Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen
Abstract
:1. Introduction
2. Results and Discussion
2.1. Analyses, DH, Molecular Weight (MW) and Amino Acid Composition of Hydrolysates
2.2. Analyses of Antioxidant Activities
2.3. Purification of the Antioxidant Peptides from SGI-2
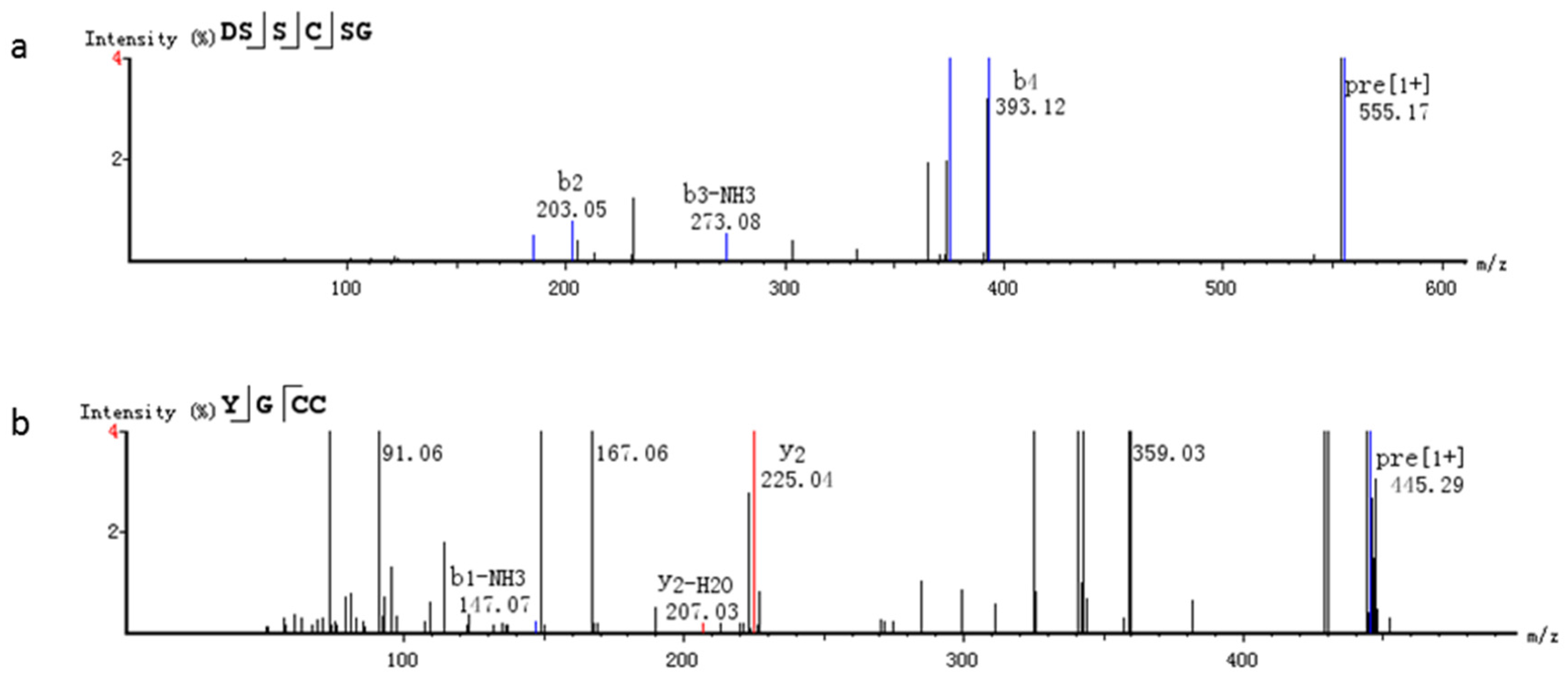
2.4. Identification of Purified Peptide
3. Materials and Methods
3.1. Materials
3.2. Preparation of Skin Collagen Hydrolysates of Alaska Pollock
3.3. Determination of Characteristics of Hydrolysates
3.3.1. Determination of the Degree of Hydrolysis (DH)
3.3.2. Molecular Weight (MW) Distribution
3.3.3. Amino Acid Composition
3.4. Antioxidative Activity Assay
3.4.1. ABTS•+ Scavenging Activity Assay
3.4.2. FRAP Assay
3.4.3. OH· Scavenging Activity Assay
3.5. Purification of Antioxidant Peptides
3.6. Analysis and Identification of Purified Peptide
3.6.1. Assay of High Resolution Mass Spectrometry (LC-ESI-LTQ-Orbitrap-MS)
3.6.2. Identification of the Key Peptides
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis inhibitory activities of collagen peptide from jellyfish (Rhopilemaesculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Duan, X.; Zhuang, Y.L. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.L.; Sun, L.P.; Zhao, X.; Hou, H.; Li, B.F. Investigation of gelatin polypeptides of jellyfish (Rhopilema esculentum) for their antioxidant activity in vitro. Food Technol. Biotechnol. 2010, 48, 222–228. [Google Scholar]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 17, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, A.; Hajji, M.; Balti, R.; Lassoued, I.; Triki-Ellouz, Y.; Nasri, M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009, 114, 1198–1205. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, C.; Jiang, A. Antioxiant peptides isolated from sea cucumber Stichopus Japonicus. Eur. Food Res. Technol. 2012, 234, 441–447. [Google Scholar] [CrossRef]
- Cheung, I.W.Y.; Cheung, L.K.Y.; Tan, N.Y.; Li-Chan, E.C.Y. The role of molecular size in antioxidant activity of peptide fractions from Pacific hake (Merluccius productus) hydrolysates. Food Chem. 2012, 134, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.; Kitts, D.D.; Li-Chan, E.C. Antioxidative and angiotensin-I-converting enzyme inhibitory potential of a Pacific Hake (Merluccius productus) fish protein hydrolysate subjected to simulated gastrointestinal digestion and Caco-2 cell permeation. J. Agric. Food Chem. 2010, 58, 1535–1542. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Li, B.; Zhao, X.; Yi, J. Physicochemical properties of gelatin gels from walleye pollock (Theragra chalcogramma) skin cross-linked by gallic acid and rutin. Food Hydrocol. 2011, 25, 907–914. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Wang, S.; Si, L.; Li, B. Immunomodulatory activity of Alaska Pollock hydrolysates obtained by glutamic acid biosensor-Artificial neural network and the identification of its active central fragmen. J. Funct. Foods 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, S.K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollock (Theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, Y.; Lu, J.; Chen, A.; Li, Y.; Zheng, G. Enzymatic hydrolysis of Alaska Pollock (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010, 90, 635–640. [Google Scholar] [PubMed]
