Anti-Diabetic Effects of CTB-APSL Fusion Protein in Type 2 Diabetic Mice
Abstract
:1. Introduction
2. Results
2.1. Analysis of Recombinant Transfer Vector pFastBac1-CTB-APSL
2.2. Analysis of Recombinant Bacmid
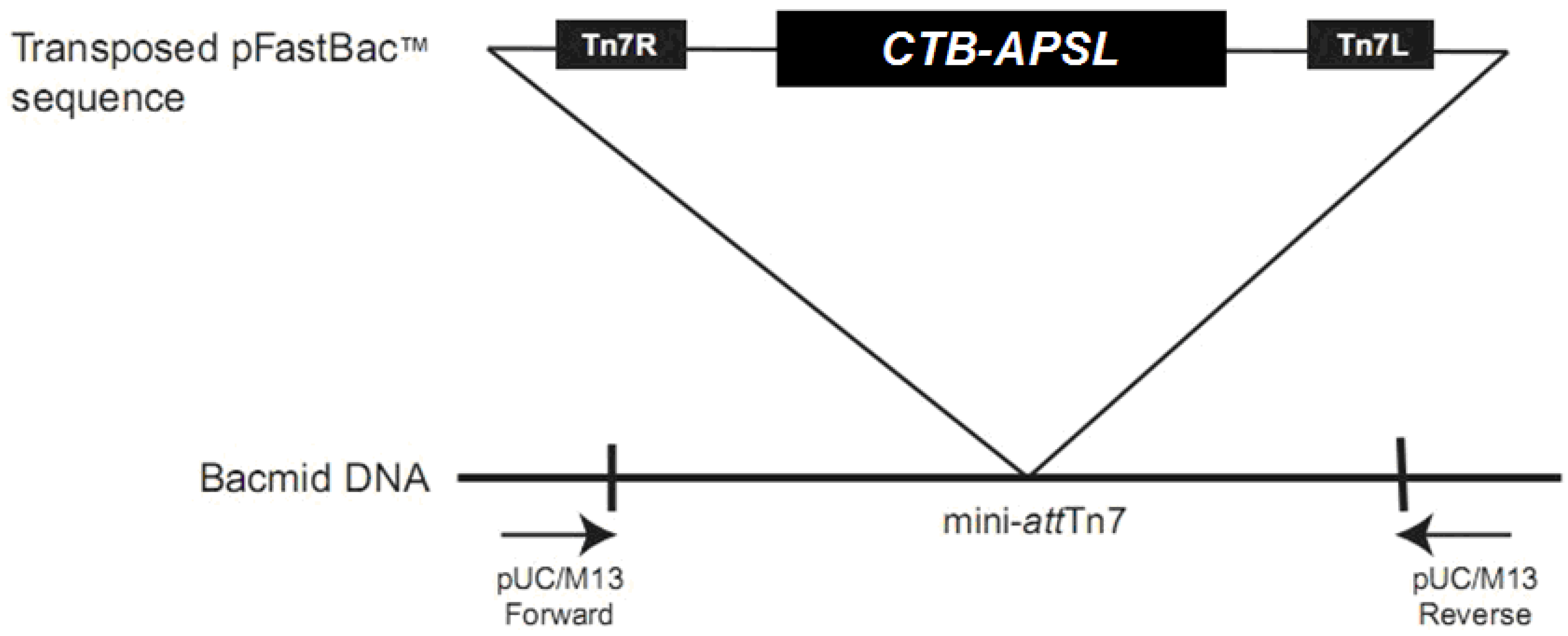
2.3. Analysis of Recombinant Virus

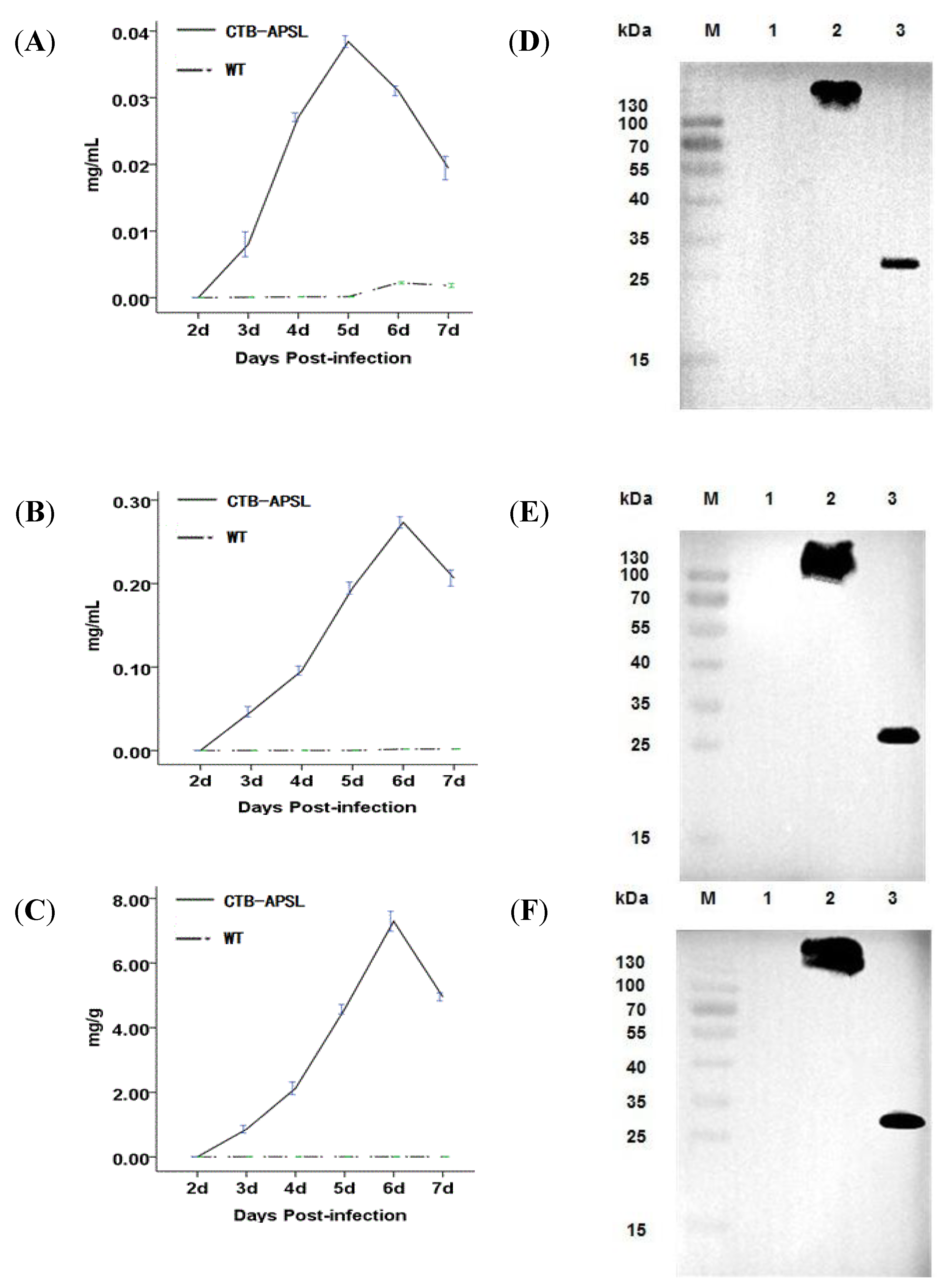
2.4. Expression of CTB-APSL Fusion Protein in Silkworm
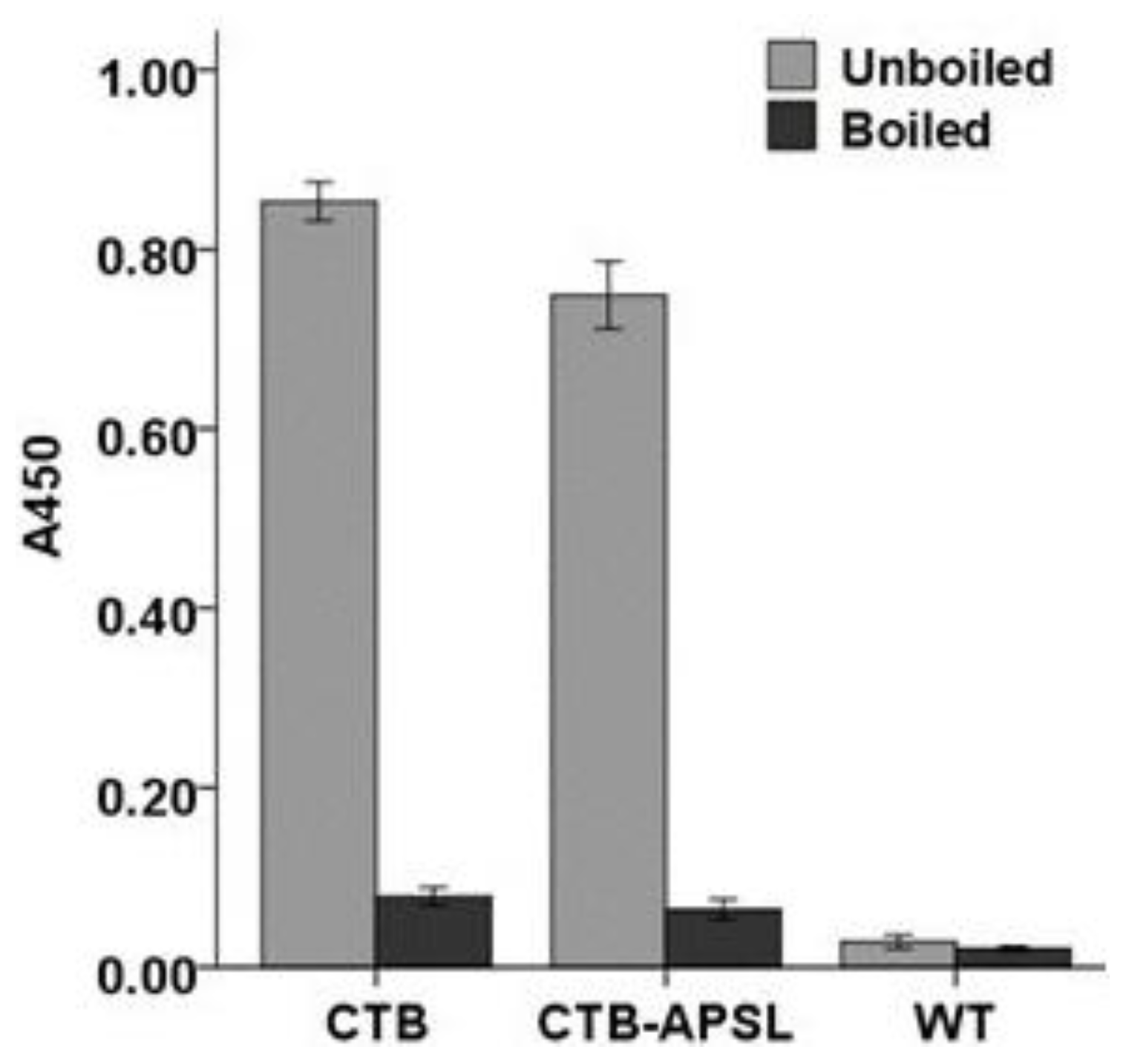
2.5. Affinity of CTB-APSL Fusion Protein for GM1 Ganglioside
2.6. Changes in Body Weight, Fasting Blood Glucose, Kidney Coefficient and Spleen Coefficient
