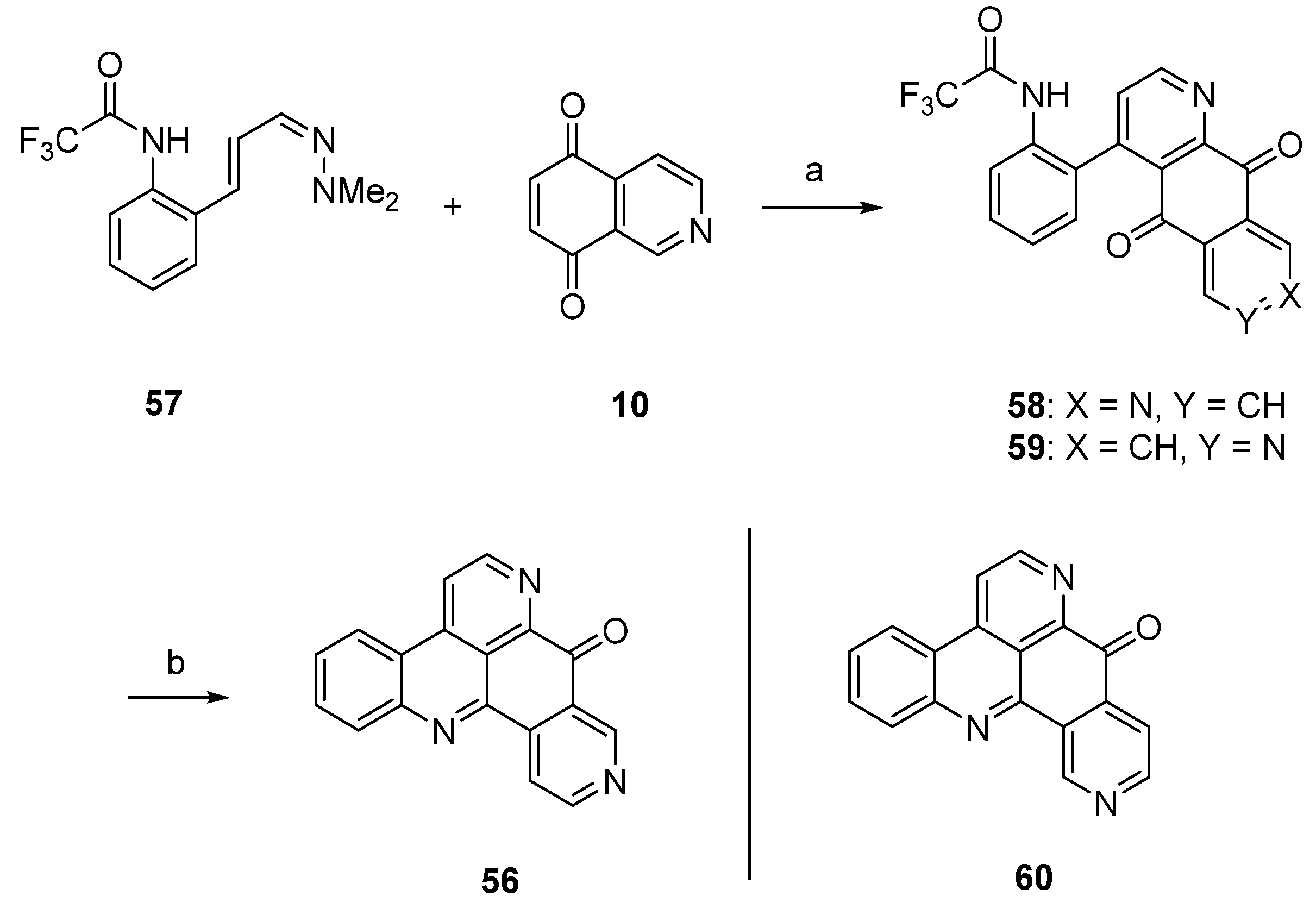
Delfourne’s first total synthesis of demethyldeoxyamphimedine (
56), which is illustrated in
Scheme 10, was published in 2002 [
12], years before this compound was identified as a natural product [
27]. This approach started with a hetero-Diels-Alder cycloaddition of isoquinoline-5,8-dione (
10) and 1-azadieene
57, both of them not commercially available. Besides the desired diazaanthranquinone
58, which was isolated in only 0.8% yield, the poorly separable regioisomer
59 was formed in 1.7% yield. The final ring closure step was accomplished under alkaline conditions to give demethyldeoxyamphimedine (
56) in almost quantitative yield. Analogous cyclization of the isomeric intermediate
59 gave the isomer
60 of the alkaloid in 91% yield.
Scheme 10.
Synthesis of demethyldeoxyamphimedine (56): (a) Toluene, N2 atmosphere (0.8%); (b) NaOH, CHCl3 (96%).
Scheme 10.
Synthesis of demethyldeoxyamphimedine (56): (a) Toluene, N2 atmosphere (0.8%); (b) NaOH, CHCl3 (96%).
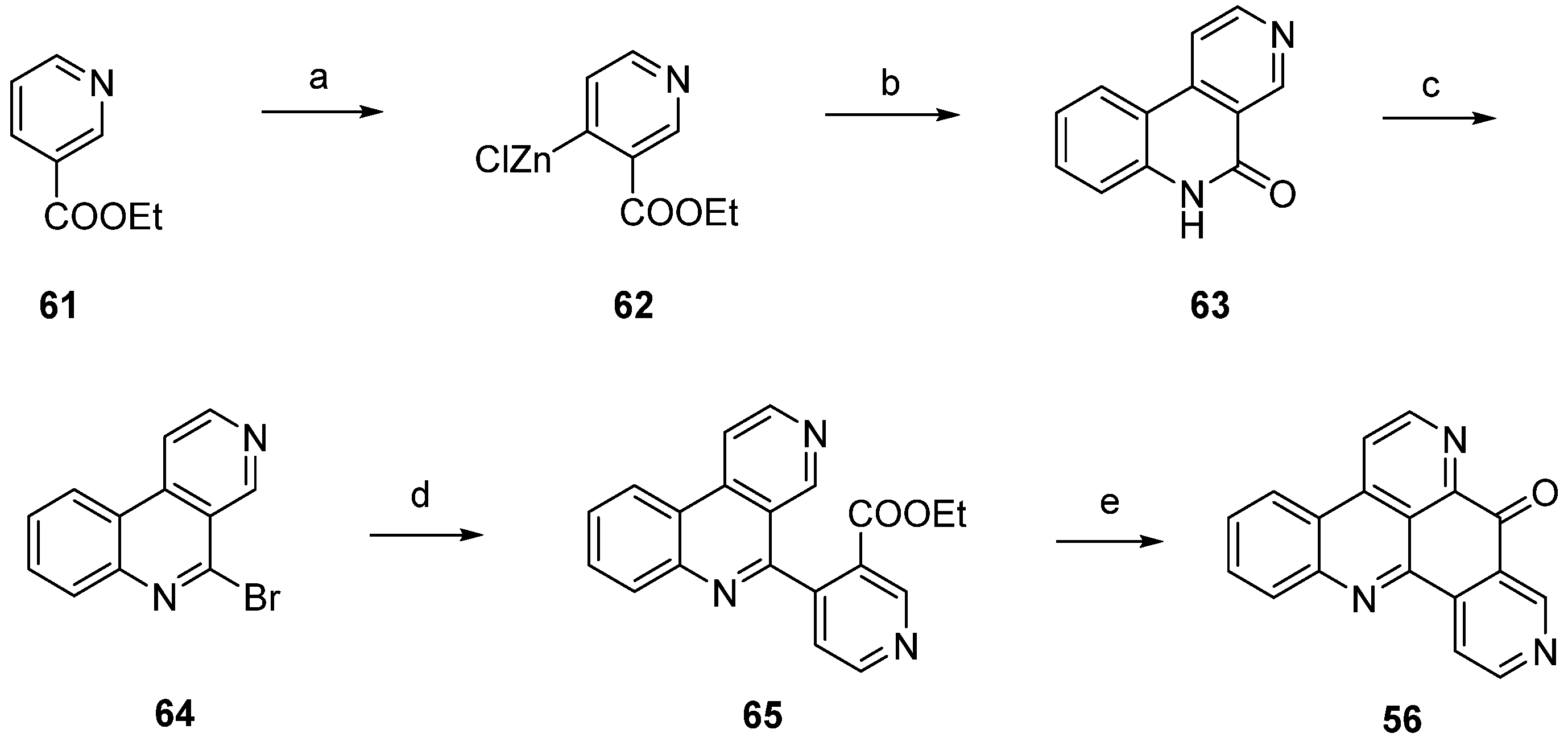
A highly effective total synthesis of demethyldeoxyamphimedine (
56) was reported by Melzer
et al. in 2014 [
26]. Regioselective direct metalation of ethyl nicotinate (
61) at C-4 using TMPMgCl·BF
3·LiCl and subsequent transmetalation with ZnCl
2 gave pyridylzinc compound
62, which was directly subjected to a Negishi cross-coupling reaction with 2-iodoaniline. The resulting biaryl (not shown in
Scheme 11) underwent spontaneous lactamization to give benzo[
c][2,7]naphthyridin-5(6
H)-one (
63). This lactam was converted to 5-bromobenzo[
c][2,7]naphthyridine (
64) with phosphoryl bromide. In another Negishi cross-coupling reaction, tricyclus
64 was coupled with organozinc intermediate
62 to give biaryl
65. The final ring closure step was achieved via direct regioselective metalation at C-4 of
65 utilizing 2.2 equivalents of Knochel’s TMPMgCl·LiCl, followed by intramolecular trapping of the ester group to give alkaloid
56 in 6.4% total yield. Thus, the authors prepared demethyldeoxyamphimedine (
56) by using only two commercial building blocks, ethyl nicotinate and 2-iodoaniline (
Scheme 11) [
26].
Scheme 11.
Synthesis of demethyldeoxyamphimedine (56) by Melzer et al: (a) TMPMgCl·BF3·LiCl, THF; then ZnCl2; (b) 2-iodoaniline, Pd(dba)2, P(2-furyl)3, THF (50% over two steps); (c) POBr3 (59%); (d) pyridylzinc compound 62, Pd(dba)2, P(2-furyl)3, THF (78%); (e) TMPMgCl·LiCl, THF (28%).
Scheme 11.
Synthesis of demethyldeoxyamphimedine (56) by Melzer et al: (a) TMPMgCl·BF3·LiCl, THF; then ZnCl2; (b) 2-iodoaniline, Pd(dba)2, P(2-furyl)3, THF (50% over two steps); (c) POBr3 (59%); (d) pyridylzinc compound 62, Pd(dba)2, P(2-furyl)3, THF (78%); (e) TMPMgCl·LiCl, THF (28%).