- You, L.; Zhao, M.; Regenstein, J.M. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Sun, L.; Bai, X.; Zhuang, Y. Effect of different cooking methods on total phenolic contents and antioxidant activities of four Boletus mushrooms. J. Food Sci. Technol. 2014, 51, 3362–3368. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Zhao, X.; Li, B. Optimization of antioxidant activity by response surface methodology in hydrolysates of jellyfish (Rhopilema esculentum) umbrella collagen. J. Zhejiang Univ. Sci. B 2009, 10, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Fan, Y.; Li, B. Purification and identification of immunomodulating peptides from enzymatic hydrolysates of Alaska pollock frame. Food Chem. 2012, 134, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wei, R.B.; Ruan, G.Q. Isolation and identification of antioxidative peptides from peptic hydrolysates of half-fin anchovy (Setipinna taty). LWT Food Sci. Technol. 2015, 60, 221–229. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Bandara, N.; Wu, J. Purification and characterization of antioxidant peptides from enzymatically hydrolyzed chicken egg white. Food Chem. 2015, 188, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Yang, H.; Sun, Y. Purification and identification of antioxidant peptides from walnut (Juglans regia L.) protein hydrolysates. Peptides 2012, 38, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Chen, T.T.; Hu, P. Purification and characterization of an antioxidant peptide (GSQ) from Chinese leek (Allium tuberosum Rottler) seeds. J. Funct. Foods 2014, 10, 144–153. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, B. Characterization of structure-antioxidant activity relationship of peptides in free radical systems using QSAR models: Key sequence positions and their amino acid properties. J. Theor. Biol. 2013, 318, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; He, J.; Zhuang, Y.; Sun, L. Purification and Identification of Antioxidant Peptides from Enzymatic Hydrolysates of Tilapia (Oreochromis niloticus) Frame Protein. Molecules 2012, 17, 12836–12850. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.S.; Nazeer, R.A.; Jaiganesh, R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides 2011, 32, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Li, B.; He, J. Structure–activity relationship study of antioxidative peptides by QSAR modeling: The amino acid next to C-terminus affects the activity. J. Pept. Sci. 2011, 17, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Zhang, H.; Wang, L.; Guo, X.N.; Wang, X.G.; Yao, H.Y. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysate by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chem. 2010, 119, 226–234. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Byun, H.G.; Kim, S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005, 16, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Wang, L.; He, Y.; Wang, Z.; Xu, J.; Ma, H. Hydrolysis kinetics and antioxidant activity of collagen under simulated gastrointestinal digestion. J. Funct. Food 2014, 11, 493–499. [Google Scholar] [CrossRef]
- Hou, H.; Li, B.; Zhao, X.; Zhang, Z.; Li, P. Optimization of enzymatic hydrolysis of Alaska pollock frame for preparing protein hydrolysates with low-bitterness. LWT Food Sci. Technol. 2011, 44, 421–428. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, Y.L.; Zhai, J. Fractionation and evaluation of radical scavenging peptides from in vitro digests of buckwheat protein. Food Chem. 2010, 118, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Aleman, A.; Gimenez, B.; Perez-Santin, E. Contribution of Leu and Hyp residues to antioxidant and ACE-inhibitory activities of peptide sequences isolated from squid gelatin hydrolysate. Food Chem. 2011, 125, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Liu, H.X.; Ji, X. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar] [CrossRef] [PubMed]
- MaxQuant Server (version 1.5.3.28). Available online: http://www.coxdocs.org/doku.php?id=maxquant:common:download_and_installation (accessed on 12 October 2016).