| Group Weight (g) | FBG (mmol/L) | GHb | Kidney Coefficient | |
|---|---|---|---|---|
| Control | 38.68 ± 1.40 *** | 5.27 ± 0.58 *** | 15.60 ± 2.27 *** | 0.0172 ± 0.0009 *** |
| Diabetic | 33.53 ± 1.84 | 18.14 ± 2.12 | 39.04 ± 5.67 | 0.0204 ± 0.0028 |
| WT | 34.33 ± 2.22 | 16.30 ± 1.97 * | 34.13 ± 3.70 * | 0.0192 ± 0.0017 |
| CTB-APSL | 36.38 ± 2.05 ** | 14.03 ± 2.09 *** | 24.15 ± 4.22 *** | 0.0182 ± 0.0013 ** |
| Metformin | 34.72 ± 1.85 | 13.49 ± 1.88 *** | 23.53 ± 3.45 *** | 0.0183 ± 0.0013 ** |
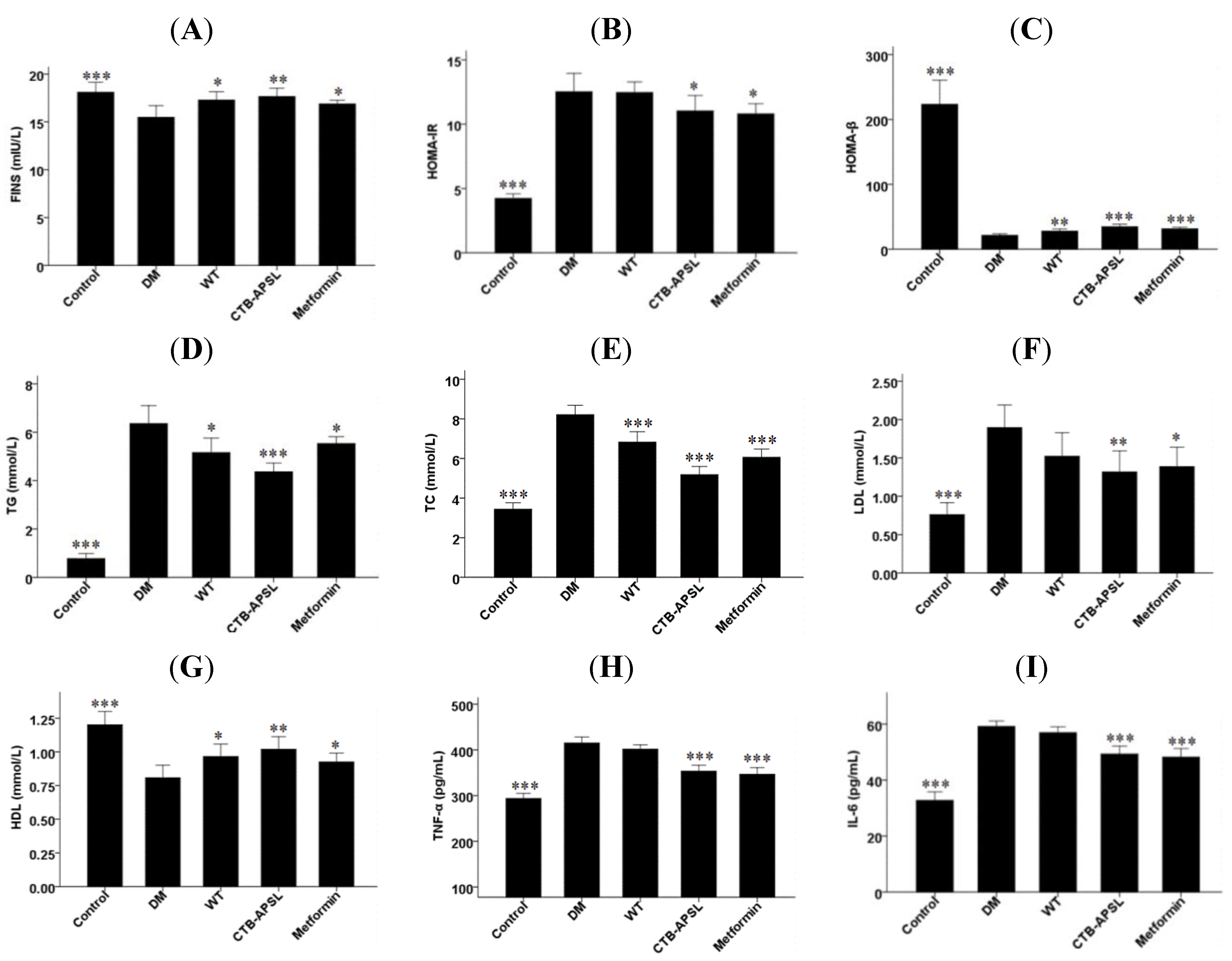
2.7. FINS, HOMA-IR, HOMA-β, TG, TC, LDL, HDL, TNF-α and IL-6 Levels in Experimental Animals
2.8. Histological Analysis of Mouse Pancreatic, Hepatic and Nephritic Tissues in Experimental Animals

3. Discussion
4. Experimental
4.1. Materials
4.2. Construction of Recombinant Transfer Vector
4.3. Transfection and Acquisition of the Recombinant Virus
4.4. Expression and Collection of the CTB-APSL Fusion Protein
4.5. Western Blotting and ELISA Assay
4.6. GM1 Binding Assay
4.7. Diabetic Mouse Models
4.8. Drug Delivery
4.9. Specimen Collection and Biochemical Indicator
4.10. Histological Analysis
4.11. Statistical Analysis
5. Conclusions
Acknowledgments
Authors Contribution
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2012, 35, S64–S71. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 5th ed.; International Diabetes Federation: Brussels, Belgium, 2011. [Google Scholar]
- Szybinski, Z. Polish Multicenter Study on Diabetes Epidemiology (PMSDE) 1998–2000. Pol. Arch. Med. Wewn. 2001, 106, 751–758. [Google Scholar]
- National Institutes of Health; National Institute of Diabetes and Digestive and Kidney Diseases. Complications of Diabetes. Available online: http://diabetes.niddk.nih.gov/complications (accessed on 6 June 2012).