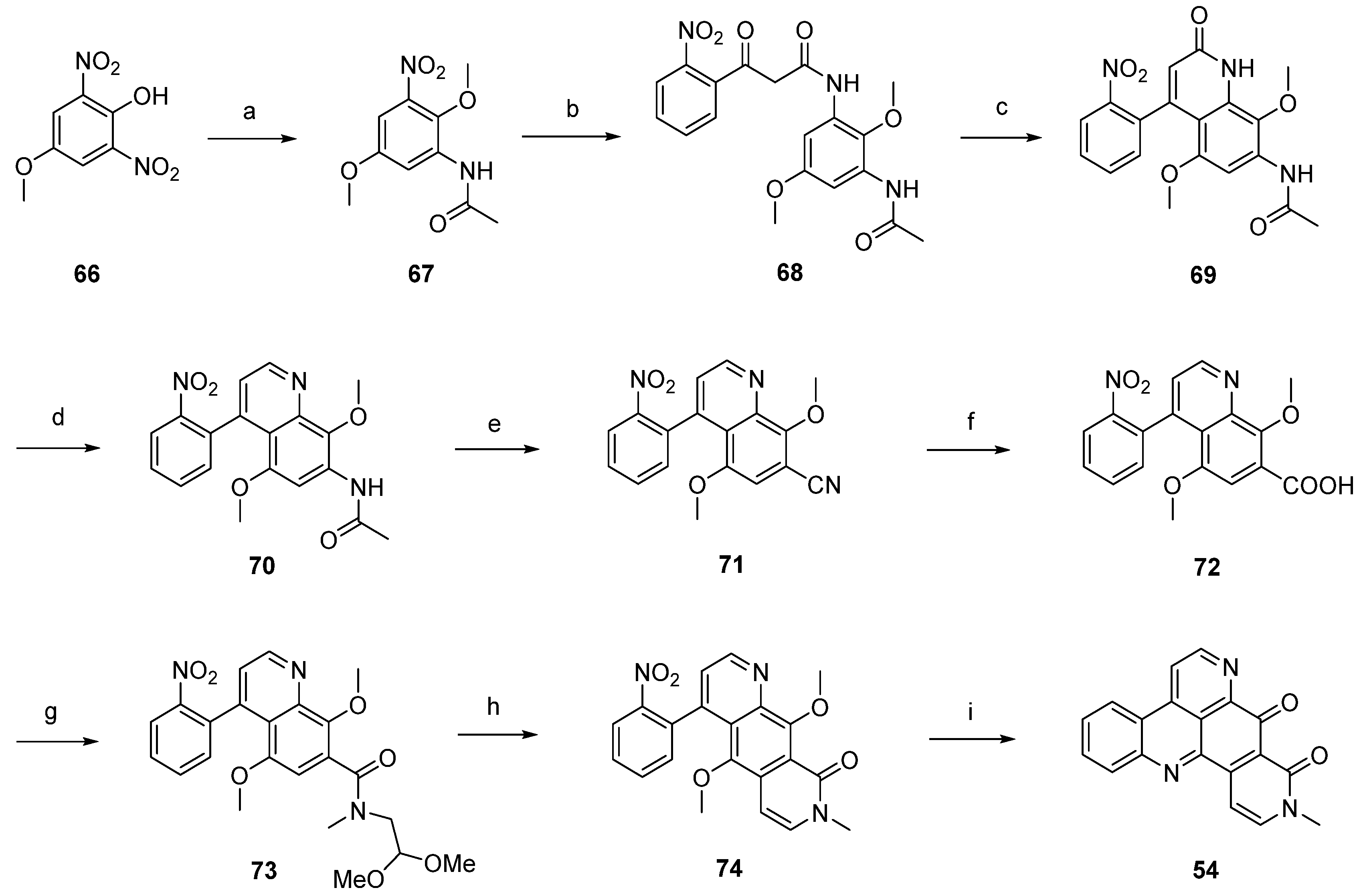
The first total synthesis of neoamphimedine (
54) was reported by Ireland in 2007 [
28]. This approach started with
O-methylation of dinitrophenol
66 with diazomethane, followed by selective reduction of one of the two nitro groups with 10% palladium on carbon and subsequent acetylation of the so-obtained amine (not shown in
Scheme 12) with acetic anhydride to afford acetanilide
67. Catalytic reduction and following conversion with ethyl (2-nitrobenzoyl)acetate furnished β-keto amide
68 in very good yields. Treatment of
68 with polyphosphoric acid gave quinolone
69 in a Knorr cyclization reaction. Quinoline
70 was obtained in a two-step protocol through treating
69 with trifluoromethanesulfonic anhydride and subsequent hydrogenolysis of the resulting triflate ester. The following transformation of the 7-acetylamino group of
70 into a nitrile was accomplished under Sandmeyer conditions after acidic hydrolysis. Next, the nitrile of
71 was hydrolyzed by heating with concentrated sulfuric acid to give the carboxylic acid
72, and further amidated with
N-methylamino acetaldehyde dimethylacetal to give carboxamide
73. Pomeranz-Fritsch–type cyclization with sulfuric acid gave oxo-diazaanthracene
74. The desired alkaloid
54 was obtained by catalytic reduction of the nitro group of
74, subsequent CAN oxidation to a tricyclic quinone, followed by intramolecular condensation with the amino group in a total yield of 2% over 12 steps (
Scheme 12) [
28].
Based on this methodology, LaBarbera published an improved total synthesis of neoamphimedine (
54) [
29]. The central quinolone intermediate
77 was synthesized in three steps, starting from nitrobenzoate
75. Catalytic hydrogenation of
75 gave the aniline
76, which was converted to
77 by treatment with Meldrum’s acid and trimethyl orthoformate. The following thermal ring closure gave 4-quinolone
78 with an overall yield of 78%. Treatment of
78 with trifluoromethanesulfonic anhydride furnished triflate ester
79, which was smoothly converted to the biaryl
81 in a Stille cross-coupling reaction with readily available trimethyl-(2-nitrophenyl)stannane (
80). Next, alkaline hydrolysis of the methyl ester group gave carboxyclic acid
72. The following steps were performed as described above [
28] and afforded neoamphimedine (
54) in 25% overall yield (
Scheme 13) [
29].
Scheme 12.
Ireland’s synthesis of neoamphimedine (54): (a) CH2N2; then 10% Pd/C in cyclohexene/EtOH; then AcOH/Ac2O (77% over two steps); (b) 10% Pd/C in cyclohexene/EtOH; then ethyl (2-nitrobenzoyl)acetate, m-xylenes (96%); (c) PPA (52%); (d) Tf2O, CH2Cl2, Et3N; then formic acid, Et3N, DMF, Pd(OAc)2, dppf (61% over two steps); (e) AcOH, H2O, H2SO4; then NaNO2; then CuCN (50%); (f) H2SO4 (80%); (g) N-methylamino acetaldehyde dimethyl acetal, EDC, CH2Cl2 (87%); (h) H2SO4 (43%); (i) 10% Pd/C, cyclohexene/EtOH; then CAN (30%).
Scheme 12.
Ireland’s synthesis of neoamphimedine (54): (a) CH2N2; then 10% Pd/C in cyclohexene/EtOH; then AcOH/Ac2O (77% over two steps); (b) 10% Pd/C in cyclohexene/EtOH; then ethyl (2-nitrobenzoyl)acetate, m-xylenes (96%); (c) PPA (52%); (d) Tf2O, CH2Cl2, Et3N; then formic acid, Et3N, DMF, Pd(OAc)2, dppf (61% over two steps); (e) AcOH, H2O, H2SO4; then NaNO2; then CuCN (50%); (f) H2SO4 (80%); (g) N-methylamino acetaldehyde dimethyl acetal, EDC, CH2Cl2 (87%); (h) H2SO4 (43%); (i) 10% Pd/C, cyclohexene/EtOH; then CAN (30%).
Scheme 13.
Improved synthesis of neoamphimedine (
54): (
a) Pd/C, H
2, MeOH; (
b) Meldrum’s acid, trimethyl orthoformate (90% over two steps); (
c) Ph
2O, reflux (87%); (
d) Tf
2O, DMAP, 2,6-lutidine, CH
2Cl
2 (92%); (
e) trimethyl-(2-nitrophenyl)stannane (
80), CuI, Pd(OAc)
2, dppe, DMF (83%); (
f) LiOH; following steps, see
Scheme 12.
Scheme 13.
Improved synthesis of neoamphimedine (
54): (
a) Pd/C, H
2, MeOH; (
b) Meldrum’s acid, trimethyl orthoformate (90% over two steps); (
c) Ph
2O, reflux (87%); (
d) Tf
2O, DMAP, 2,6-lutidine, CH
2Cl
2 (92%); (
e) trimethyl-(2-nitrophenyl)stannane (
80), CuI, Pd(OAc)
2, dppe, DMF (83%); (
f) LiOH; following steps, see
Scheme 12.
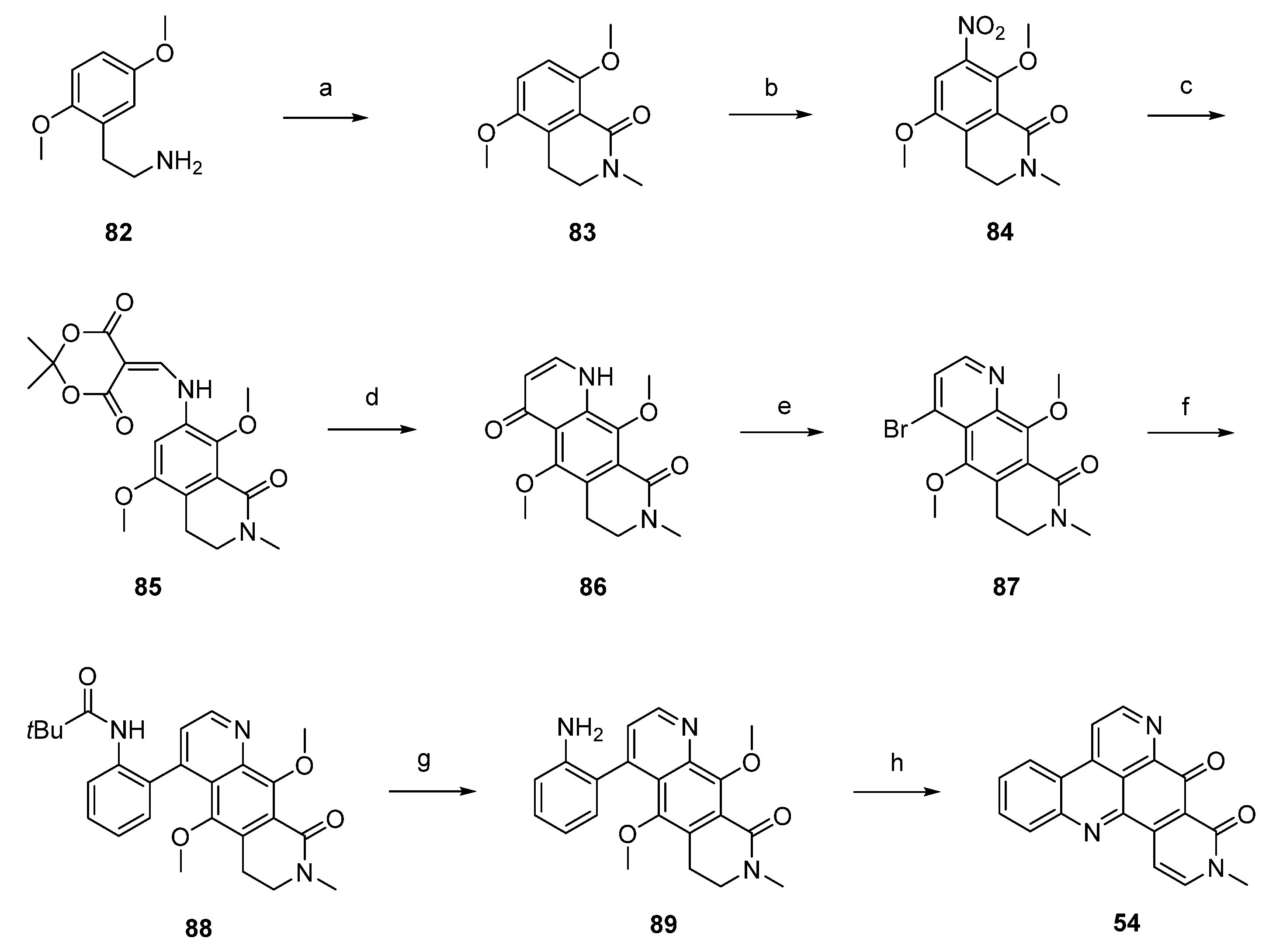
Another synthetic approach to neoamphimedine (
54) was reported by Nakahara
et al. in 2012 [
30]. Commercially available 2,5-dimethoxyphenethylamine (
82) was
N-acylated with ethyl chloroformate and triethylamine.