- Orsini Delgado, M.C.; Nardo, A.; Pavlovic, M.; Rogniaux, H.; Añón, M.; Tironi, V.A. Identification and characterization of antioxidant peptides obtained by gastrointestinal digestion of amaranth proteins. Food Chem. 2016, 197, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
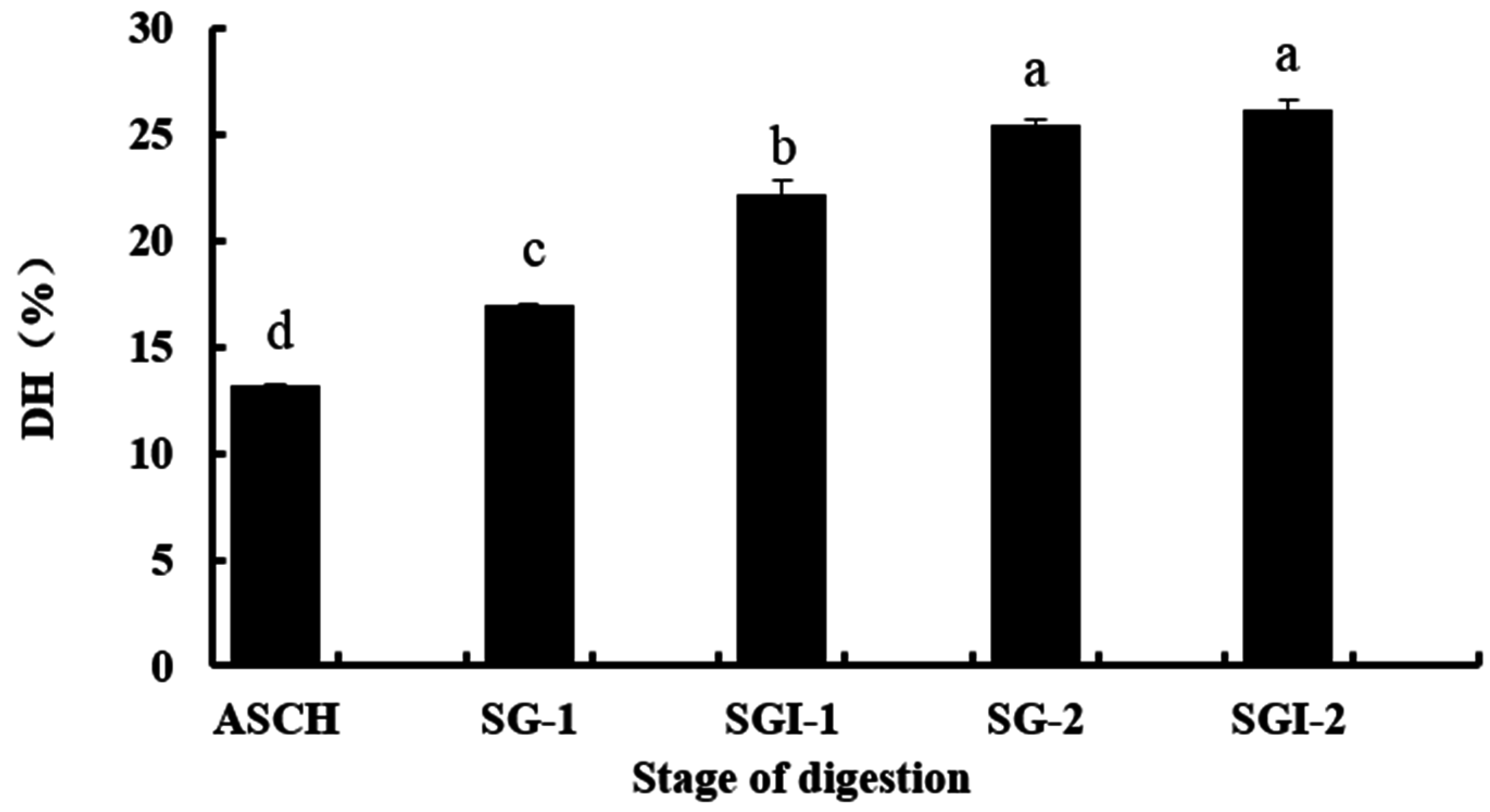
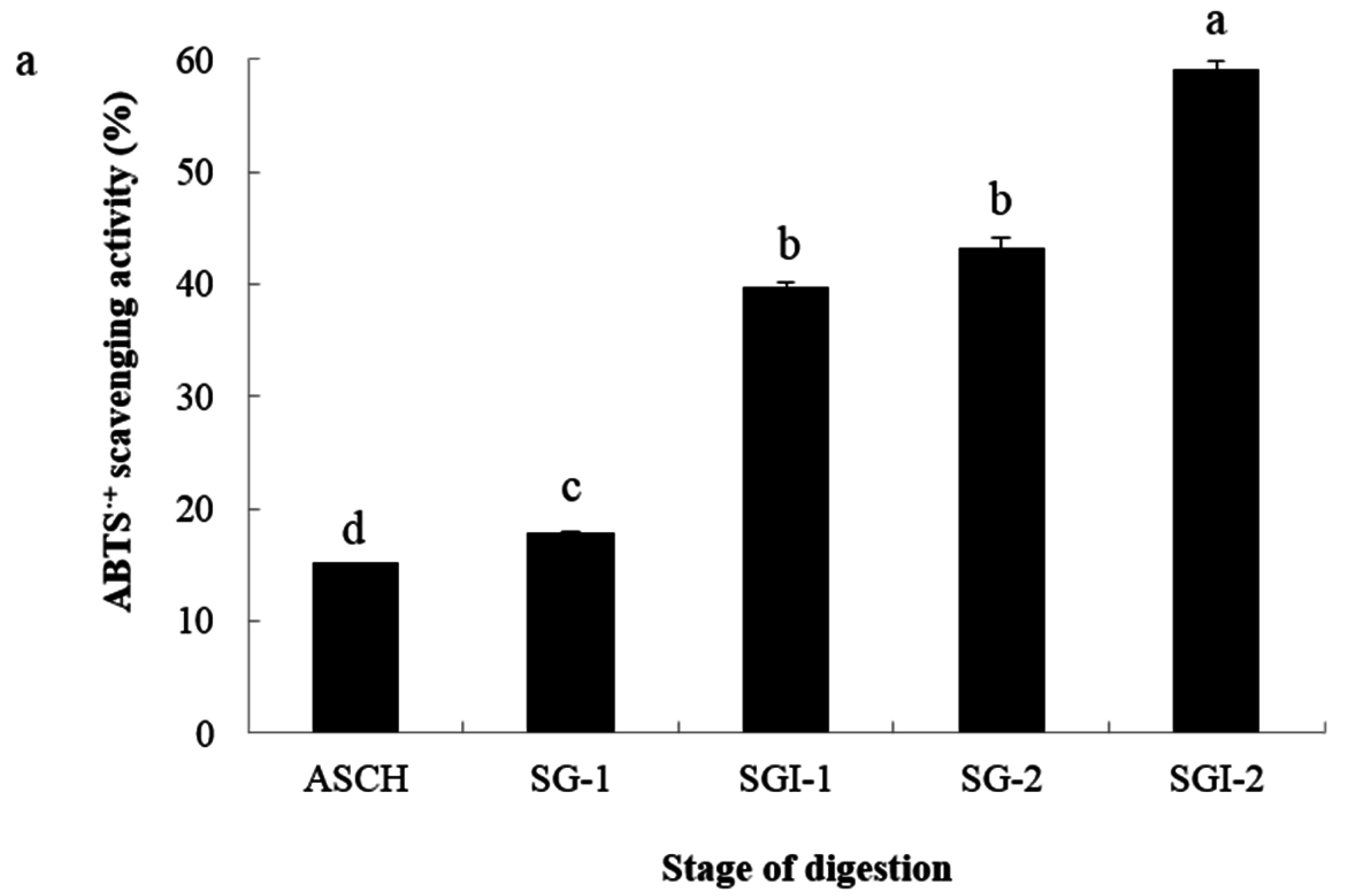