- International Diabetes Federation. Global Diabetes Plan 2011–2021. Available online: http://www.idf.org/global-diabetes-plan-2011-2021 (accessed on 18 September 2011).
- Hutton, J.C.; Davidson, H.W. Cytokine-induced dicing and splicing in the β-cell and the immune response in type 1 diabetes. Diabetes 2010, 59, 335–336. [Google Scholar] [CrossRef]
- Lundgren, V.M.; Isomaa, B.; Lyssenko, V.; Laurila, E.; Korhonen, P.; Groop, L.C.; Tuomi, T. GAD antibody positivity predicts type 2 diabetes in an adult population. Diabetes 2010, 59, 416–422. [Google Scholar] [CrossRef]
- Kahn, S.E. The importance of β-cell failure in the development and progression of type 2 diabetes. J. Clin. Endocr. Metab. 2001, 86, 4047–4058. [Google Scholar]
- Spander, B.D. Structure and function of cholera toxin and related Escherichia coli heat-lable enterotoxin. Microbiol. Rev. 1992, 56, 622–643. [Google Scholar]
- Sun, J.B.; Holmgren, J.; Czerkinsky, C. Cholera toxin B subunit: An efficient transmucosal carrier-dilivery system for induction of peripheral immunological tolerance. Proc. Natl. Acad. Sci. USA 1994, 91, 10795–10799. [Google Scholar] [CrossRef]
- Arakawa, T.; Yu, J.; Chong, D.K.; Hough, J.; Engen, P.C.; Langridge, W.H. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 1998, 16, 934–938. [Google Scholar] [CrossRef]
- Limaye, A.; Koya, V.; Samsam, M.; Daniell, H. Receptor-mediated oral delivery of a bioencapsulated green fluorescent protein expressed in transgenic chloroplasts into the mouse circulatory system. FASEB J. 2006, 20, 37–46. [Google Scholar]
- LaBrecque, D.R.; Pesch, L.A. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J. Phys. 1975, 248, 273–284. [Google Scholar]
- Labrecque, D.R.; Steele, G.; Fogertym, S.; Wilson, M.; Barton, J. Purification and physical-chemical characterization of hepatic stimulator substance. Hepatology 1987, 7, 100–106. [Google Scholar] [CrossRef]
- Huang, F.; Lv, Z.; Li, Q.; Wei, L.; Zhang, L.; Wu, W. Study on hepatoprotective effect of peptide S-8300 from shark liver. World J. Gastroenterol. 2005, 11, 1809–1812. [Google Scholar]
- Ou, Y.; Li, Q.; Lv, Z.; WU, W.; Wang, Q. Purification and characterization of hepatocyte regeneration stimulatory factor from shark liver. J. Chin. Pharm. Sci. 2003, 12, 175–180. [Google Scholar]
- Hagiya, M.; Francavilla, A.; Polimeno, L.; Ihara, I.; Sakai, H.; Seki, T.; Shimonishi, M.; Poter, K.A.; Starzl, T.E. Cloning and sequence analysis of the rat augmenter of liver regeneration (ALR) gene: Expression of biologically active recombinant ALR and demonstration of tissue distribution. Proc. Natl. Acad. Sci. USA 1994, 91, 8142–8146. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.; Wu, W. Purification and characterization of a new peptide (S-8300) from shark liver. J. Food Biochem. 2010, 34, 962–970. [Google Scholar] [CrossRef]
- Lv, Z.; Ou, Y.; Li, Q.; Zhang, W.; Ye, B.; Wu, W. Expression, purification and bioactivities analysis of recombinant active peptide from shark liver. Mar. Drugs 2009, 7, 258–267. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Chen, J.; Zhang, W.; Sheng, Q.; Chen, J.; Yu, W.; Nie, Z.; Zhang, Y.; Wu, W.; et al. A shark liver gene-derived active peptide expressed in the silkworm, Bombyx mori: Preliminary studies for oral administration of the recombinant protein. Mar. Drugs 2013, 11, 1492–1505. [Google Scholar] [CrossRef]