N-Methylation of the so-obtained
N-carbethoxy derivative (not shown in
Scheme 14) was performed with methyl iodide and sodium hydride. Subsequent Bischler-Napieralski–type cyclization with triflic anhydride and
N,
N-dimethylaminopyridine (DMAP) furnished quinolone
83 in 74% overall yield. Following regioselective nitration of
83 with cupric nitrate trihydrate in acetic anhydride afforded
84 in excellent yield. Catalytic hydrogenation of the nitro group utilizing 10% palladium on carbon and subsequent reaction with Meldrum’s acid and trimethyl orthoformate yielded enamine
85, which was cyclized to acridinedione
86 by heating in diphenyl ether. Subsequent bromination with phosphoryl bromide afforded
87, and Suzuki cross-coupling with 2-(pivaloylamino)phenylboronic acid gave intermediate
88 in high yield. Acid-mediated removal of the pivaloyl protective group gave amine
89. Conversion to the alkaloid
54 was performed in two steps:
O-demethylation with BBr
3 gave a hydroquinone intermediate which underwent cyclization and dehydrogenation in the dihydropyridone ring upon oxidation with CAN (31% yield over both steps). Using nitric acid as the oxidant led to a significant loss in yield (6%). The total yield of neoamphimedine (
54) over 12 steps was 6% (
Scheme 14) [
30].
Scheme 14.
Synthesis of neoamphimedine (54): (a) ClCO2Et, Et3N, THF; then MeI, NaH, THF; then DMAP, (CF3SO2)O, CH2Cl2 (74% over three steps); (b) Cu(NO2)2∙3H2O, Ac2O (96%); (c) Pd/C, H2, MeOH; then Meldrum’s acid, trimethyl orthoformate (81% over two steps); (d) Ph2O, reflux (83%); (e) POBr3, THF (70%); (f) 2-(pivaloylamino)phenylboronic acid, Pd(PPh3)4, K2CO3, H2O, EtOH, toluene (90%); (g) H2SO4/H2O (64%); (h) BBr3, CH2Cl2; then CAN/H2O, MeCN (31%).
Scheme 14.
Synthesis of neoamphimedine (54): (a) ClCO2Et, Et3N, THF; then MeI, NaH, THF; then DMAP, (CF3SO2)O, CH2Cl2 (74% over three steps); (b) Cu(NO2)2∙3H2O, Ac2O (96%); (c) Pd/C, H2, MeOH; then Meldrum’s acid, trimethyl orthoformate (81% over two steps); (d) Ph2O, reflux (83%); (e) POBr3, THF (70%); (f) 2-(pivaloylamino)phenylboronic acid, Pd(PPh3)4, K2CO3, H2O, EtOH, toluene (90%); (g) H2SO4/H2O (64%); (h) BBr3, CH2Cl2; then CAN/H2O, MeCN (31%).
4. Eilatin-Type Pyridoacridines
The symmetrical alkaloid eilatin (
90,
Figure 4) is the only known heptacyclic member of the pyridoacridine alkaloid class and was isolated in 1988 by Rudi
et al. from the tunicate
Eudistoma sp. [
31]. Since synthetic approaches towards eilatin (
90) have not been reviewed before, this chapter will close this gap and gives a short overview on all published syntheses since its isolation.
Figure 4.
Structure of eilatin (90).
Figure 4.
Structure of eilatin (90).
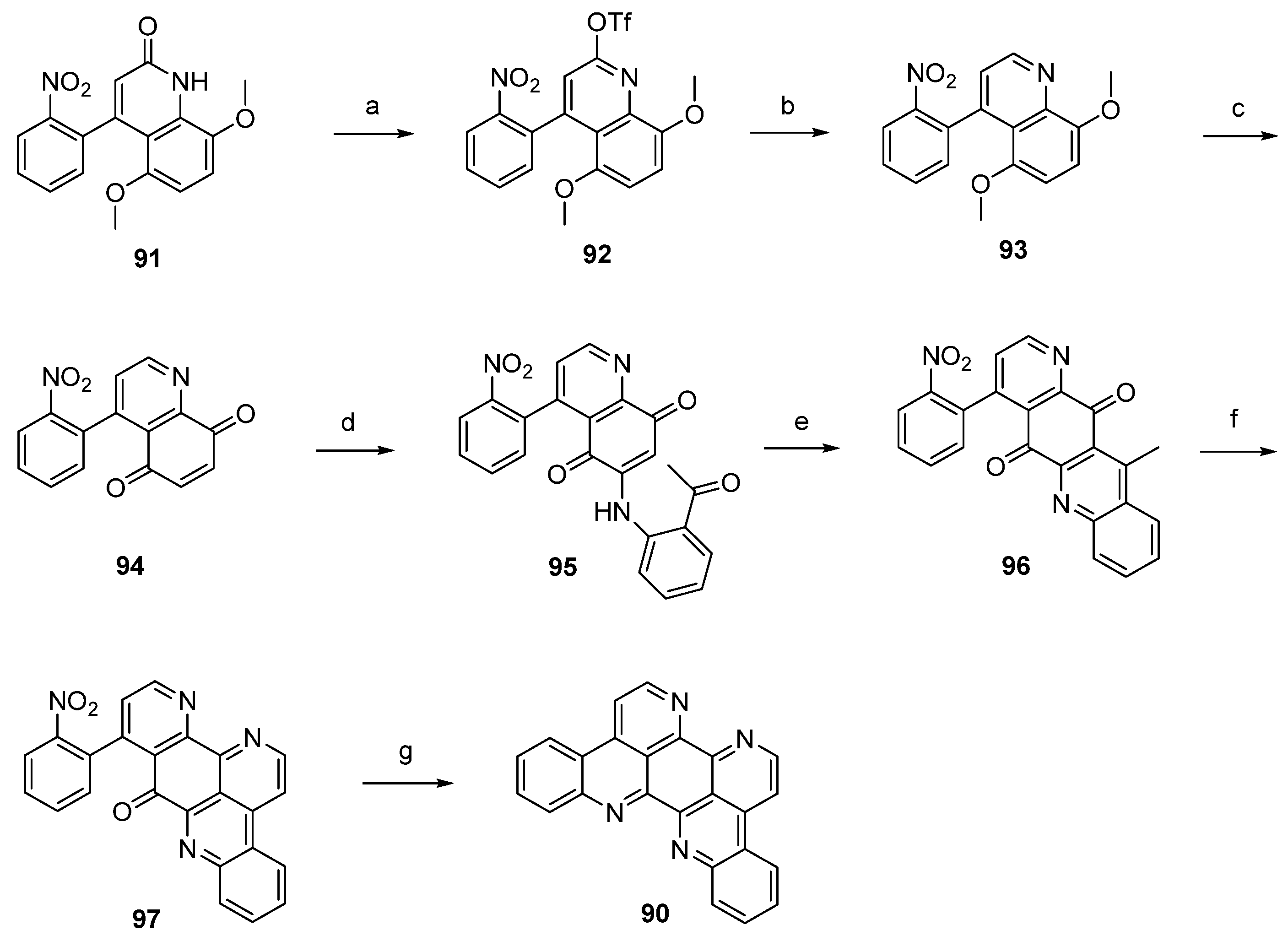
The first total synthesis of eilatin (
90), which is illustrated in
Scheme 15, was reported by the Kubo group in 1993 [
32]. The alkaloid was obtained in seven steps (overall yield 13%) from intermediate
91, which had been developed before as an intermediate for a total synthesis of amphimedine (
1) [
33]. Arylquinolone
91 was converted to the aryl triflate
92 with trifluoromethanesulfonic anhydride, and subsequent palladium-catalyzed reductive removal of the sulfonyloxy group with triethylammonium formate furnished 4-arylquinoline
93. Following oxidative demethylation of
93 using CAN gave
p-quinone
94. The next three steps follow Bracher’s ascididemine synthesis (see
Scheme 1), thus arylaminoquinoline-5,8-dione
95 was prepared by regioselective oxidative amination of
p-quinone
94 with 2-aminoacetophenone in the presence of CeCl
3. Next, an acid-catalyzed cyclization step was performed furnishing tetracyclic quinone
96, and the annulation of ring G was achieved using condensation with
N,
N-dimethylformamide diethyl acetal followed by ammonium chloride treatment to afford pentacyclus
97. The final ring closure step was accomplished through catalytic hydrogenation of the nitro compound
97 with 10% palladium on carbon and spontaneous cyclocondensation to give eilatin (
90) (
Scheme 15) [
32].
Scheme 15.
Synthesis of eilatin (90): (a) Tf2O, CH2Cl2, Et3N (93%); (b) formic acid, Et3N, Pd(OAc)2, dppf, DMF (87%); (c) CAN, acetonitrile/H2O (60%); (d) CeCl3·7H2O, 2-aminoacetophenone, EtOH (54%); (e) conc. H2SO4/AcOH (83%); (f) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (75% over two steps); (g) 10% Pd/C, EtOH (85%).
Scheme 15.
Synthesis of eilatin (90): (a) Tf2O, CH2Cl2, Et3N (93%); (b) formic acid, Et3N, Pd(OAc)2, dppf, DMF (87%); (c) CAN, acetonitrile/H2O (60%); (d) CeCl3·7H2O, 2-aminoacetophenone, EtOH (54%); (e) conc. H2SO4/AcOH (83%); (f) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (75% over two steps); (g) 10% Pd/C, EtOH (85%).
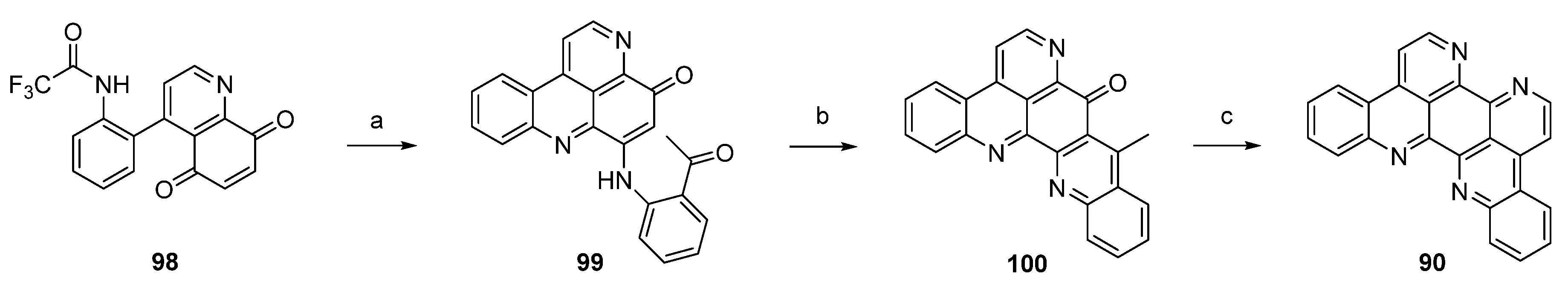
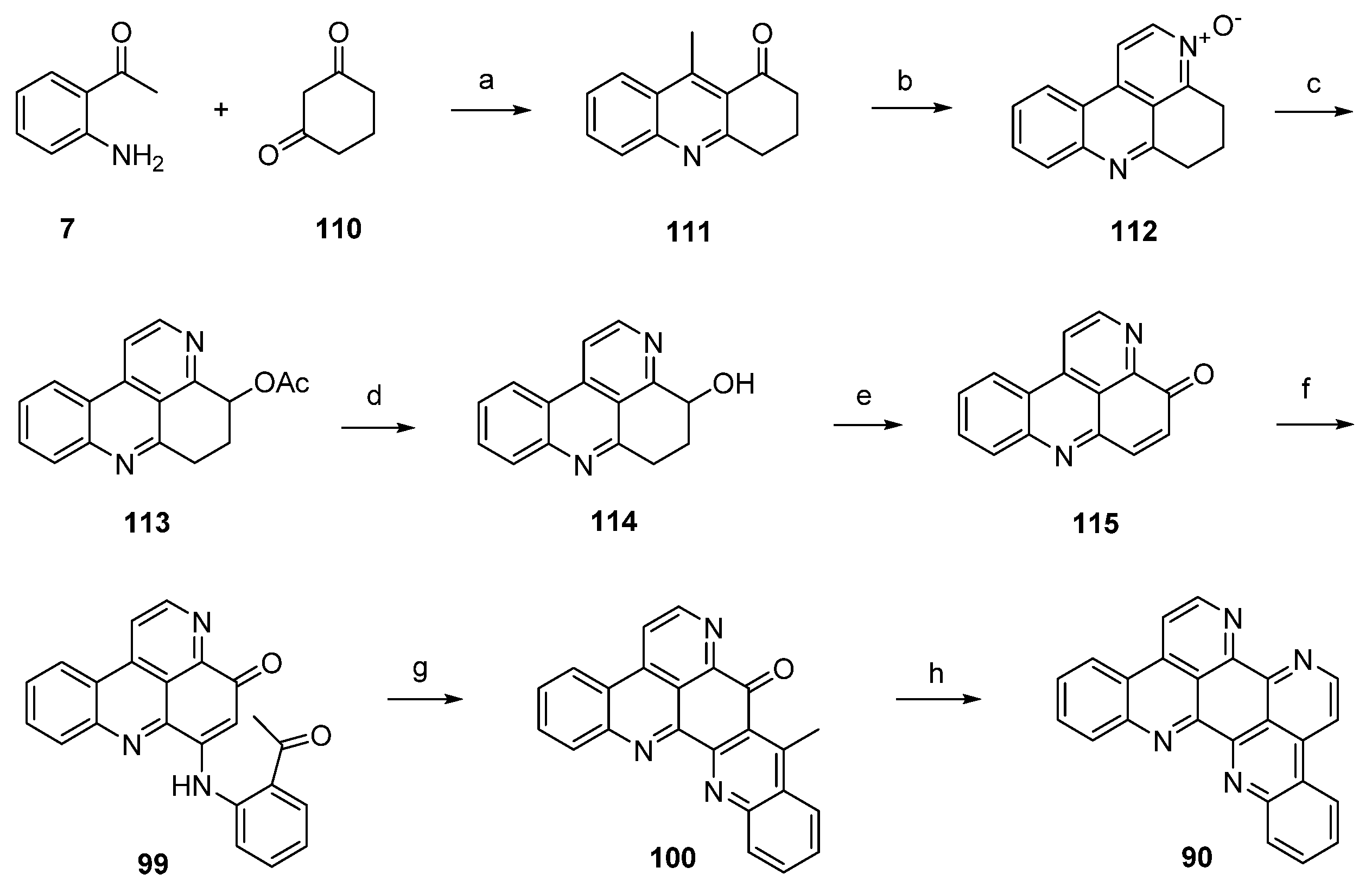
In a closely related approach, the same authors synthesized eilatin (
90) starting from quinolone-5,8-dione
98 bearing a trifluoroacetyl-protected amino group at the phenyl ring. Regioselective CeCl
3-catalyzed oxidative amination of
98 and 2-aminoacetophenone furnished, obviously under spontaneous
N-deprotection/cyclocondensation, the tetracyclic aminoquinone
99. After an acid-catalyzed cyclization step, which afforded hexacyclus
100, eilatin (
90) was obtained in a one-pot annulation of ring G using
N,
N-dimethylformamide diethyl acetal and ammonium chloride (
Scheme 16) [
32].