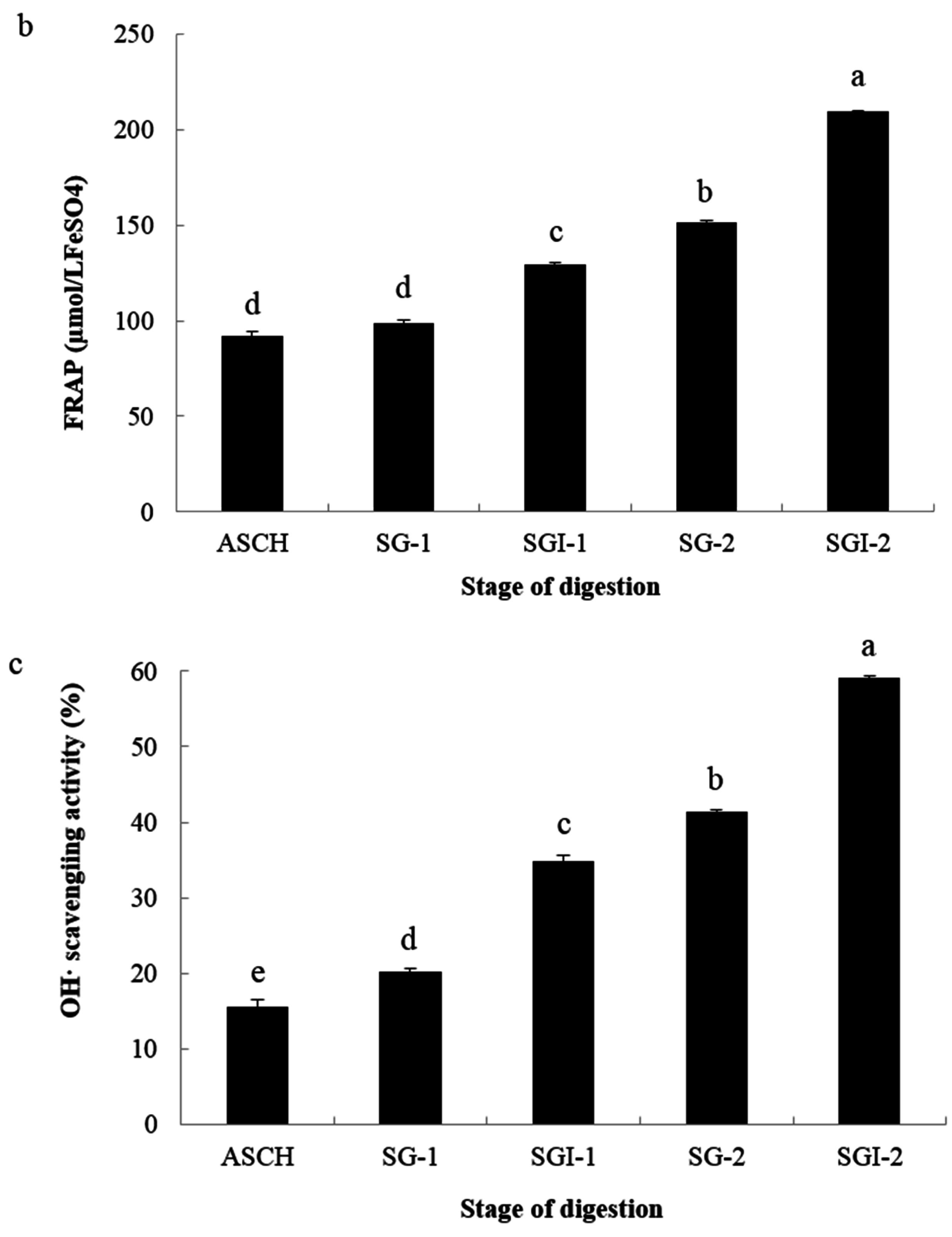
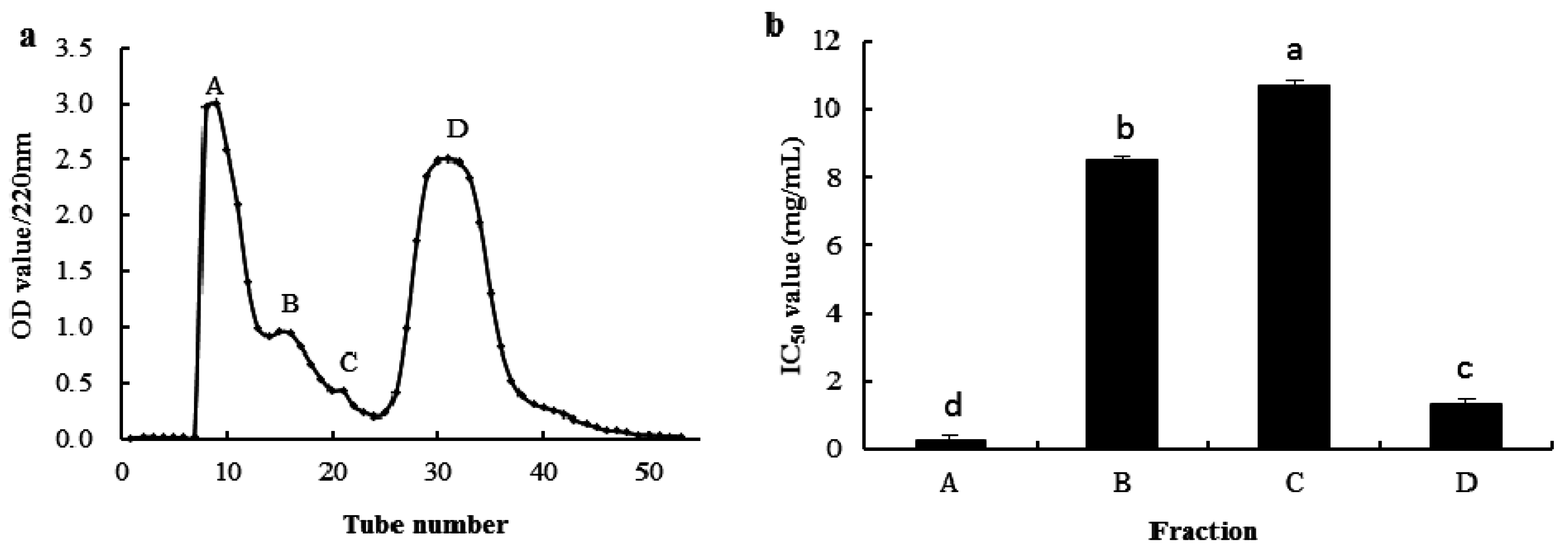

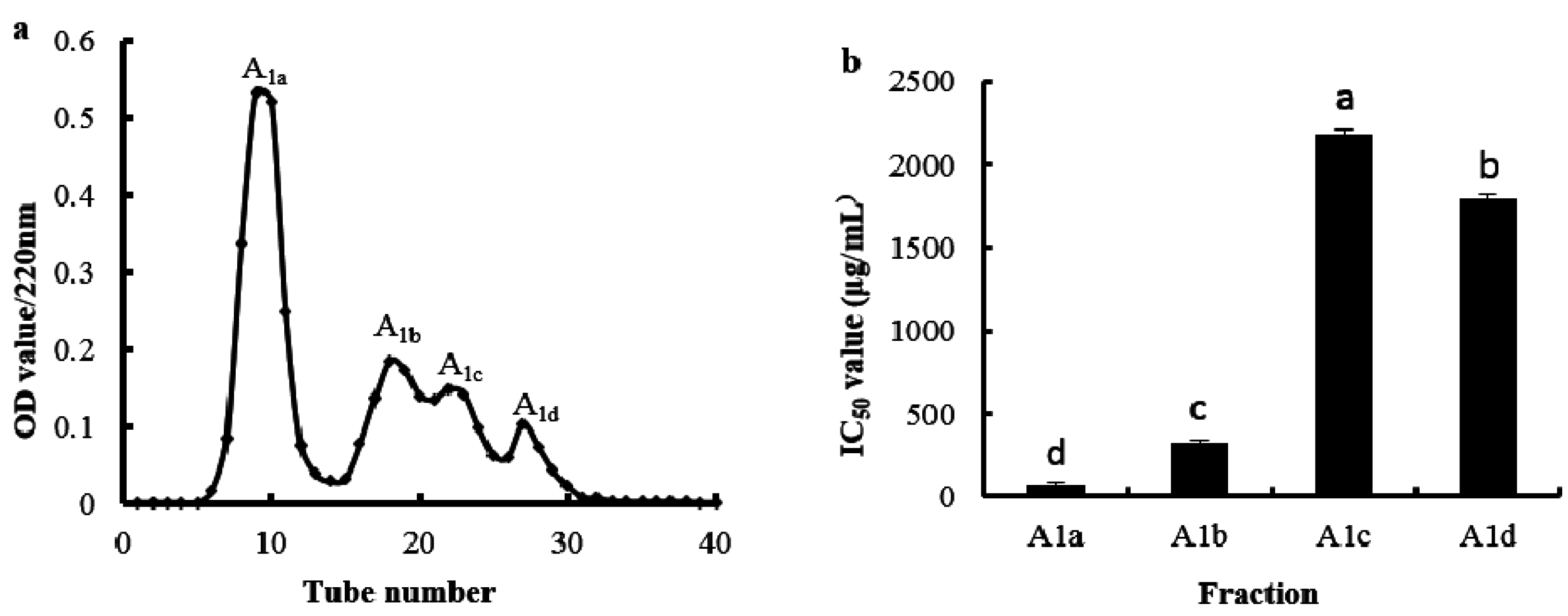
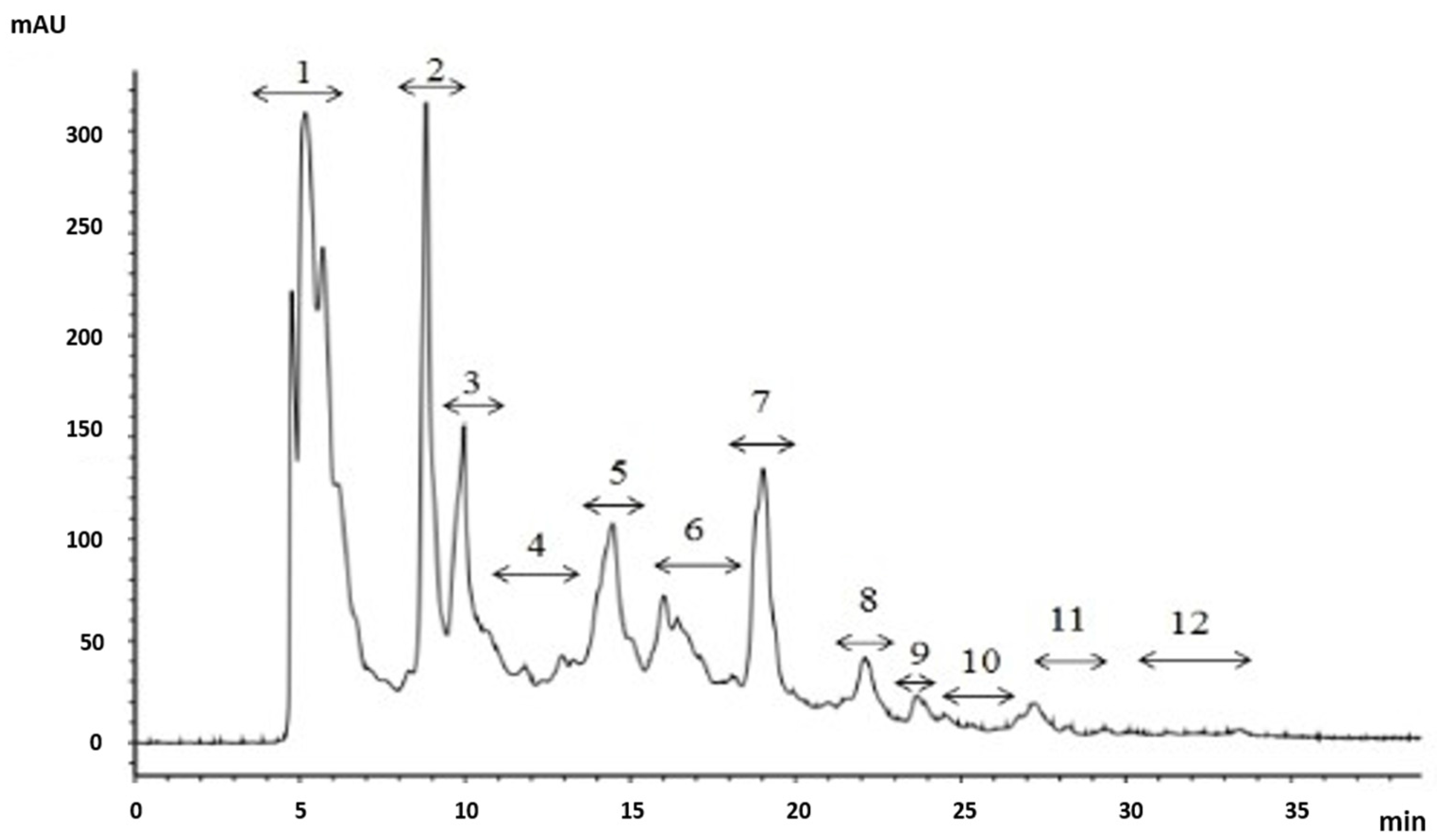
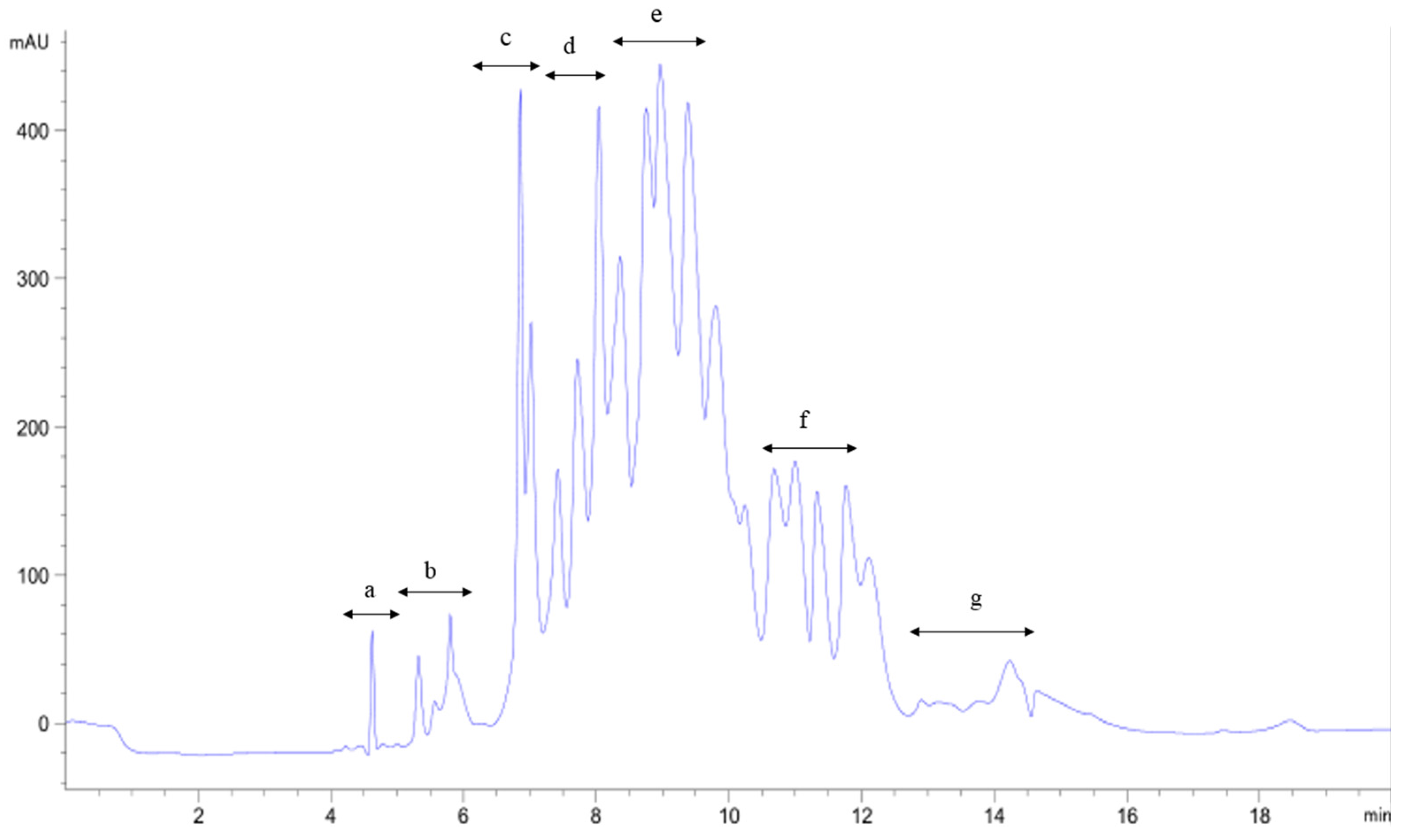
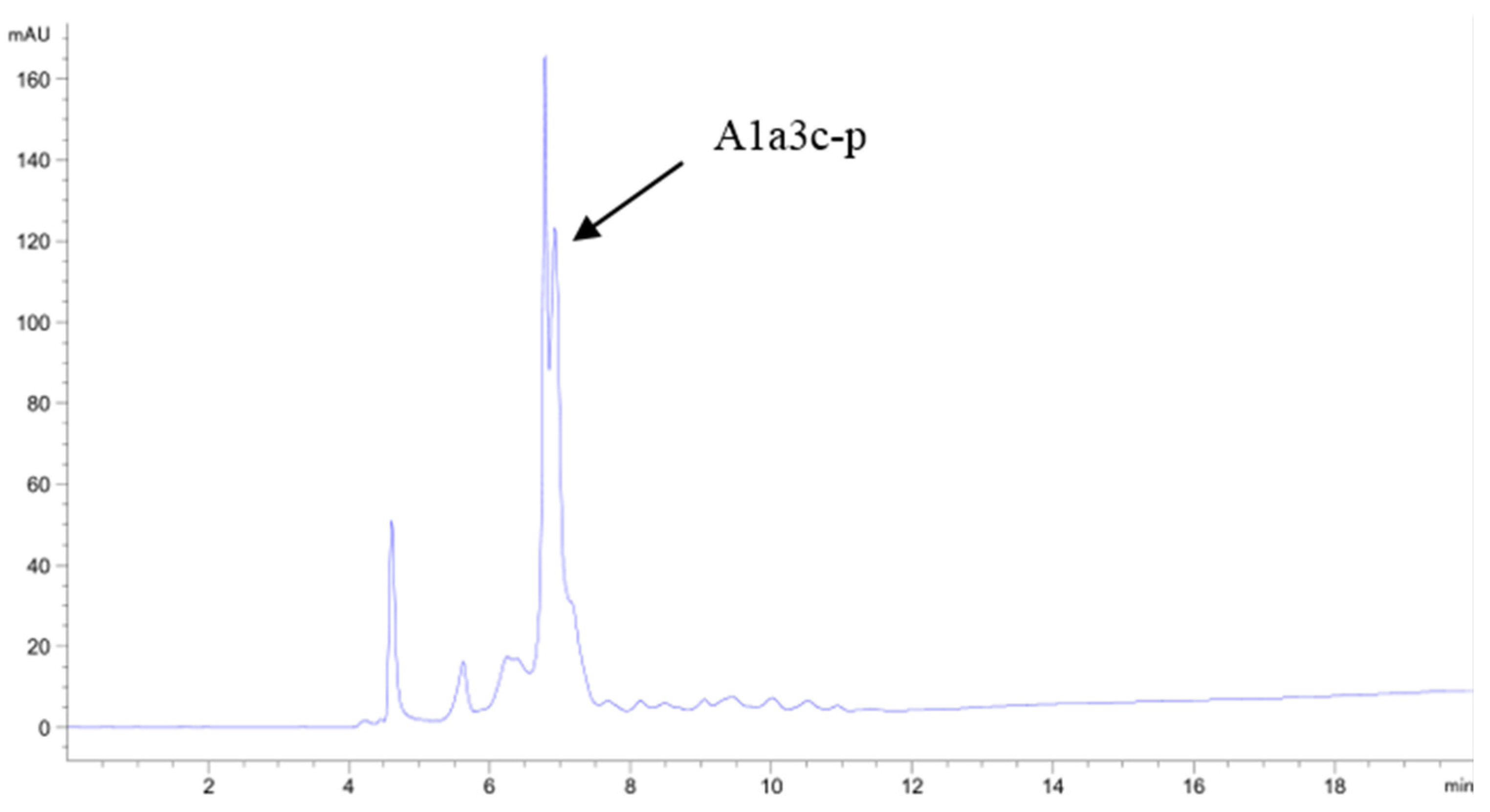
| Hydrolysates | ASCH | SGI-1 | SGI-2 | |||
|---|---|---|---|---|---|---|
| Num | MW (Da) | Content (%) * | MW (Da) | Content (%) | MW (Da) | Content (%) |