- Orit, P.H.; Philip, Z. Acute and chronic complication of type 2 diabetes mellitus in children and adolescents. Lancet 2007, 369, 1823–1831. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. Br. Med. J. 2000, 321, 405–412. [Google Scholar] [CrossRef]
- World Health Organization. Preventing Chronic Diseases: A Vital Investment; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Mehnert, H. Metformin, the rebirth of a biguanide: Mechanism of action and place in the prevention and treatment of insulin resistance. Exp. Clin. Endocrinol. Diabetes 2001, 109, 259–264. [Google Scholar] [CrossRef]
- Raptis, S.A.; Dimitriadis, G.D. Oral hypoglycemic agents: Insulin secretagogues, alpha-glucosidase inhibitors and insulin sensitizers. Exp. Clin. Endocrinol. Diabetes 2001, 109, 265–287. [Google Scholar] [CrossRef]
- Rai, A.K.; Rai, D.K. Spectroscopic studies of some antidiabetic drugs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2003, 59, 1673–1680. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy. Diabetes Care 2006, 8, 1963–1972. [Google Scholar]
- Yi, P.; Park, J.S.; Melton, D.A. Betatrophin: A hormone that controls pancreatic β cell proliferation. Cell 2013, 153, 747–758. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, M.; An, W. Increased hepatic apoptosis in high-fat diet-induced NASH in rats may be associated with down regulation of hepatic stimulator substance. J. Mol. Med. 2011, 89, 1207–1217. [Google Scholar] [CrossRef]
- Lupi, R.; Dotta, F.; Marselli, L.; Del Guerra, S.; Masini, M.; Santangelo, C.; Patané, G.; Boggi, U.; Piro, S.; Anello, M.; et al. Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence the beta-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 2002, 51, 1437–1442. [Google Scholar] [CrossRef]
- Piro, S.; Anello, M.; Pietro, C.D.; Lizzio, M.N.; Patanè, G.; Rabuazzo, A.M.; Vigneri, R.; Purrello, M.; Purrello, F. Chronic exposure to free fatty acid or high glucose induces apoptosis in rat pancreatic islets: possible role of oxidative stree. Metabolism 2002, 51, 1340–1347. [Google Scholar] [CrossRef]
- Pickup, J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004, 27, 813–823. [Google Scholar] [CrossRef]
- Ortis, F.; Naamane, N.; Flamez, D.; Ladrière, L.; Moore, F.; Cunha, D.A.; Colli, M.L.; Thykjaer, T.; Thorsen, K.; Ørntoft, T.F.; et al. Cytokines interleukin-1β and tumor necrosis factor-α regulate different transcriptional and alternative splicing networks in primary β-cells. Diabetes 2010, 59, 358–374. [Google Scholar] [CrossRef]
- Steer, S.A.; Scarim, A.L.; Chambers, K.; Corbett, J.A. Interleukin-1 stimulates beta-cell necrosis and release of the immunological adjuvant HMGB1. PLoS Med. 2006, 3, e171. [Google Scholar] [CrossRef]
- Vincent, J.A.; Mohr, S. Inhibition of caspase21/interleukin-1β signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes 2007, 56, 224–230. [Google Scholar] [CrossRef]