Scheme 16.
Synthesis of eilatin (90): (a) CeCl3·7H2O, 2-aminoacetophenone, EtOH (52%); (b) conc. H2SO4/AcOH (75%); (c) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (41% over two steps).
Scheme 16.
Synthesis of eilatin (90): (a) CeCl3·7H2O, 2-aminoacetophenone, EtOH (52%); (b) conc. H2SO4/AcOH (75%); (c) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl, AcOH (41% over two steps).
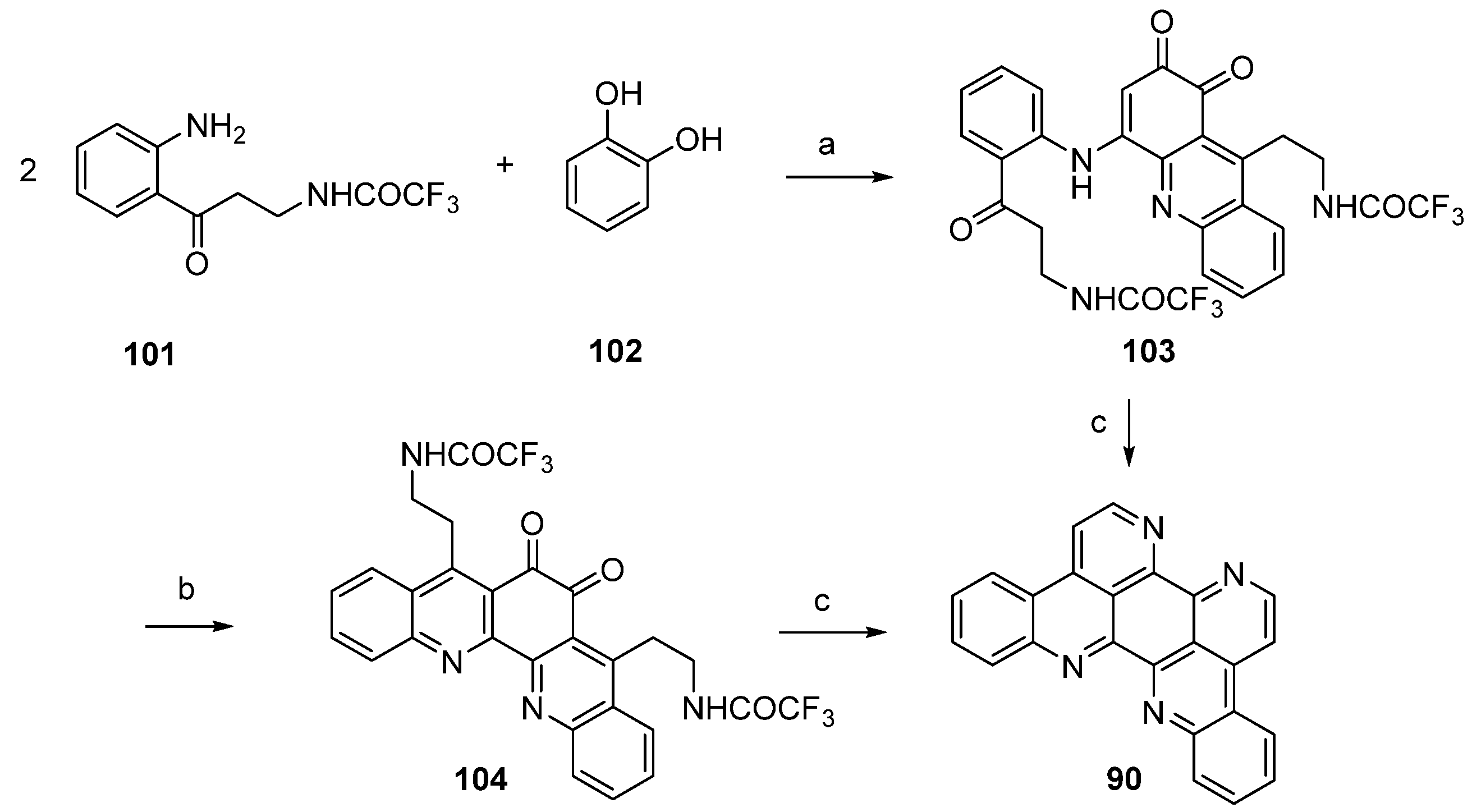
Kashman’s biomimetic approach towards eilatin (
90) suggests that kynuramine and
o-benzoquinone (or the corresponding catechol,
102), both natural products, can be considered as potential biosynthetic precursors of this alkaloid. In the first step, the monoprotected trifluoroacetyl kynuramine
101 was reacted with catechol (
102) under oxidative conditions to give compound
103 in very low yield. Treatment of
103 with ammonia in methanol and DMAP directly furnished alkaloid
90. An alternative route towards eilatin (
90) was accomplished by the treatment of
103 first with BF
3 etherate. The so-obtained pentacyclus
104 was converted to eilatin (
90) under alkaline conditions (NH
3-MeOH) (
Scheme 17) [
34]. No yields were given for the final steps in both approaches.
Scheme 17.
Biomimetic synthesis of eilatin (
90): (
a) NaIO
3, EtOH (15%); (
b) BF
3·OEt
2, CH
2Cl
2 (no yield given); (
c) NH
3, MeOH (no yield given) [
34].
Scheme 17.
Biomimetic synthesis of eilatin (
90): (
a) NaIO
3, EtOH (15%); (
b) BF
3·OEt
2, CH
2Cl
2 (no yield given); (
c) NH
3, MeOH (no yield given) [
34].
In this and an accompanying paper [
35] the Kashman group described several unsuccessful approaches to eilatin (
90). One of these attempts is illustrated in
Scheme 18. In a conversion closely related to the one described in
Scheme 17, compound
105 was prepared from 2-aminoacteophenone (
7) and catechol (
102) under oxidative conditions. Ring closure was accomplished by treating
105 with BF
3-etherate and this furnished the symmetrical pentacyclic dibenzo-1,10-phenanthroline-5,6-dione
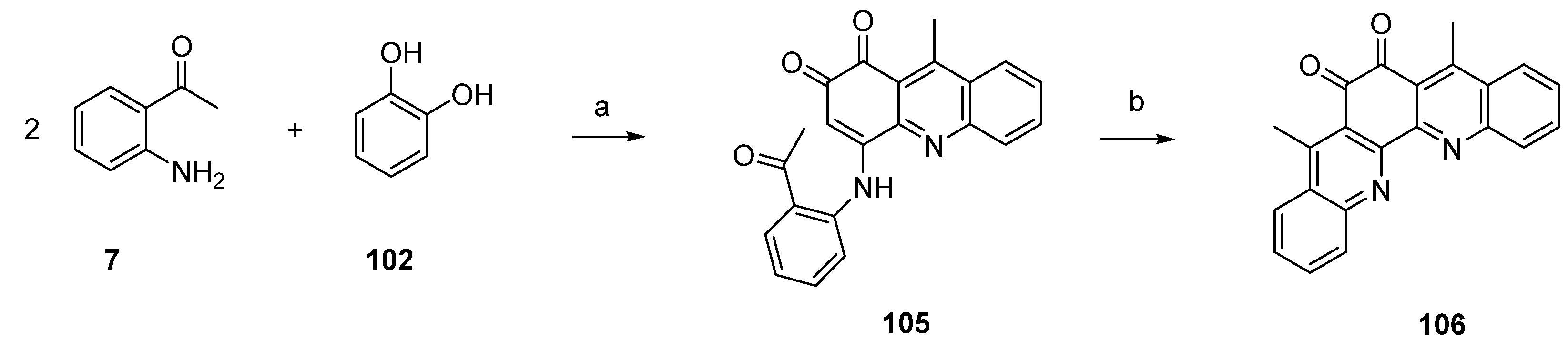
106 in high yield. A completion of this synthesis (annulation of rings F and G of eilatin) has not yet been published.
Scheme 18.
Synthesis of postulated eilatin precursor 106: (a) NaIO3, EtOH (60%); (b) BF3·OEt2, CH2Cl2 (70%).
Scheme 18.
Synthesis of postulated eilatin precursor 106: (a) NaIO3, EtOH (60%); (b) BF3·OEt2, CH2Cl2 (70%).
The synthetic strategy employed by Glazer and Tor [
36] for the preparation of a Ru
II-eilatin complex started with “pre-eilatin” (
107), a formal seco analogue of the alkaloid, which was obtained in 54% yield by Pd-catalyzed homo-coupling of 4-bromobenzo[
c][2,7]naphthyridine (
42). Treatment of “pre-eilatin” (
107) with Ru(bpy)
2Cl
2·5H
2O in ethylene glycol and water gave the dark-red Ru
II complex
108 ([Ru(bpy)
2(pre-eilatin)]
2+), which was converted into the deep-green [Ru(bpy)
2(eilatin)]
2+ complex
109 by exposure to palladium on carbon in ethylene glycol and acetone at elevated temperatures in almost quantitative yield. Separation of free eilatin from the complex
109 is not mentioned in the publication. It is noteworthy that treatment of the free “pre-eilatin” (
107) ligand with Pd/C did not yield eilatin (
90) (
Scheme 19). This approach is quite short, but affords stoichiometric amounts of the expensive Ru complex for the key step.
Scheme 19.