| 1 | 3198.76 | 66.82 | 1552.34 | 74.66 | 1026.26 | 59.49 |
| 2 | 2245.49 | 16.45 | 976.83 | 18.04 | 640.53 | 18.34 |
| 3 | 647.39 | 8.09 | 505.59 | 5.08 | 284.97 | 16.60 |
| 4 | 199.63 | 8.08 | 180.09 | 2.15 | 96.58 | 4.56 |
| Amino Acids | ASCH | SGI-1 | SGI-2 |
|---|---|---|---|
| Asp | 54.19 | 56.37 | 56.81 |
| Thr | 25.56 | 26.43 | 27.37 |
| Ser | 64.78 | 64.81 | 62.17 |
| Glu | 75.24 | 76.53 | 76.38 |
| Gly | 314.42 | 325.46 | 319.66 |
| Ala | 109.31 | 107.95 | 105.06 |
| Cys | 31.80 | 19.17 | 23.18 |
| Val | 17.90 | 19.44 | 20.56 |
| Met | 15.01 | 14.53 | 14.39 |
| Ile | 11.82 | 12.49 | 13.42 |
| Leu | 22.25 | 22.71 | 23.58 |
| Tyr | 3.40 | 3.77 | 4.90 |
| Phe | 13.04 | 12.81 | 13.89 |
| Lys | 27.95 | 28.70 | 30.59 |
| NH3 | 57.51 | 54.29 | 69.71 |
| His | 8.83 | 8.87 | 9.17 |
| Arg | 53.01 | 52.55 | 35.25 |
| Pro | 93.99 | 93.13 | 93.90 |
| THAA* | 315.11 | 302.22 | 307.98 |
| Total | 1000 | 1000 | 1000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Chang, W.; Ma, Q.; Zhuang, Y. Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen. Mar. Drugs 2016, 14, 186. https://doi.org/10.3390/md14100186
Sun L, Chang W, Ma Q, Zhuang Y. Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen. Marine Drugs. 2016; 14(10):186. https://doi.org/10.3390/md14100186
Chicago/Turabian StyleSun, Liping, Weidan Chang, Qingyu Ma, and Yongliang Zhuang. 2016. "Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen" Marine Drugs 14, no. 10: 186. https://doi.org/10.3390/md14100186