- Tsiavou, A.; Hatziagelaki, E.; Chaidaroglou, A.; Manginas, A.; Koniavitou, K.; Degiannis, D.; Raptis, S.A. TNF-α, TGF-β, IL-10, IL-6, gene polymorphisms in latent autoimmune diabetes of adults (LADA) and type 2 diabetes mellitus. Clin. Immunol. 2004, 24, 591–599. [Google Scholar] [CrossRef]
- Miao, Y.; Zhang, Y.; Nakagaki, K.; Zhao, T.; Zhao, A.; Meng, Y.; Nakagaki, M.; Park, E.Y.; Maenaka, K. Expression of spider flagelliform silk protein in Bombyx mori cell line by a novel Bac-to-Bac/BmNPV baculovirus expression system. Appl. Microbiol. Biotechnol. 2006, 71, 192–199. [Google Scholar] [CrossRef]
- Carbonell, L.F.; Miller, L.K. Baculovirus interaction with nontarget organisms: A virus-borne reporter gene is not expressed in two mammalian cell lines. Environ. Microbiol. 1987, 53, 1412–1417. [Google Scholar]
- Herrington, D.A.; Losonsdy, G.A.; Smith, G.; Vovovitz, F.; Cochran, M.; Jackson, K.; Hoffman, S.L.; Gordon, D.M.; Levine, M.M.; Edelman, R. Safety and immunogenicity in volunteers of a recombinant Plasmodium falciparum circumsporozoite protein malaria vaccine produced in Lepidopteran cells. Vaccine 1992, 10, 841–846. [Google Scholar] [CrossRef]
- Yue, W.; Miao, Y.; Li, X.; Wu, X.; Zhao, A.; Nakagaki, M. Cloning and expression of manganese superoxide dismutase of the silkworm, Bombyx mori by Bac-to-Bac/BmNPV Baculovirus expression system. Appl. Microbiol. Biotechnol. 2006, 73, 181–186. [Google Scholar] [CrossRef]
- Kato, T.; Kajikawa, M.; Maenaka, K.; Park, E.Y. Silkworm expression system as a platform technology in life science. Appl. Microbiol. Biotechnol. 2010, 85, 459–470. [Google Scholar] [CrossRef]
- Chen, L.; Song, L.; Li, T.; Zhu, J.; Xu, J.; Zheng, Q.; Yu, R. A new antiproliferative and antioxidant peptide isolated from Arca subcrenata. Mar. Drugs 2013, 11, 1800–1814. [Google Scholar] [CrossRef]
- Giri, A.; Ohshima, T. Bioactive marine peptides: Nutraceutical value and novel approaches. Adv. Food Nutr. Res. 2012, 65, 73–105. [Google Scholar] [CrossRef]
- Zhang, J.; Pritchard, E.; Hu, X.; Valentin, T.; Panilaitis, B.; Omenetto, F.G.; Kaplan, D.L. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc. Natl. Acad. Sci. USA 2012, 109, 11981–11986. [Google Scholar]
- Ryu, K.S.; Lee, H.S.; Kim, K.Y.; Kim, M.J.; Kang, P.D.; Chun, S.N.; Lim, S.H.; Lee, M.L. Anti-diabetic effects of the silkworm (Bombyx mori) extracts in the db/db mice. Planta Med. 2012, 78, PI458. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, Y.; Gao, Z.; Guo, Q.; Wang, T.; Lu, C.; Chen, Y.; Sheng, Q.; Chen, J.; Nie, Z.; Zhang, Y.; et al. Anti-Diabetic Effects of CTB-APSL Fusion Protein in Type 2 Diabetic Mice. Mar. Drugs 2014, 12, 1512-1529. https://doi.org/10.3390/md12031512
Liu Y, Gao Z, Guo Q, Wang T, Lu C, Chen Y, Sheng Q, Chen J, Nie Z, Zhang Y, et al. Anti-Diabetic Effects of CTB-APSL Fusion Protein in Type 2 Diabetic Mice. Marine Drugs. 2014; 12(3):1512-1529. https://doi.org/10.3390/md12031512
Chicago/Turabian StyleLiu, Yunlong, Zhangzhao Gao, Qingtuo Guo, Tao Wang, Conger Lu, Ying Chen, Qing Sheng, Jian Chen, Zuoming Nie, Yaozhou Zhang, and et al. 2014. "Anti-Diabetic Effects of CTB-APSL Fusion Protein in Type 2 Diabetic Mice" Marine Drugs 12, no. 3: 1512-1529. https://doi.org/10.3390/md12031512