Synthesis of RuII-eilatin complex 109: (a) Pd(OAc)2, Bu4NBr, K2CO3, iPrOH, DMF (b) Ru(bpy)2Cl2·5H2O, ethylene glycol, water (62%); (c) Pd/C, ethylene glycol-acetone (97%).
Scheme 19.
Synthesis of RuII-eilatin complex 109: (a) Pd(OAc)2, Bu4NBr, K2CO3, iPrOH, DMF (b) Ru(bpy)2Cl2·5H2O, ethylene glycol, water (62%); (c) Pd/C, ethylene glycol-acetone (97%).
A divergent synthesis leading to both eilatin (
90) and its isomer isoeilatin (
119) was published by Plodek and Bracher in 2015 [
37]. This approach started with a Friedländer reaction of 2-aminoacetophenone (
7) and 1,3-cyclohexanedione (
110) to afford acridone
111. Following one-pot annulation of a pyridine-
N-oxide ring was accomplished by condensation of the acidic methyl group of
111 with
N,
N-dimethylformamide diethyl acetal under controlled conditions (55 °C) and subsequent ring closure with hydroxylamine hydrochloride to give
N-oxide
112. In a Boekelheide rearrangement, this
N-oxide was converted to the acetoxy compound
113 by heating with acetic anhydride. Alkaline ester hydrolysis of
113 afforded alcohol
114 in 84% yield. Subsequent oxidation of this alcohol under mild conditions with MnO
2 under concomitant ring dehydrogenation furnished pyridoacridone
115 in 66% yield. Further conversion to eilatin (
90) was performed in analogy to Bracher’s total synthesis of ascididemin (
3) [
7]. Thus, regioselective oxidative amination of
115 with 2-aminoacetophenone under CeCl
3 catalysis gave arylamino derivative
99. Next, acid-catalyzed cyclization furnished hexacyclic compound
100 in almost quantitative yield. The final annulation of ring G was performed in a one-pot reaction by condensation of the acidic methyl group with
N,
N-dimethylformamide diethyl acetal, followed by heating with ammonium chloride/glacial acetic acid to give
90. Hence, alkaloid
90 was synthesized in seven steps with an overall yield of 6.9% from only two building blocks (1,3-cyclohexanedione, 2-aminoacetophenone) (
Scheme 20) [
37].
Scheme 20.
Synthesis of eilatin (90): (a) Reflux (54%); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH2OH·HCl (41% over two steps); (c) acetic anhydride (66%); (d) 10% NaOH in H2O/MeOH (84%); (e) MnO2 (66%); (f) 2-aminoacetophenone, CeCl3·7H2O (74%); (g) 10% H2SO4 in AcOH (96%); (h) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl (65% over two steps).
Scheme 20.
Synthesis of eilatin (90): (a) Reflux (54%); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH2OH·HCl (41% over two steps); (c) acetic anhydride (66%); (d) 10% NaOH in H2O/MeOH (84%); (e) MnO2 (66%); (f) 2-aminoacetophenone, CeCl3·7H2O (74%); (g) 10% H2SO4 in AcOH (96%); (h) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl (65% over two steps).
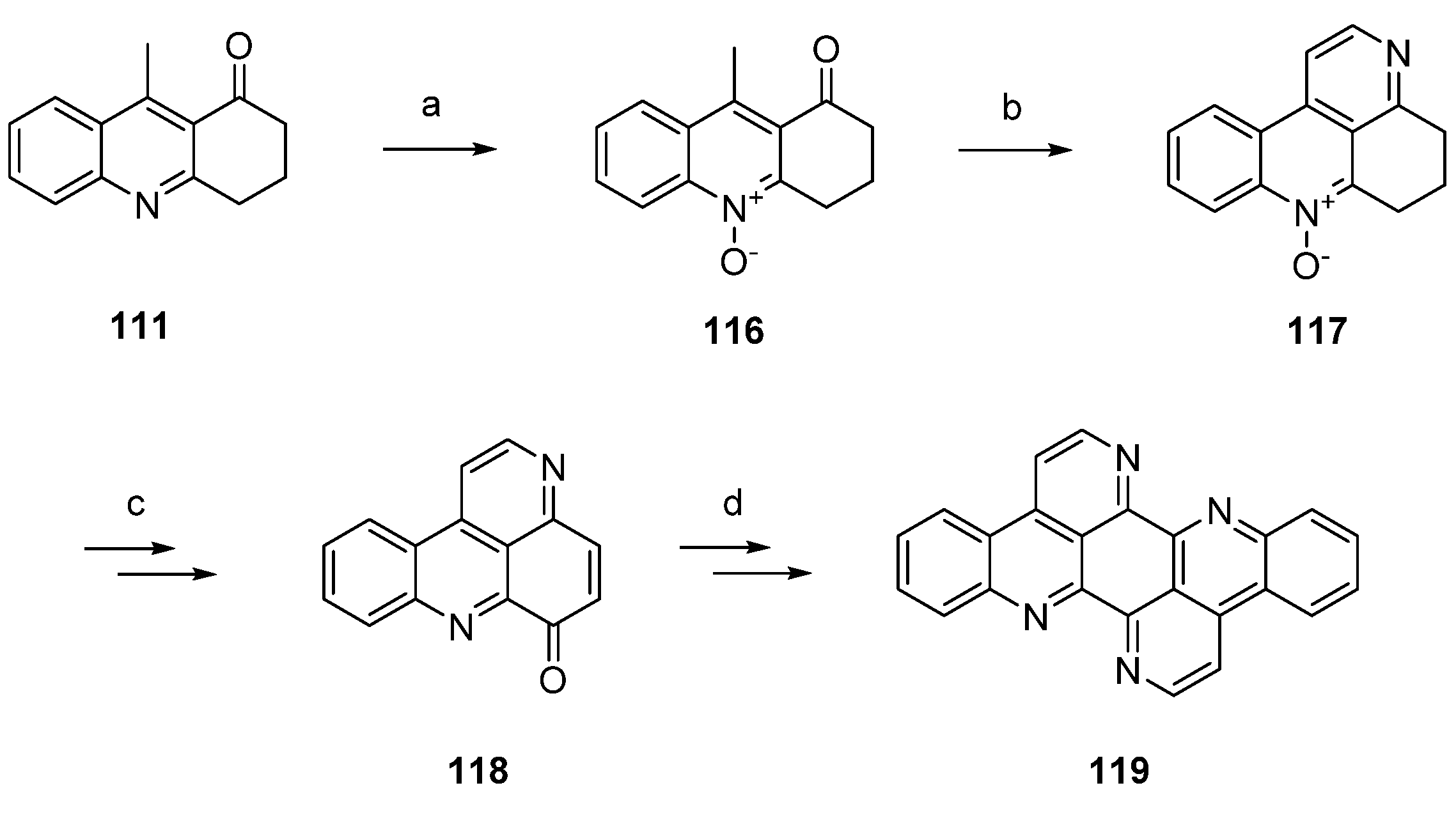
Isoeilatin (
119), a synthetic isomer of
90, was prepared from the same building blocks by using different reaction conditions. In this synthesis, acridone
111 was first treated with
meta-chloroperoxybenzoic acid (
mCPBA) to furnish
N-oxide
116. An annulation of a pyridine ring through condensation with
N,
N-dimethylformamide diethyl acetal and subsequent ring closure with ammonium chloride gave tetracyclic compound
117, an isomer of the
N-oxide
112 utilized in the synthesis of eilatin (
90), in 38% yield. In analogy to the above-mentioned protocol,
N-oxide
117 was subjected to Boekelheide rearrangement, ester hydrolysis and oxidation with MnO
2 to furnish pyridoacridone
118. The following steps (oxidative amination, acid-catalyzed cyclization, annulation of the seventh ring using
N,
N-dimethylformamide diethyl acetal and ammonium chloride) were performed in full analogy to the above-mentioned total synthesis of eilatin (
90) [
37]. Isoeilatin (
119) was obtained in eight steps with 5.1% total yield (
Scheme 21) [
37].
Scheme 21.
Synthesis of isoeilatin (119): (a) mCPBA, CH2Cl2 (88%); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl (38% over two steps); (c) acetic anhydride (80%); then 10% NaOH in H2O/MeOH; then MnO2, toluene (50% over two steps); (d) 2-aminoacetophenone, CeCl3·7H2O; then 10% H2SO4 in AcOH; then N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl (38% over four steps).
Scheme 21.
Synthesis of isoeilatin (119): (a) mCPBA, CH2Cl2 (88%); (b) N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl (38% over two steps); (c) acetic anhydride (80%); then 10% NaOH in H2O/MeOH; then MnO2, toluene (50% over two steps); (d) 2-aminoacetophenone, CeCl3·7H2O; then 10% H2SO4 in AcOH; then N,N-dimethylformamide diethyl acetal, DMF; then NH4Cl (38% over four steps).
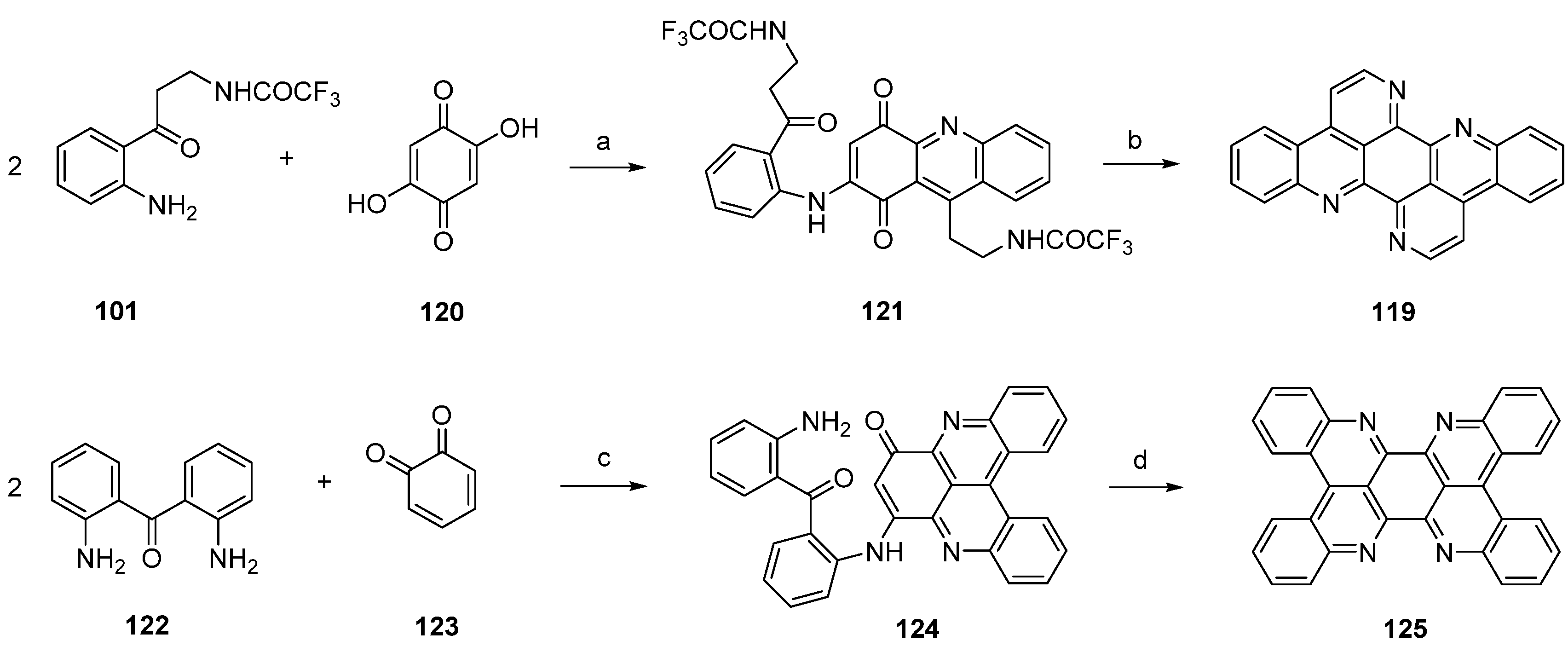
Another approach towards isoeilatin (
119) was reported by Kashman [
38] in a paper published in 1994. Reaction of monoprotected trifluoroacetyl kynuramine
101 and 2,5-dihydroxy-1,4-cyclohexanedione (
120) under acidic conditions (AcOH/Et
3N) furnished isoeilatin precursor
121 in 7% yield. Following treatment of with ammonia in methanol provided isoeilatin (
119) (
Scheme 22).
The authors also described the synthesis of a dibenzo analogue of eilatin (
125) in this publication [
38]. For this purpose, 2,2′-diaminobenzophenone (
122) was reacted with
o-benzoquinone (
123) in the presence of CeCl
3 to give compound
124. Subsequent treatment with acid (AcOH/H
2SO
4/Et
3N) of
124 afforded the highly symmetrical “dibenzoeilatin” (
125) (
Scheme 22) [
38].
Scheme 22.
Synthesis of isoeilatin (119): (a) AcOH/Et3N (7%); (b) NH3 in methanol (43%). Synthesis of dibenzoeilatin (125); (c) CeCl3·7H2O, EtOH (36%); (d) AcOH/H2SO4/Et3N (no yield given).
Scheme 22.
Synthesis of isoeilatin (119): (a) AcOH/Et3N (7%); (b) NH3 in methanol (43%). Synthesis of dibenzoeilatin (125); (c) CeCl3·7H2O, EtOH (36%); (d) AcOH/H2SO4/Et3N (no yield given).
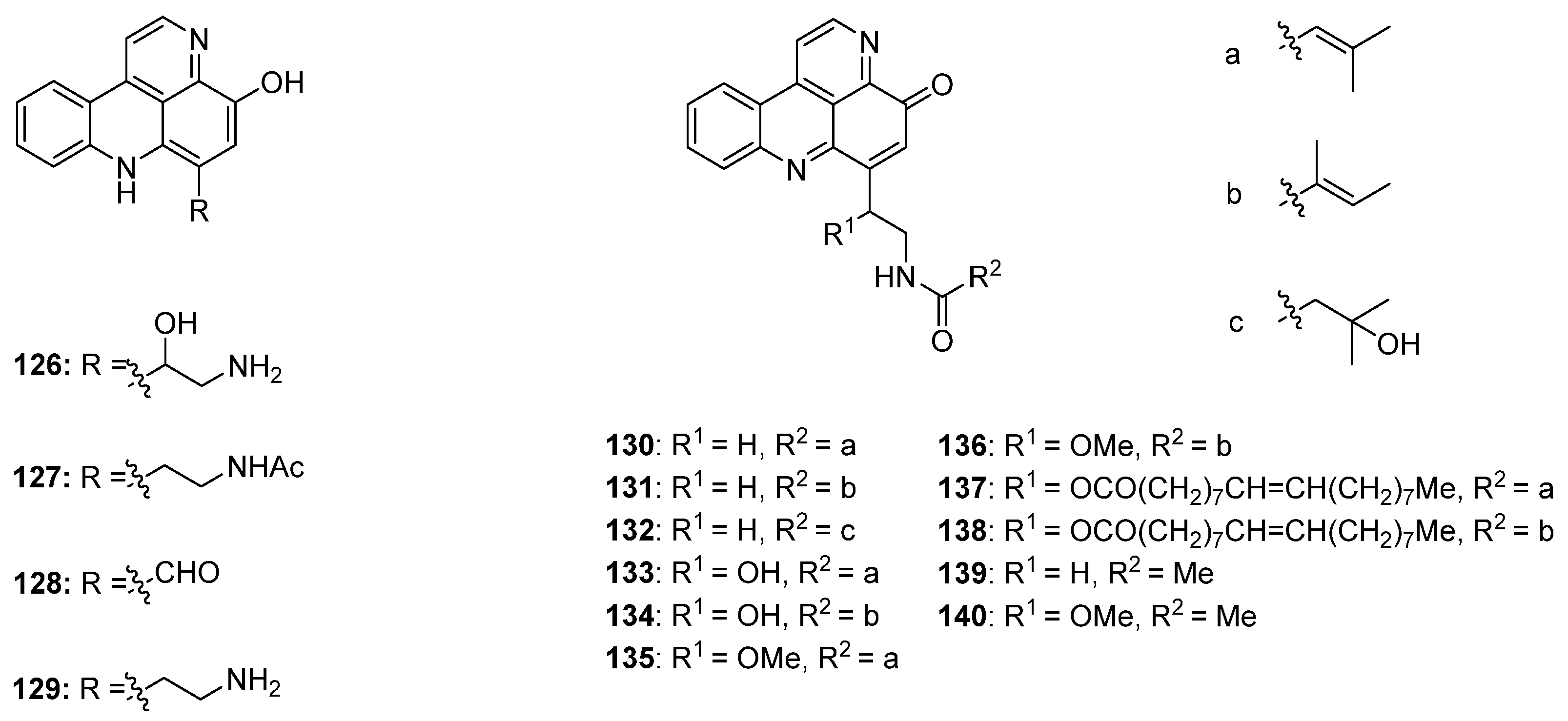
5. Styelsamine- and Cystodytin-Type Pyridoacridines
Styelsamine- and cystodytin-type pyridoacridines are teracyclic alkaloids bearing a functionalized side-chain at C-6. In contrast to cystodytin-type alkaloids, which possess the pyrido[4,3,2-
mn]acridin-4-one skeleton, styelsamine alkaloids are characterized by the hydroxylated pyrido[4,3,2-
mn]acridine core. Until today, four stylesamine-type (stylesamines A–D;
126–
129) [
39] and 11 cystodytin-type alkaloids (cystodytin A–K;
130–
140) [
40,
41,
42,
43] have been isolated and characterized (
Figure 5).
Figure 5.
Structures of the styelsamines A–D (126–129) and cystodytins A–K (130–140).
Figure 5.
Structures of the styelsamines A–D (126–129) and cystodytins A–K (130–140).
Based on Skyler and Heathcock’s biomimetic approach [
44] to stylesamine B (
127) and cystodytin J (
139), Fong and Copp reported the synthesis of a series of side-chain substituted analogues of styelsamine and cystodytin alkaloids in 2013 [
45]. The key step of this biomimetic strategy is a CeCl
3-catalyzed oxidative coupling of kynuramine (
141) and functionalized dopamine analogues
142–
147 in the presence of silver oxide. While kynuramine (
141) was prepared from a
N-protected tryptamine
via oxidative cleavage of the pyrrole ring, the dopamine analogues
142–
147 were synthesized from 3,4-dimethoxyphenethylamine by
N-acylation and subsequent
O-demethylation (both syntheses are not shown in
Scheme 23).
In situ oxidation of the catechol moiety of the dopamines with silver oxide gives
ortho-quinones, which undergo CeCl
3-mediated nucleophilic addition of the aromatic amino group of kynuramine (
141) (the more basic aliphatic side-chain amino group is prevented from this reaction by protonation) and re-oxidation to an aminoquinone. Subsequent treatment with 6 M hydrochloric acid resulted in cyclization to the acridines and, finally, closure of the fourth ring in an imine formation/tautomerization sequence related to a protocol that is known from Kashman’s biomimetic ascididemin synthesis [
13]. The stylesamine-type pyridoacridines
127,
148–
152 were isolated in yields of 6% to 20%. Subsequent oxidation of
127,
148–
152 with one equivalent of silver oxide in bicarbonate-buffered methanol furnished cystodytin-type pyridoacridines
139,
153–
157 in 13%–79% yields (
Scheme 23) [
45].
Scheme 23.
Synthesis of styelsamine (127, 148–152) and cystodytine analogues (139, 153–157): (a) CeCl3·7H2O, Ag2O, MeOH/AcOH (2:1), then 6 M HCl (6%–20%); (b) Ag2O (one equiv.), MeOH (13%–79%).
Scheme 23.
Synthesis of styelsamine (127, 148–152) and cystodytine analogues (139, 153–157): (a) CeCl3·7H2O, Ag2O, MeOH/AcOH (2:1), then 6 M HCl (6%–20%); (b) Ag2O (one equiv.), MeOH (13%–79%).
In 2002, Skyler and Heathcock [
3] reported that treatment of stylesamine B (
127) with hydrochloric acid (4 M) in methanol afforded the alkylamino analogue of stylesamine D
158 in quantitative yield. Repeating this experiment, Copp isolated, besides the desired product
158, the unexpected
O-methyl analogue
159. With this
O-methyl analogue
159 in hand, the authors were able to prepare the
N-acyl analogues
160–
162. While acrylamide
160 and 2-phenylacetamide
161 were synthesized by treatment of
159 with appropriate carboxylic acids in DMF and PyBOP, 3-phenylpropanamide
162 was prepared from
159 by reaction with dihydrocinnamoyl chloride in THF (
Scheme 24) [
45].
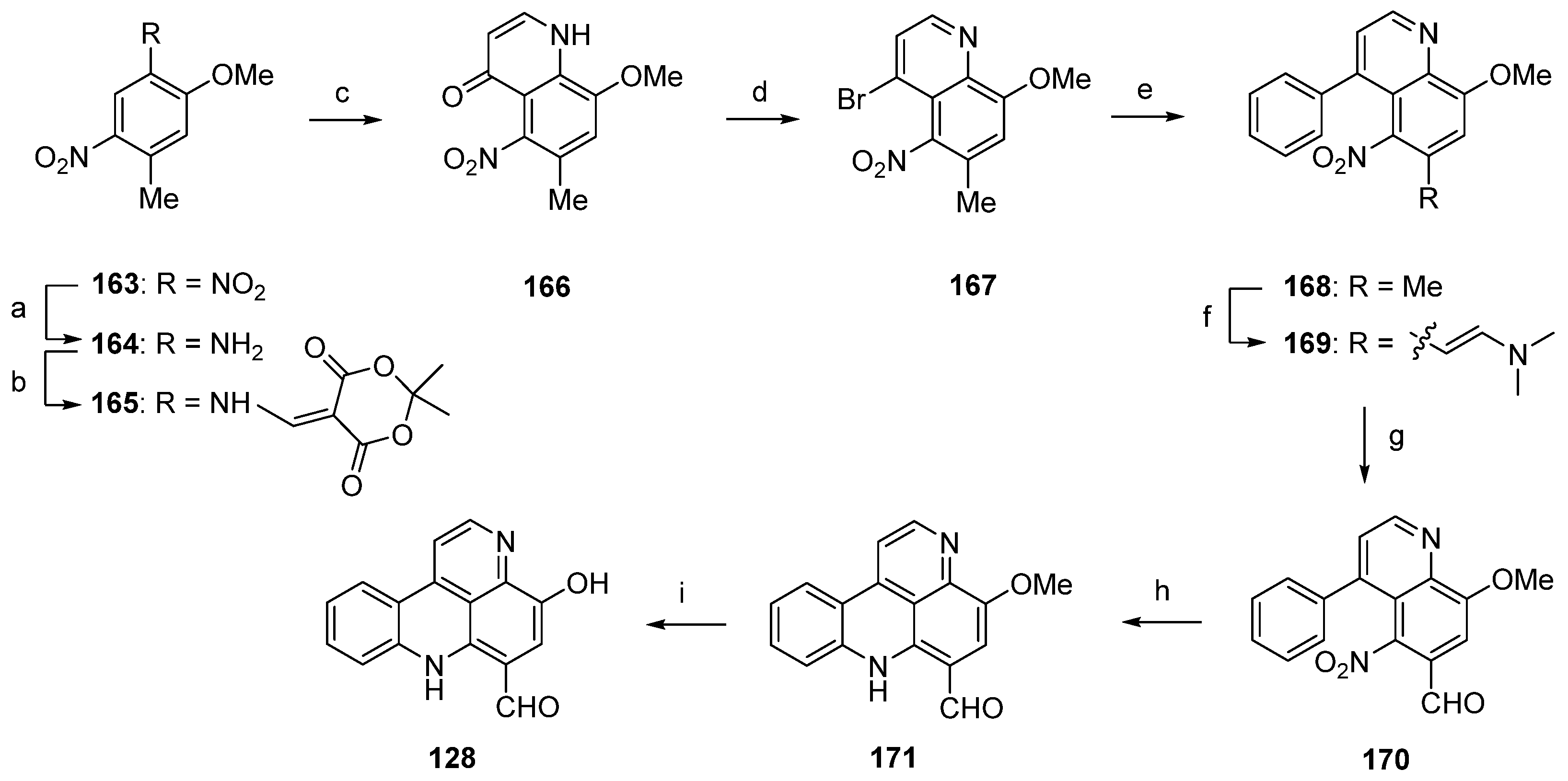
In 2003, Nakahara and Kubo reported the first total synthesis of styelsamine C (
128) [
46,
47]. This nine-step approach with an overall yield of 16% started with selective transfer hydrogenation of 5-methoxy-2,4-dinitrotoluene (
163) using cyclohexene and 10% palladium on carbon catalyst to give 2-methoxy-4-methyl-5-nitroaniline (
164). The following treatment with Meldrum’s acid furnished enaminone
165, which was cyclized in refluxing diphenyl ether to afford 4-quinolone
166 (47% yield over three steps). Reaction of
166 with phosphoryl bromide gave 4-bromoquinoline
167 in 78% yield. The 4-phenylquinoline
168 was obtained in almost quantitative yield by the Suzuki cross-coupling reaction of
167 with phenylboronic acid under standard conditions. The acidic methyl group at C-6 of the nitroquinoline
168 was converted to an aldehyde in two steps. Treatment of
168 with
N,
N-dimethylformamide dimethyl acetal at 170 °C (in the full paper [
47] the authors present different conditions,
N,
N-dimethylformamide dimethyl acetal at 145 °C, but the same yield) furnished enamine
169 in 91% yield. Subsequent oxidative cleavage with NaIO
4 in THF/water afforded aldehyde
170 in 90% yield. The final cyclization step was achieved
via an intramolecular nitrene insertion reaction by the heating of
170 with triethyl phosphite, yielding the tetracyclic compound
171 in 65% yield. Styelsamine C (
128) was finally prepared by
O-demethylation of
171 with boron tribromide in 86% yield (
Scheme 25) [
46,
47].
Scheme 24.
Synthesis of analogues of styelsamine alkaloids: (a) 4 M HCl/MeOH (1:1) (158, 60% and 159, 45%); (b) for 160 and 161: corresponding carboxylic acid, DMF, CH2Cl2, Et3N, PyBOP (160, 88% and 161, 48%); for 162: dihydrocinnamoyl chloride, THF, Et3N (43%).
Scheme 24.
Synthesis of analogues of styelsamine alkaloids: (a) 4 M HCl/MeOH (1:1) (158, 60% and 159, 45%); (b) for 160 and 161: corresponding carboxylic acid, DMF, CH2Cl2, Et3N, PyBOP (160, 88% and 161, 48%); for 162: dihydrocinnamoyl chloride, THF, Et3N (43%).
Scheme 25.
Synthesis of styelsamine C (128): (a) 10% Pd/C, cyclohexene/EtOH (60%); (b) Meldrum’s acid, trimethyl orthoformate (94%); (c) diphenyl ether, reflux (83%); (d) POBr3 (78%); (e) phenylboronic acid, EtOH/toluene, K2CO3, Pd(PPh3)4 (94%); (f) N,N-dimethylformamide dimethyl acetal, 170 °C (91%); (g) NaIO4, THF/H2O (90%); (h) P(OEt)3 (65%); (i) BBr3 in CH2Cl2 (86% over two steps).
Scheme 25.
Synthesis of styelsamine C (128): (a) 10% Pd/C, cyclohexene/EtOH (60%); (b) Meldrum’s acid, trimethyl orthoformate (94%); (c) diphenyl ether, reflux (83%); (d) POBr3 (78%); (e) phenylboronic acid, EtOH/toluene, K2CO3, Pd(PPh3)4 (94%); (f) N,N-dimethylformamide dimethyl acetal, 170 °C (91%); (g) NaIO4, THF/H2O (90%); (h) P(OEt)3 (65%); (i) BBr3 in CH2Cl2 (86% over two steps).
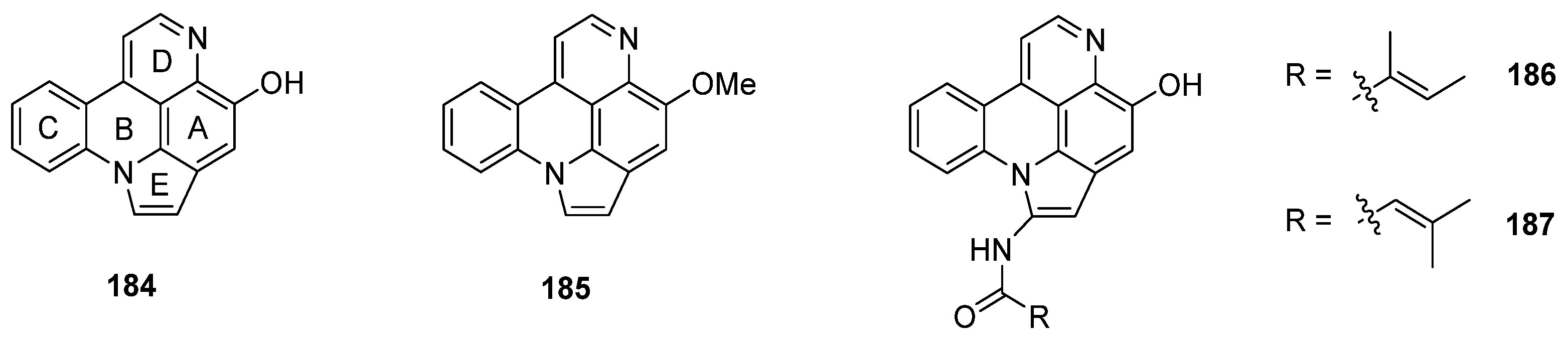
7. Arnoamine-Type Pyridoacridines
Arnoamines A–D (
184–
187) are a unique type of pyridoacridine alkaloids, since they bear a pyrrole ring fused to rings A and B of the pyrido[4,3,2-
mn]acridine skeleton. Isolated in 1998 (arnoamines A and B) and 2013 (arnoamines C and D) from two different ascidians (
Cystodytes species), these pentacycles are up to today the only known representatives of this pyridoacridine subclass (
Figure 7) [
50,
51].
Figure 7.
Structures of arnoamines A (184), B (185), C (186), and D (187).
Figure 7.
Structures of arnoamines A (184), B (185), C (186), and D (187).
The first total syntheses of arnoamines A (
184) and B (
185) were reported in 2000 [
52] and have already been described in Delfourne’s review [
6]. The key steps of this approach were the thermolysis of an arylaminomethylene in Meldrum’s acid derivative for construction of the quinoline scaffold (rings A and D), a Suzuki cross-coupling reaction of a functionalized 4-bromoquinoline (
188) for the introduction of the phenyl ring (C), annulation of the pyrrole ring
via Fischer indole synthesis, followed by cyclization under intramolecular
N-arylation of the pyrrole ring (E) [
52].
A related approach to arnoamine B (
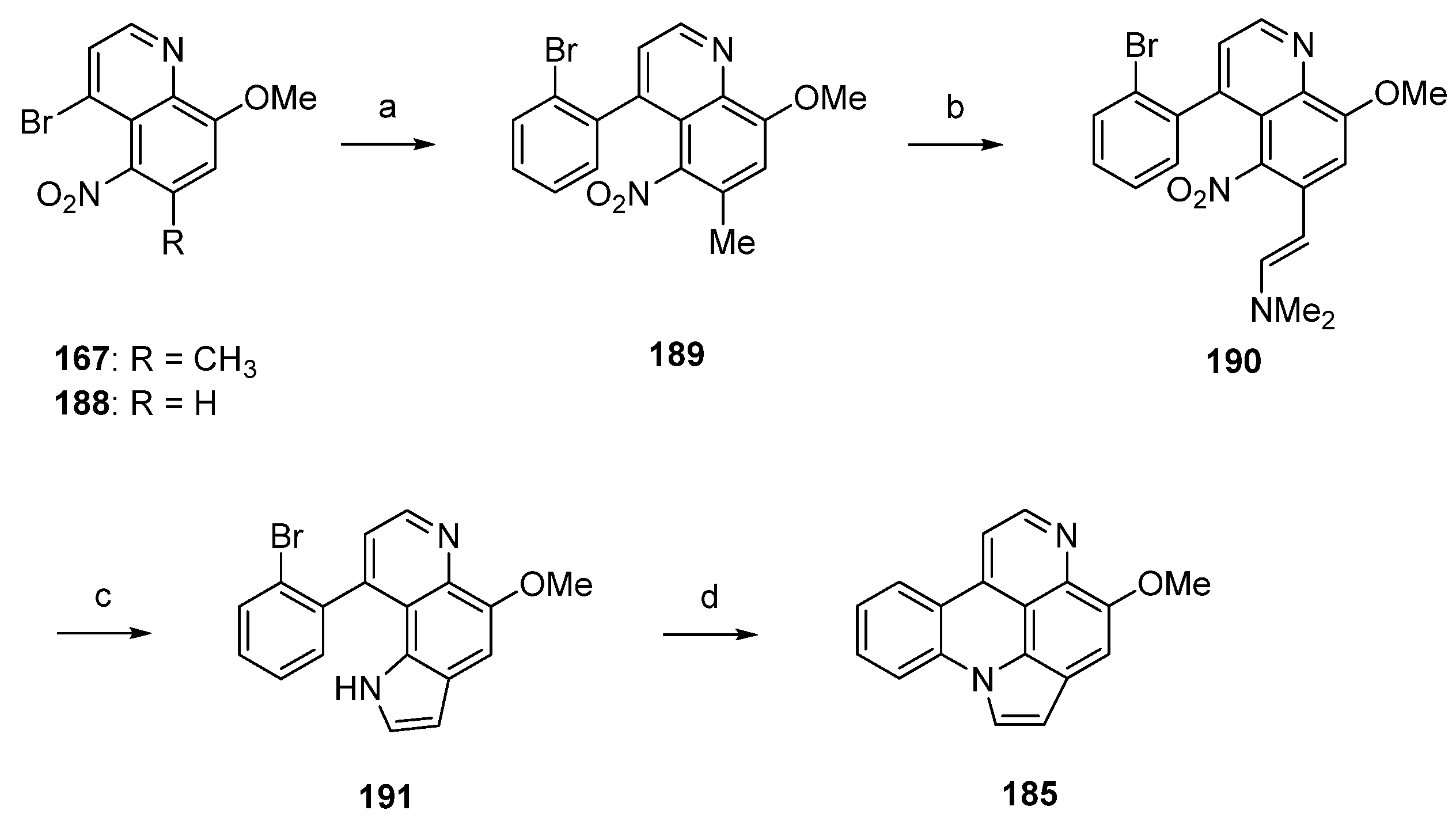
185) was reported by Nakahara
et al. in 2007 [
53]. Starting from readily available 5-nitroquinoline
167 (see
Scheme 25), alkaloid
185 was prepared in four steps with an overall yield of 12%. In the first step,
167 was subjected to a palladium(0)-catalyzed Suzuki cross-coupling reaction to give 4-(2-bromophenyl)quinoline
189 in 85% yield. Annulation of the pyrrole ring was accomplished in low yield (17% over two steps) utilizing the Leimgruber-Batcho protocol by condensation of the CH-acidic 6-methyl group of
189 with
N,
N-dimethylformamide dimethyl acetal to give enamine
190 and subsequent reductive cyclization with zinc powder in 80% aqueous acetic acid. Despite the low yield, this protocol is more convenient than Delfourne’s protocol starting from the des-methyl compound
188 and utilizing a Fischer synthesis for construction of the pyrrole ring. Finally, cyclization to arnoamine B (
185) was accomplished in 81% yield under intramolecular
N-arylation of the pyrrole moiety of
191 under palladium(II) acetate catalysis (
Scheme 27) [
53].
Scheme 27.
Synthesis of arnoamine B (185): (a) from 167: 2-bromophenylboronic acid, H2O/toluene, K2CO3, Pd(PPh3)4 (85%); (b) N,N-dimethylformamide dimethyl acetal, DMF (83%); (c) Zn, AcOH/H2O (21%); (d) Pd(OAc)2, P(CMe3)3, K2CO3, xylene (81%).
Scheme 27.
Synthesis of arnoamine B (185): (a) from 167: 2-bromophenylboronic acid, H2O/toluene, K2CO3, Pd(PPh3)4 (85%); (b) N,N-dimethylformamide dimethyl acetal, DMF (83%); (c) Zn, AcOH/H2O (21%); (d) Pd(OAc)2, P(CMe3)3, K2CO3, xylene (81%).
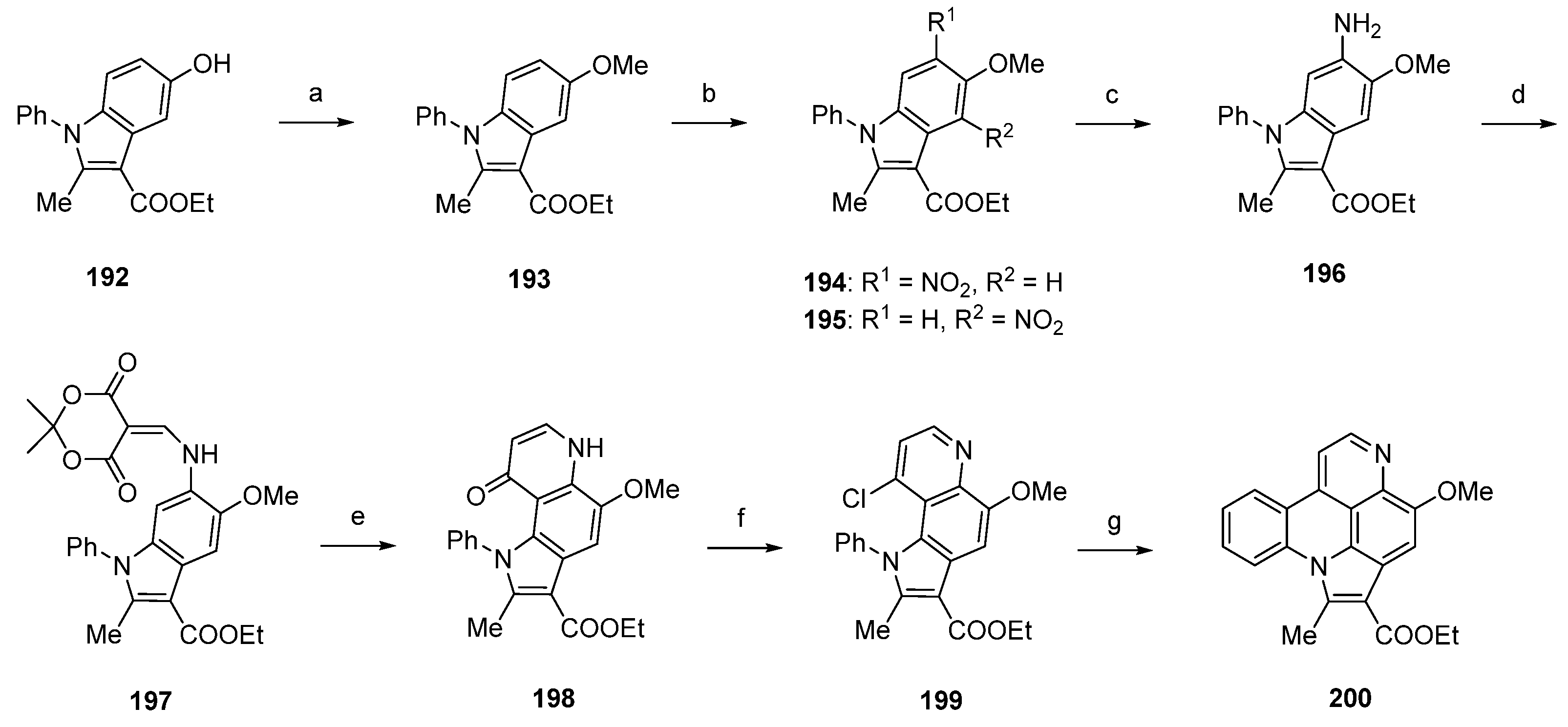
Using a different approach, starting from a
N-phenylindole
192, Radchenko
et al. worked out a simple and effective route to the pyrido[4,3,2-
mn]pyrrolo[3,2,1-
de]acridine core of arnoamine-type pyridoacridines [
54]. Synthesis of the arnoamine B analogue
200 started with
O-methylation of
192 with dimethyl sulfate and sodium hydroxide to afford methyl ether
193 in almost quantitative yield. The following nitration with nitric acid (75%) in acetic anhydride furnished a mixture of nitroindoles
194 and
195 in 63% and 30% yield. Raney nickel-catalyzed hydrogenation of
194 gave primary amine
196, which was converted to enamine
197 by treatment with Meldrum’s acid and trimethyl orthoformate in 95% yield. Subsequent thermal cyclization in refluxing diphenyl ether furnished pyrroloquinolone
198 in 80% yield. Arnoamine B analogue
200 was finally obtained by treating
198 with phosphoryl chloride (giving
199) and subsequent radical intramolecular cyclization using tributyltin hydride and azobis(isobutyronitrile) in 93% yield over two steps (
Scheme 28) [
54].
Scheme 28.
Synthesis of arnoamine B analogue 200: (a) Me2SO4, 2 M NaOH, H2O, dioxane (95%); (b) HNO3, Ac2O (194, 63% and 195, 30%); (c) from 194: H2, Raney nickel, isopropanol (no yield given); (d) Meldrum’s acid, trimethyl orthoformate (95%); (e) diphenyl ether, reflux (80%); (f) POCl3 (96%); (g) Bu3SnH, AIBN, benzene (97%).
Scheme 28.
Synthesis of arnoamine B analogue 200: (a) Me2SO4, 2 M NaOH, H2O, dioxane (95%); (b) HNO3, Ac2O (194, 63% and 195, 30%); (c) from 194: H2, Raney nickel, isopropanol (no yield given); (d) Meldrum’s acid, trimethyl orthoformate (95%); (e) diphenyl ether, reflux (80%); (f) POCl3 (96%); (g) Bu3SnH, AIBN, benzene (97%).
In close relationship to this approach, Nakahara
et al. achieved the synthesis of arnoamine B (
185) in five steps with an overall yield of 33% [
55]. This total synthesis starts with a CuI-catalyzed
N-arylation reaction of 5-methoxy-6-nitroindole (
201) with iodobenzene in toluene to give indole
202 in 80% yield. Following catalytic hydrogenation over 10% palladium on carbon in methanol yielded 6-aminoindole
203. Annulation of the 4-pyridone ring was accomplished following the methods described above by treating
203 with Meldrum’s acid in trimethyl orthoformate (66% yield over two steps), followed by thermal cyclization of
204 upon heating in refluxing diphenyl ether. Subsequent treatment with phosphoryl chloride afforded chloro compound
205 in 70% yield over two steps. The final intramolecular cyclization step was performed using a large excess of tributyltin hydride and azobis(isobutyronitrile) in toluene to furnish the desired alkaloid
185 in 90% yield (
Scheme 29) [
55].
Arnoamines C (186) and D (187) have not been synthesized until today.
Scheme 29.
Synthesis of arnoamine B (185) by Kubo: (a) iodobenzene, CuI, (CH2NHMe)2, K3PO4, toluene (80%); (b) 10% Pd-C, methanol; (c) Meldrum’s acid, trimethyl orthoformate (66% over two steps); (d) Ph2O, reflux; then POCl3 (70% over two steps); (e) Bu3SnH (30 equiv.), AIBN (15 equiv.), toluene (90%).
Scheme 29.
Synthesis of arnoamine B (185) by Kubo: (a) iodobenzene, CuI, (CH2NHMe)2, K3PO4, toluene (80%); (b) 10% Pd-C, methanol; (c) Meldrum’s acid, trimethyl orthoformate (66% over two steps); (d) Ph2O, reflux; then POCl3 (70% over two steps); (e) Bu3SnH (30 equiv.), AIBN (15 equiv.), toluene (90%).
8. Subarine—A Formal Seco Analogue of Pyridoacridines
Subarine (
212), a marine pyridyl benzo[
c][2,7]naphthyridine alkaloid, is closely related to the pyridoacridine family, as it is formally a seco analogue of ascididemin-type alkaloids. This alkaloid was prepared following two different approaches by the Delfourne [
56] and Bracher [
57] groups.
Delfourne’s total synthesis of subarine (
212) was accomplished in four steps with an overall yield of 70% starting from intermediate 1,10-phenanthrolin-4-ol (
206), itself obtained in four steps from commercially available 8-aminoquinoline. Reaction of
206 with phosphoryl bromide and phosphorus tribromide gave the corresponding bromo derivative
207. Subsequent oxidative cleavage of
207 with potassium permanganate under alkaline conditions afforded the binicotinic-type dicarboxylic acid
208. The following conversion to diester
209 was accomplished by treatment of
208 with DCC and methanol. Palladium-catalyzed Stille cross-coupling reaction with
N-(
tert-butoxycarbonyl)-2-(trimethylstannyl)aniline afforded the expected phenyl-binicotinate
210, together with the already cyclized lactam
211. Subarine (
212) was finally obtained by treating
211 with trifluoroacetic acid. In the case of
210, synthesis was accomplished through cleavage of the Boc protecting group and a subsequent intramolecular cyclization reaction (
Scheme 30) [
56].
Scheme 30.
Synthesis of subarine (212) by Delfourne: (a) POBr3, PBr3 (79%); (b) KMnO4, KOH, H2O; (c) DCC, MeOH (88% over two steps); (d) 2-(NHBoc)C6H4SnMe3, Pd(PPh3)4, 1,4-dioxane (210, 63% ; 211, 18%); (e) Et3N, CH2Cl2 (98% for both reactions).
Scheme 30.
Synthesis of subarine (212) by Delfourne: (a) POBr3, PBr3 (79%); (b) KMnO4, KOH, H2O; (c) DCC, MeOH (88% over two steps); (d) 2-(NHBoc)C6H4SnMe3, Pd(PPh3)4, 1,4-dioxane (210, 63% ; 211, 18%); (e) Et3N, CH2Cl2 (98% for both reactions).
A significantly shorter synthetic route towards the alkaloid subarine (
212) was reported by Lotter and Bracher in 2009 [
57]. This four-step approach starts with the oxidation of 1,10-phenanthroline (
213) using potassium permanganate. The so-obtained dicarboxylate
214 was esterificated by treatment with sulfuric acid in methanol to give the symmetric diester
215 in high yield. Amidation of
215 with 2-haloanilines under Weinreb conditions (trimethylaluminum, heptane) furnished the mono-2-haloanilides
216 and
217 in moderate yields, accompanied by the corresponding dianilides. The final cyclization step was accomplished by treating 2-iodoanilide
216 with tributyltin hydride and a catalytic amount of azobis(isobutyronitrile) in benzene to afford subarine (
212) in very poor yield [
57]. Significant amounts of deiodination product
218 were obtained in this reaction. Unfortunately, this approach gives only a very poor overall yield (
Scheme 31).
Scheme 31.
Synthesis of subarine (212) by Lotter and Bracher: (a) KMnO4, KOH, H2O (b) H2SO4, MeOH (73% over two steps); (c) 2-iodoaniline/2-bromoaniline, CH2Cl2, Me3Al, heptane (216, 40%; 217, 35%); (d) Bu3SnH, AIBN, benzene (7% from 216).
Scheme 31.
Synthesis of subarine (212) by Lotter and Bracher: (a) KMnO4, KOH, H2O (b) H2SO4, MeOH (73% over two steps); (c) 2-iodoaniline/2-bromoaniline, CH2Cl2, Me3Al, heptane (216, 40%; 217, 35%); (d) Bu3SnH, AIBN, benzene (7% from 216).